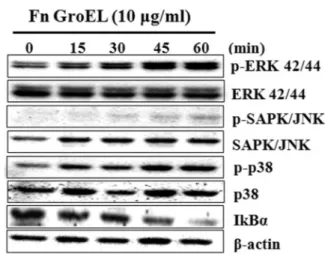
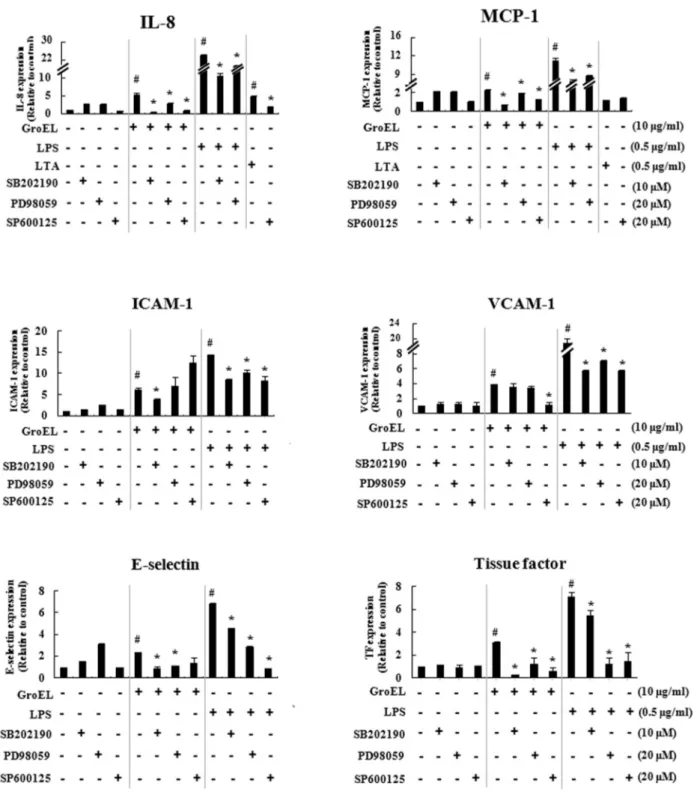
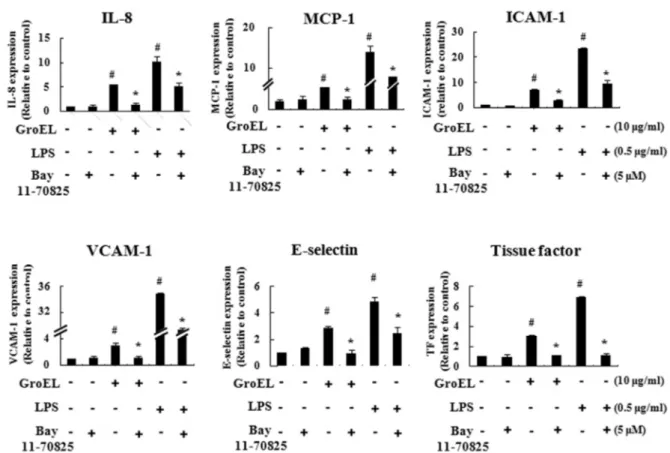
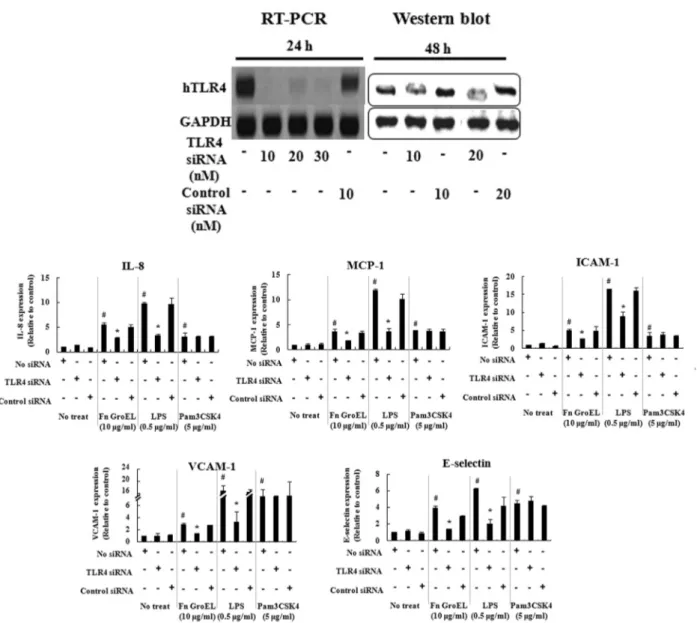
*Correspondence to: Bong-Kyu Choi, Department of Oral Microbiology and Immunology, School of Dentistry, Seoul National University, 28 Yongon-Dong, Chongno-Gu, Seoul 110-749, Republic of Korea, TEL: 82-2-740-8640, Fax: 82-2-743-0311, E-mail: bongchoi@snu.ac.kr
Fusobacterium nucleatum GroEL signaling via Toll-like receptor 4 in human microvascular endothelial cells
Hae-Ri Lee
1, and Bong-Kyu Choi
2,3*
1
Korea Centers for Disease Centers for Disease Control and Prevention, Cheongwon-gun, Chungbuk, Korea
2
Department of Oral Microbiology and Immunology, School of Dentistry, Seoul National University, Seoul, Korea
3