62
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
BLOOD RESEARCH Volume 55ㆍNumber 1ㆍMarch 2020
Letters to the Editor
Clinical significance of
myeloproliferative neoplasms with JAK2 V617F mutations and major BCR-ABL1 translocations:
a literature review with case presentation
TO THE EDITOR: It is well known that BCR-ABL1 trans- locations and JAK2V617F mutations are mutually exclusive events in myeloproliferative neoplasms (MPN) [1]. However, the coexistence of both abnormalities has been reported in several cases [1-6]. Until now, two studies have reported the co-occurrence of BCR-ABL1 translocation and JAK2V617F mutation in Korea [3, 4]. Here, we discuss the clinical sig- nificance through a literature review, especially focusing on cases reported in Korea with a case presentation.
A 68-year-old woman was admitted with increased hemo- globin (Hb) and leukocyte count. Complete blood count (CBC) at admission showed the following: white blood cells, 17.06×109/L; Hb, 17.3 g/dL; and platelets, 245×109/L. Peripheral blood smear demonstrated leukoerythroblastosis with the presence of few tear drop cells. Differential counts of leuko- cytes were as follows: myelocytes 1%, metamyelocytes 1%, neutrophil 84%, lymphocytes 9%, monocytes 4%, eosino- phil 1%, and rare nucleated red blood cells. Her serum lactate dehydrogenase level was elevated (313 IU/L, refer- ence range, 0–250 IU/L) while her serum erythropoietin level was decreased (3.06 mIU/L, reference range, 3.7–37.5 mIU/L). An abdominopelvic computed tomography showed splenomegaly. We could not acquire sufficient information from the bone marrow aspirate, because it was diluted.
However, bone marrow biopsy revealed hypercellularity and a slightly increased number of megakaryocytes (3.4/high power field) with dense clustering and certain degree of atypia (Fig. 1). Diffuse myelofibrosis, with bone marrow fibrosis (MF) grade 2, was detected (Fig. 1). Reverse-tran- scriptase PCR (RT-PCR) assay for the BCR-ABL1 fusion transcript showed the presence of a major BCR-ABL1 fusion
transcript (b13a2 type), which was confirmed by sequencing (Fig. 1). The fusion transcript level was 1.23% on the interna- tional scale (IS). An allele-specific PCR assay for the JAK2V617F mutation was positive for a heterozygous type JAK2V617F mutation with an allelic burden of 79.20%, which was confirmed by sequencing (Fig. 1). The chromoso- mal analysis revealed 46,XX karyotype[20]. Interphase fluo- rescent in situ hybridization for BCR-ABL1 translocation was nuc ish (ABL1, BCR)×2 (ABL1 con BCR×1) [13/500], representing a normal result (below the cutoff level). The final diagnosis revealed MPN, with concurrent JAK2V617F mutation and major BCR-ABL1 translocation. The patient was only treated with hydroxyurea (500 mg, twice a day).
After 10 months of treatment, her CBC became normal (5.35×109/L white blood cells, a Hb of 14.9 g/dL, and 185×109/L platelets) and BCR-ABL1 transcripts level was 0.016% IS.
Detecting BCR-ABL1 translocation and JAK2V617F mu- tation simultaneously at the time of diagnosis is a rare event.
Recent studies have reported that about 0.2–0.5% of MPN patients are positive for both BCR-ABL1 and JAK2V617F [6, 7]. It has been suggested that most MPN cases with concurrent BCR-ABL1 and JAK2V617F consist of two un- related clones [2, 6, 8].
The coexistence of these abnormalities may affect labo- ratory findings and patient diagnosis. Park et al. [3] reported two cases of MPN, with a relative JAK2V617F dominancy over BCR-ABL1 transcripts. Their laboratory findings re- sembled essential thrombocythemia (ET) and primary mye- lofibrosis (PMF). Our patient showed a relative dominancy of JAK2V617F, with the laboratory diagnosis resembling PMF. In contrast, the case reported by Yi and Kim [4]
showed relatively high levels of BCR-ABL1 transcripts.
Laboratory features of both CML and PMF were detected (Table 1). Soderquist et al. [8] have reported that coexistence cases show mixed megakaryocytes characteristics that in- clude both dwarf megakaryocytes, seen in CML, and bulbous megakaryocytes, seen in PMF. Therefore, it is essential to perform tests for BCR-ABL1 and JAK2V617F simultaneously with quantitation, in order to avoid misinterpretation and obtain an accurate diagnosis of MPN.
The molecular abnormality profile can influence the
bloodresearch.or.kr Blood Res 2020;55:62-69.
Letters to the Editor 63
Fig. 1. Bone marrow biopsy showing (A) hypercellular marrow with increased megakaryocytes and atypia (H&E stain, ×400) and (B) myelofibrosis grade MF-2 demonstrated by reticulin staining (reticulin stain, ×200). Reverse transcriptase (RT)-PCR analysis of BCR-ABL1 translocation using a HemaVision kit showing a single 397 bp band in the M6B split out PCR, indicating a fusion transcript with BCR-ABL1 (C). Sequencing analysis of the RT-PCR product showing breakpoint located at exon 13 in the BCR gene and exon 2 in the ABL gene (D). Sequencing analysis of JAK2V617F showing a heterozygous JAK2V617F mutation (E).
choice of treatment modalities. Cases reported by Park et al. [3] and our patient showed acceptable responses to hydroxyurea. However, the case reported by Yi and Kim [4] showed treatment responses to dasatinib (a tyrosine kin- ase inhibitor), hydroxyurea, and ruxolitinib (a JAK1/2 ty- rosine kinase inhibitor) (Table 1). Other studies [6, 9] have reported successful responses to the simultaneous use of tyrosine kinase inhibitors and hydroxyurea or ruxolitinib in patients with concomitant BCR-ABL1 and JAK2V617F.
Therefore, detection of molecular abnormalities is important for appropriate treatment, especially when a decision must be made in the event of therapeutic failure and drug replacement.
Another issue is the impact of molecular abnormalities on patient outcomes. Soderquist et al. [8] have reported that seven of 11 MPN patients with concurrent BCR-ABL1 and JAK2V617F showed progression to myelofibrosis, sug- gesting that these patients are more prone to disease progression. The coexistence of two molecular abnormalities might accelerate the progression. Nevertheless, cases re- ported in Korea have shown successful responses without death, at the time of publication [3, 4]. However, data con-
cerning the progression rate and survival of patients with concomitant BCR-ABL1 and JAK2V617F mutations are cur- rently insufficient.
In summary, it is important to recognize the possibility of the coexistence of a BCR-ABL1 translocation and JAK2V617F mutation in MPN, because they can affect hem- atologic features and treatment strategy. However, it is un- clear if MPNs with concomitant BCR-ABL1 translocations and JAK2V617F mutations reflect a novel clinical entity with distinct features and outcomes. Further investigation with a large cohort is needed.
Bohyun Kim1, Kyu Taek Lee2, Young Ahn Yoon1, Young-Jin Choi1
1Department of Laboratory Medicine, 2Division of Hematology
& Oncology, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
Blood Res2020;55:62-69. bloodresearch.or.kr
64 Letters to the Editor
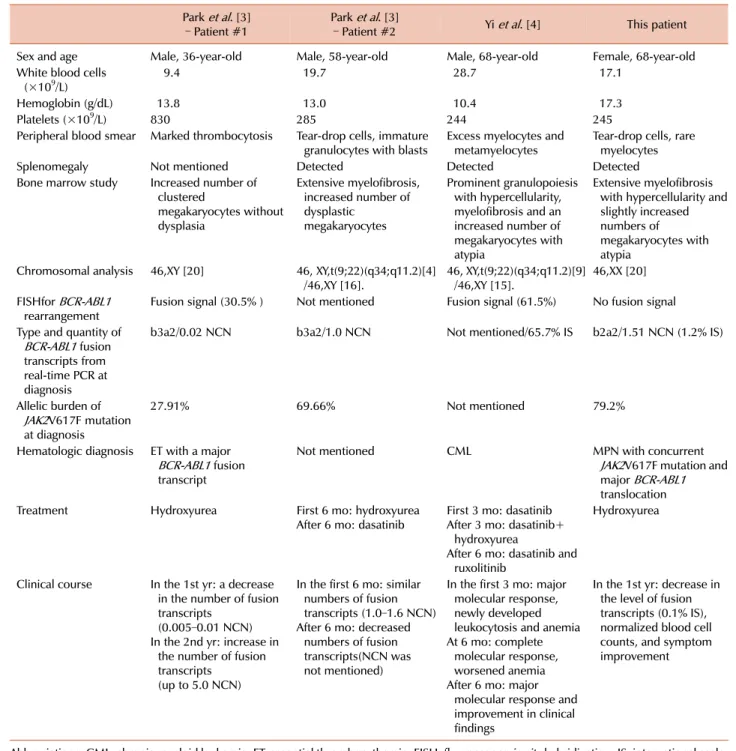
Table 1. Clinical and hematologic features in Korean MPN patients with concurrent BCR-ABL1 translocation and JAK2V617F mutation.
Park et al. [3]
– Patient #1 Park et al. [3]
– Patient #2 Yi et al. [4] This patient
Sex and age Male, 36-year-old Male, 58-year-old Male, 68-year-old Female, 68-year-old White blood cells
(×109/L) 9.4 19.7 28.7 17.1
Hemoglobin (g/dL) 13.8 13.0 10.4 17.3
Platelets (×109/L) 830 285 244 245
Peripheral blood smear Marked thrombocytosis Tear-drop cells, immature
granulocytes with blasts Excess myelocytes and
metamyelocytes Tear-drop cells, rare myelocytes
Splenomegaly Not mentioned Detected Detected Detected
Bone marrow study Increased number of clustered
megakaryocytes without dysplasia
Extensive myelofibrosis, increased number of dysplastic
megakaryocytes
Prominent granulopoiesis with hypercellularity, myelofibrosis and an increased number of megakaryocytes with atypia
Extensive myelofibrosis with hypercellularity and slightly increased numbers of megakaryocytes with atypia
Chromosomal analysis 46,XY [20] 46, XY,t(9;22)(q34;q11.2)[4]
/46,XY [16]. 46, XY,t(9;22)(q34;q11.2)[9]
/46,XY [15]. 46,XX [20]
FISHfor BCR-ABL1
rearrangement Fusion signal (30.5% ) Not mentioned Fusion signal (61.5%) No fusion signal Type and quantity of
BCR-ABL1 fusion transcripts from real-time PCR at diagnosis
b3a2/0.02 NCN b3a2/1.0 NCN Not mentioned/65.7% IS b2a2/1.51 NCN (1.2% IS)
Allelic burden of JAK2V617F mutation at diagnosis
27.91% 69.66% Not mentioned 79.2%
Hematologic diagnosis ET with a major BCR-ABL1 fusion transcript
Not mentioned CML MPN with concurrent
JAK2V617F mutation and major BCR-ABL1 translocation
Treatment Hydroxyurea First 6 mo: hydroxyurea
After 6 mo: dasatinib First 3 mo: dasatinib After 3 mo: dasatinib+
hydroxyurea
After 6 mo: dasatinib and ruxolitinib
Hydroxyurea
Clinical course In the 1st yr: a decrease in the number of fusion transcripts
(0.005–0.01 NCN) In the 2nd yr: increase in
the number of fusion transcripts
(up to 5.0 NCN)
In the first 6 mo: similar numbers of fusion transcripts (1.0–1.6 NCN) After 6 mo: decreased
numbers of fusion transcripts(NCN was not mentioned)
In the first 3 mo: major molecular response, newly developed leukocytosis and anemia At 6 mo: complete
molecular response, worsened anemia After 6 mo: major
molecular response and improvement in clinical findings
In the 1st yr: decrease in the level of fusion transcripts (0.1% IS), normalized blood cell counts, and symptom improvement
Abbreviations: CML, chronic myeloid leukemia; ET, essential thrombocythemia; FISH, fluorescence in situ hybridization; IS, international scale;
MPN, myeloproliferative neoplasms; NCN, normalized copy number; PCR, polymerase chain reaction.
Correspondence to: Bohyun Kim Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, 31 Suncheonhyang 6-gil, Dongnam-gu, Cheonan 31151, Korea
E-mail: bhkim@schmc.ac.kr
Received on Nov. 29, 2019; Revised on Jan. 10, 2020; Accepted on Jan. 13, 2020 https://doi.org/10.5045/br.2020.55.1.62
AuthorsÊ Disclosures of Potential Conflicts of Interest No potential conflicts of interest relevant to this article were reported.
REFERENCES
1. Xiao X, Zhang Y, Zhang GS, Zheng WL, Xiao L, Liu SF.
Coexistence of JAK2V617F mutation and BCR-ABL1 transcript in two Chinese patients with chronic myelogenous leukemia.
bloodresearch.or.kr Blood Res 2020;55:62-69.
Letters to the Editor 65
Acta Haematol 2012;127:47-9.
2. Martin-Cabrera P, Haferlach C, Kern W, Schnittger S, Haferlach T. BCR-ABL1-positive and JAK2 V617F-positive clones in 23 pa- tients with both aberrations reveal biologic and clinical importance. Br J Haematol 2017;176:135-9.
3. Park SH, Chi HS, Cho YU, et al. Two cases of myeloproliferative neoplasm with a concurrent JAK2 (V617F) mutation and BCR/ABL translocation without chronic myelogenous leukemia pheno- type acquisition during hydroxyurea treatment. Ann Lab Med 2013;33:229-32.
4. Yi JH, Kim HR. Coexistence of BCR/ABL1-positive chronic mye- loid leukemia and JAK2 V617F-mutated myelofibrosis success- fully treated with dasatinib and ruxolitinib. Blood Res 2019;
54:77-9.
5. Bader G, Dreiling B. Concurrent JAK2-positive myeloprolifer- ative disorder and chronic myelogenous leukemia: a novel en- tity? A case report with review of the literature. J Investig Med High Impact Case Rep 2019;7:2324709619832322.
6. Lewandowski K, Gniot M, Wojtaszewska M, et al. Coexistence of JAK2 or CALR mutation is a rare but clinically important event in chronic myeloid leukemia patients treated with tyrosine kin- ase inhibitors. Int J Lab Hematol 2018;40:366-71.
7. Agarwal R, Blombery P, McBean M, et al. Clinicopathological differences exist between CALR- and JAK2-mutated myelopro- liferative neoplasms despite a similar molecular landscape: data from targeted next-generation sequencing in the diagnostic laboratory. Ann Hematol 2017;96:725-32.
8. Soderquist CR, Ewalt MD, Czuchlewski DR, et al. Myelopro- liferative neoplasms with concurrent BCR-ABL1 translocation and JAK2 V617F mutation: a multi-institutional study from the bone marrow pathology group. Mod Pathol 2018;31:690-704.
9. Zhou A, Knoche EM, Engle EK, Fisher DA, Oh ST. Concomitant JAK2 V617F-positive polycythemia vera and BCR-ABL-positive chronic myelogenous leukemia treated with ruxolitinib and dasatinib. Blood Cancer J 2015;5:e351.
Daratumumab in dialysis-dependent multiple myeloma
TO THE EDITOR: Daratumumab is an IgG1 kappa mono- clonal antibody against CD38, overexpressed by myeloma cells. It acts by several mechanisms, including triggering complement-dependent cytotoxicity and antibody-depend- ent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and apoptosis [1]. The combination of dar- atumumab, lenalidomide, and dexamethasone has demon- strated remarkable overall response rates (92.9%) in patients with relapsed myeloma [2]. Daratumumab clinical trials have excluded patients with a creatinine clearance <20 mL/min [3]. Data on daratumumab therapy in renal failure patients requiring dialysis are scarce, even though pharma- cokinetic data suggest that it can be safely used without
dose modification in patients with creatinine clearance <30 mL/min [4]. Here, we present our experience with dar- atumumab in two patients with severe renal impairment.
A 36-year-old female with no previous comorbidities, presented with anemia and renal failure (serum creatinine, 8.4 mg/dL; urine output, 700 mL/d). The M component was 0.22 g/dL, serum kappa () light chain was 3,650 mg/L, and the lambda light chain () was 7.41 mg/L. The difference in free light chain (dFLC) was 3,642 mg/L. Bone marrow evaluation demonstrated 50% clonal plasma cells. The mye- loma fluorescence in-situ hybridization (FISH) panel was negative. The patient was diagnosed with IgG kappa multiple myeloma R-ISS stage III with severe renal impairment re- quiring dialysis and started on a cyclophosphamide, bortezo- mib, dexamethasone (CyBorD) regimen. After 2 weeks of CyBorD therapy, the patient developed hyperemesis due to cyclophosphamide. Cyclophosphamide was replaced with thalidomide. Bortezomib, thalidomide, and dexamethasone (VTD) were administered for 2 weeks, following which thalidomide was discontinued owing to severe myalgia.
Next, the ABCD regimen, comprising liposomal doxor- ubicin, bortezomib, cyclophosphamide (100 mg D1-D15), and dexamethasone (40 mg weekly), was initiated. The pa- tient tolerated the ABCD therapy well, and disease evalua- tion performed after two ABCD cycles presented stable dis- ease (>3,650 mg/L; , 7.41 mg/L and M spike of 0.24 g/dL), with a continuing need for dialysis. As no disease response was observed, daratumumab (16 mg/kg/dose week- ly ×8 doses, followed by 2-weekly ×8 doses, then monthly), lenalidomide (5 mg), and dexamethasone were administered.
To avoid fluid overload, the daratumumab infusion was administered after the dialysis session, and the infusion rate did not exceed 100 mL/h. No infusion reactions or cytopenia were observed. The patient was dialysis-independent after the fourth daratumumab dose, reporting a serum creatinine stabilized at 4.3 mg/dL. After the ninth daratumumab dose, disease evaluation demonstrated a Very Good Partial Response (VGPR) including M band of 0.12 g/dL, –22.1 mg/L, –12.9 mg/L (/ ratio 1.73), and dFLC of 9.2 mg/L.
The patient underwent an autologous stem cell transplant with high dose melphalan (140 mg/m2). Hematopoietic stem cell (HSC) mobilization was performed with the granulocyte colony-stimulating factor and upfront plerixafor. The total HSC dose collected after two sessions of apheresis was 1.52×106 cells/kg. Neutrophil engraftment was achieved on day 12, and platelet engraftment was achieved on day 14.
The patient was restarted on monthly daratumumab in- jections from day 60 post-transplant, indicating a stringent complete response (, 2.8 mg/L; , 1.8 mg/L and ratio 2.1) on the day 100 evaluation, with continuing complete re- mission observed 21 months post-transplant on monthly daratumumab.
The second patient, a 53-year-old female, with no pre- vious comorbidities, was diagnosed with light chain mye- loma RISS-III with renal failure requiring hemodialysis (serum creatinine, 7.9 mg/dL; urine output, 200 mL/d). The