C
ANCERPREVENTION RESEARCH □ REVIEW ARTICLE □
181 책임저자:고경혁, 660-751, 진주시 칠암동 92
경상대학교 의학전문대학원 병리학교실 Tel: 055-751-8761, Fax: 055-759-7952 E-mail: gyunghko@gnu.ac.kr
접수일:2010년 5월 11일, 1차 수정일:2010년 8월 18일, 2차 수정일:2010년 8월 23일, 게재승인일:2010년 8월 25일
Correspondence to:Gyung Hyuck Ko
Department of Pathology, School of Medicine, Gyeongsang National University, 92, Chilam-dong, Jinju 660-751, Korea
Tel: +82-55-751-8761, Fax: +82-55-759-7952 E-mail: gyunghko@gnu.ac.kr
A Composite Peripheral T-Cell Lymphoma and Anaplastic Variant of Diffuse Large B-Cell Lymphoma
Jong Sil Lee1,3,4, Gyung Hyuck Ko1,3,4, Jung Wook Yang1, Dong Chool Kim1,3,4, Jeong Hee Lee1,3,4 and Gyeong-Won Lee2,3,4
1Department of Pathology, 2Division of Hematology-Oncology, Department of Internal Medicine, Gyeongsang National University School of Medicine, 3Institute of Health Science, Gyeongsang National University School of Medicine,
4Gyeongnam Regional Cancer Center, Jinju 660-751, Korea
We report a rare case of a composite lymphoma consisting of peripheral T-cell lymphoma (PTCL), not otherwise specified (NOS), and an anaplastic variant of diffuse large B-cell lymphoma (DLBCL) associated with Epstein-Barr virus (EBV) infections in the 47-year-old man. The patient initially sought care at a local hospital with a single enlarged cervical lymph node. Histologic examination disclosed EBV-associated PTCL. Chemotherapy and autologous stem cell transplantation resulted in complete remission. After 5 years, the patient was readmitted due to generalized lymphadenophathy. The biopsy of the cervical lymph node showed PTCL. He underwent chemotherapy treatment, but an inguinal lymph node grew in size. The lymph node showed partial involvement of DLBCL in addition to PTCL. Many lymphocytes in the B-cell lymphoma and T-cell lymphoma showed positivity for EBV. This case suggests that EBV may have contributed to the development of a composite EBV-associated B-cell lymphoma and T-cell lymphoma. (Cancer Prev Res 15, 181-184, 2010)
Key Words: Peripheral T-cell lymphoma, Diffuse, Large B-Cell, Lymphoma, Epstein-Barr virus infections
INTRODUCTION
A composite lymphoma is defined as two or more morph- ologically and immunophenotypically distinct lymphoma clones in the same organ or tissue.1-5) The combination might include a Hodgkin’s lymphoma with a B-cell or a T-cell non-Hodg- kin’s lymphoma (NHL), a B-cell NHL with a T-cell NHL, or two distinct B-cell or T-cell NHLs at the same anatomic site.2,3) The simultaneous development of EBV-associated B-cell lymphoma in a nodal T-cell lymphoma other than an an- gioimmunoblastic T-cell lymphoma (AILT) has rarely been reported.6,7) In this paper, we describe a patient with an EBV- associated composite lymphoma in a lymph node consisting of a peripheral T-cell lymphoma (PTCL), not otherwise specified
(NOS), and an anaplastic variant of diffuse large B-cell lym- phoma (DLBCL). We hypothesize that EBV may have had a role in the pathogenesis of the B-cell lymphoma in this patient.
CASE REPORT
A 47-year-old man who developed cervical lymph node en- largement was given a diagnosis of EBV-associated peripheral T-cell lymphoma in 2004. He was treated with a combination chemotherapy regimen consisting of cyclophosphamide, doxoru- bicin, vincristine, and prednisone plus etoposide (CHOEP), and he also underwent autologous stem cell transplantation. He had a complete remission. Six years later, in 2010, he was admitted to our hospital due to generalized lymphadenophathy. The cervical node biopsy showed peripheral T-cell lymphoma. He
182 Cancer Prevention Research Vol. 15, No. 3, 2010
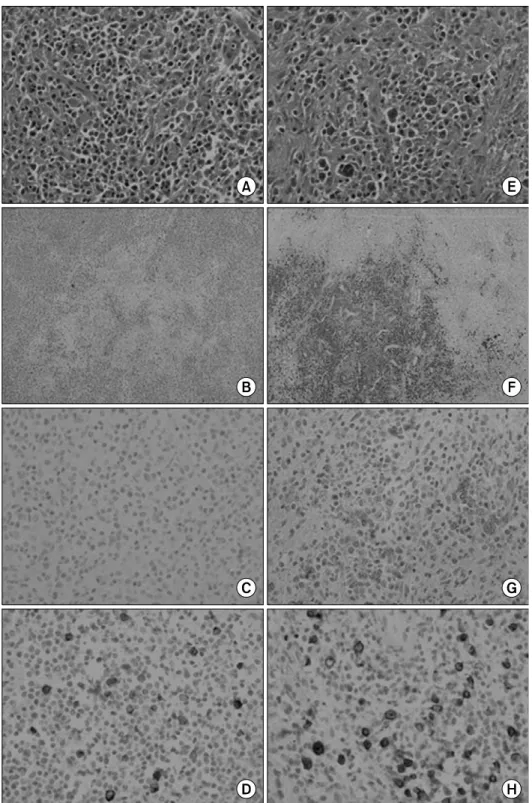
Fig. 1. Histological examinations of inguinal lymph node. T-cell area (A∼D) and anaplastic B-cell area (E∼H). H&E (A, E) and im- munohistochemical stain with an- ti-CD3 antibody (B), anti-CD20 antibody (F), anti-CD30 antibody (C, G), and LMP-1 (D, H) (A, C, D, E, G, H ×400 & B, F ×40).
underwent chemotherapy, but an inguinal lymph node en- larged. For diagnosis, we took a biopsy of the inguinal node.
The lymph node was infiltrated diffusely by small to medium- sized lymphocytes with mildly irregular nuclei (Fig. 1A) with a focus of many large cells (Fig. 1E). The diagnosis of PTCL, NOS, was favored because there was only scant proliferation
of high endothelial venules and follicular dendritic cells, a polymorphous infiltrate of small to medium-sized T-cells with mildly irregular nuclei was evident, and only a few reactive inflammatory cells were present (Fig. 1A). Immunohistochemi- cal analysis was performed on an Autostainer 360 (Thermo Scientific, Fremont, CA) with an UltraVision Detection System
Jong Sil Lee, et al:Composite T and B-Cell Lymphoma 183
Table 1. Results of immunostaining experiments and In Situ Hybridization (ISH) analysis of Epstein-Barr Virus-Encoded Small RNAs (EBER)
Antibodies T-Cell Anaplastic
and ISH area B-Cell area
CD3 + -
CD20 - +
CD30 - +
EBER ISH (+) +
LMP-1 (+) +
EBER ISH: EBV-encoded RNA in situ hybridization, LMP1:
latent membrane protein 1, +: positive, (+): Focally positive,
-: Negative.
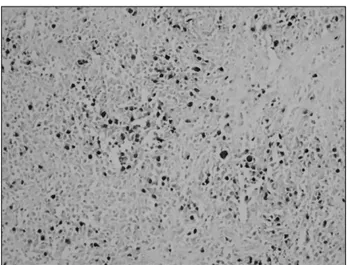
Fig. 2. In situ hybridization for Epstein-Barr virus (EBV)-encod- ed nuclear RNAs. Many lymphocytes in B-cell lymphoma showed strong nuclear staining for EBV (×200).
Anti-Mouse HRP/DAB system (Thermo scientific), preceded by microwave antigen retrieval in citrate buffer. The biopsy was analyzed (clone and dilution in parentheses) for CD3 (PS1; 1:
1,500; Zymed, San Francisco, CA), CD20 (L26; 1:2,000;
DAKO, Carpinteria, CA), CD21 (2G9; 1:300; Thermo Fisher Scientific, Fremont, CA), CD30 (BerH2; 1:1,500; Thermo Fisher Scientific), and EBV latent membrane protein 1 LMP-1 (CS1-4; 1:200; Novocastra) expression. In situ hybridization was performed on paraffin sections with a fluorescein-conju- gated Epstein-Barr virus probe to EBV small RNAs (EBERs) (Leica) and Mayer’s hematoxylin counterstaining. The T-cell lymphoma phenotype was CD3+ (Fig. 1B), CD20-, CD10
-, CD30- (Fig. 1C), and LMP-1+ (Fig. 1D). The B-cell lymphoma had the marker phenotype of CD20+ (Fig. 1F), CD3-, CD10-, and CD30+ (Fig. 1G), and LMP-1+ (Fig.
1H), (Table 1). In situ hybridization for EBER showed strong nuclear positivity in the B-cell lymphoma (Fig. 2) and scattered expression in the T-cell lymphoma (Table 1). We therefore dia- gnosed the tumor as an EBV-associated lymphoma comprising both PTCL, NOS and the anaplastic variant of DLBCL.
DISCUSSION
A number of hypotheses have been suggested to explain the existence of composite lymphomas.2,3) The first hypothesis is simple coincidence. The second hypothesis is the malignant transformation of a stem cell with the capacity to differentiate into both B-cell and T-cell lymphomas. The third hypothesis postulates the simultaneous but independent development of two neoplasms because of a shared genetic predisposition. A fourth possibility is the presence of immune dysregulation with
the capacity to induce mixed neoplastic clones. A fifth possibili- ty is immunodeficiency. The sixth hypothesis speculates that exposure to chemotherapy for one tumor induces a secondary neoplasm. The final hypothesis is that exposure to both a virus and carcinogen can result in a composite neoplasm.
In recent studies, four patterns of EBV latency have been recognized:8,9) type 1, which shows exclusive expression of EBV-encoded nuclear antigen 1 small non-polyadenylated RNA (EBER) molecules and EBV-encoded nuclear antigen 1 (EBNA-1); type II, which shows additional expression of latent membrane proteins (LMP); type III, which shows expression of all six EBNA, all three LMP, and the two EBER; and type IV latency, which is less strictly defined.
The development of simultaneous EBV-associated B-cell lymphoma in nodal PTCL, NOS is very rare. The coexistence of peripheral T-cell lymphoma, NOS and an anaplastic variant of diffuse large B-cell lymphoma in the same lymph node is unlikely to be coincidental. Tobinai et al.10) described EBV- positive B-cell lymphoma with adult T-cell leukemia/lymphoma and suggested that the immunodeficient state of the patient with adult T-cell leukemia permitted the emergence of EBV-related B-cell lymphoma. Zettl et al.6) reported EBV-as- sociated B-cell lymphoproliferative disorders in angioimmuno- blastic T-cell lymphoma (AILT) and PTCL, NOS. Three of these cases were peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) with coexistent EBV-associated diffuse large B-cell lymphoma (DLBCL).6) Hirose et al.7) reported an Epstein-Barr virus-associated composite lymphoma composed of
184 Cancer Prevention Research Vol. 15, No. 3, 2010
peripheral T-cell lymphoma and an anaplastic variant of a diffuse large B-cell lymphoma and strongly expressing p53 protein. These cases suggest that partial reactivation of EBV in the lymph node may have helped the development of anaplastic DLBCL. Our patient had initially been diagnosed with EBV-associated PTCL. After 6 years, he had generalized lymphadenopathy, and was diagnosed with a peripheral T-cell lymphoma, NOS in a cervical lymph node and an EBV-as- sociated lymphoma comprising both PTCL, NOS and the anaplastic variant of DLBCL in an inguinal lymph node. We hypothesize that disease-related and therapy-induced immuno- suppression in T-cell lymphoma permitted clonal expansion of an EBV+ B-cell clone, with subsequent development of large B-cell lymphoma.
In conclusion, we presented a rare case of an EBV-associated composite lymphoma consisting of peripheral T-cell lymphoma, not otherwise specified, and an anaplastic variant of diffuse large B-cell lymphoma. This case suggests that EBV may contribute to the development of simultaneous EBV-associated B-cell lymphoma in a T-cell lymphoma.
REFERENCES
1) Kim H. Composite lymphoma and related disorders. Am J Clin Pathol 99, 445-451, 1993.
2) Ioachim HL, Medeiros LJ. Lymph node pathology. 4th ed.
Lippincott, Williams & Wilkins a Wolters Kluwer business, pp 452-459, 2009.
3) Mokhtar NM. Journal of the Egyptian Nat. Cancer Inst 19, 171-175, 2007.
4) Tachikawa Y, Shiratsuchi M, Sada E, Idutsu K, Kiyasu J, Karube K, Ohshima K, Nishimura J, Takayanagi R, Abe Y.
Composite gastrointestinal lymphoma consisting of diffuse large B-cell lymphoma and peripheral T-cell lymphoma. Int J Hematol 90, 275-277, 2009.
5) Xu Y, McKenna RW, Hoang MP, Collins RH, Kroft SH.
Composite Angioimmunoblastic T-Cell Lymphoma and Dif- fuse Large B-Cell Lymphoma. Am J Clin Pathol 118, 848-854, 2002.
6) Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, Kirchner T, Ott M, Müller-Hermelink HK, Ott G. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angio- immunoblastic T-cell lymphoma and peripheral T-cell ly- mphoma, unspecified. Am J Clin Pathol 117, 368-379, 2002.
7) Hirose Y, Fukushima T, Masaki Y, Shimoyama K, Karasawa H, Ogawa N, Wano Y. Epstein-Barr virus-associated com- posite lymphoma composed of peripheral T-cell lymphoma and an anaplastic variant of a diffuse large B-cell type of non- Hodgkin's lymphoma and strongly expressing p53 protein. Int J Hematol 79, 260-265, 2004.
8) Oyama T, Ichimura K, Suzuki R, Suzumiya J, Ohshima K, Yatabe Y, Yokoi T, Kojima M, Kamiya Y, Taji H, Kagami Y, Ogura M, Saito H, Morishima Y, Nakamura S. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopatholo- gical study of 22 patients. Am J Surg Pathol 27, 16-26, 2003.
9) Sugita Y, Terasaki M, Niino D, Ohshima K, Fumiko A, Shigemori M, Sato Y, Asano N. Epstein-Barr virus-associated primary central nervous system lymphomas in immunocom- petent elderly patients: analysis for latent membrane protein-1 oncogene deletion and EBNA-2 strain typing. J Neurooncol May 9, 2010 [Epub ahead of print]
10) Tobinai K, Ohtsu T, Hayashi M, Kinoshita T, Matsuno Y, Mukai K, Shimoyama M. Epstein-Barr virus (EBV) genome carrying monoclonal B-cell lymphoma in a patient with adult T-cell leukemia-lymphoma. Leuk Res 15, 837-846, 1991.