저작자표시-비영리-동일조건변경허락 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 동일조건변경허락. 귀하가 이 저작물을 개작, 변형 또는 가공했을 경우 에는, 이 저작물과 동일한 이용허락조건하에서만 배포할 수 있습니다.
Investigation on the Molecular Mechanism of
Photorejuvenation in Photodynamic Therapy
by
Sue Kyung Kim
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Investigation on the Molecular Mechanism of
Photorejuvenation in Photodynamic Therapy
(Focused on Immunomodulatory Effects by
Fibroblast-Keratinocyte-Melanocyte Interaction)
by
Sue Kyung Kim
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for
the Degree of Ph.D. of Medicine
Supervised by
You Chan Kim, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
인준서: 별도 용지
This certifies that the dissertation
of Sue Kyung Kim is approved.
SUPERVISORY COMMITTEE
You Chan Kim
You-Sun Kim
Seonghyang Sohn
Jai Sung Noh
Chun Wook Park
The Graduate School, Ajou University
December, 13rd, 2013
감사의
글
논문이 완성될 때까지 끊임없는 관심과 지도를 주신 지도교수 김유찬
교수님께 깊이 감사 드립니다. 또한 연구가 진행되는 동안 많은 조언과
격려를 주신 김유선 교수님과 손성향 교수님께도 감사의 말씀을 드립니다.
그리고 실험에 익숙하지 않은 저를 위해 함께 애써주신 이원경
선생님께도 감사 드립니다.
언제나 저의 든든한 버팀목이 되어 주시는 부모님, 사랑하고 감사합니다.
가족을
위해 항상 애쓰는 고마운 남편과 항상 나에게 웃음을 주는 예쁜
우리
딸, 그리고 뱃속에서 엄마와 같이 공부해준 앞으로 태어날 우리 아가
에게도
깊은 사랑의 마음을 전합니다.
저자 씀
i
-ABSTRACT-
Investigation on the Molecular Mechanism of Photorejuvenation in
Photodynamic Therapy (Focused on Immunomodulatory Effects by
Fibroblast-Keratinocyte-Melanocyte Interaction)
Background: The photoaging of the skin is characterized by wrinkles, mottled pigmentation, telangiectasia and malignancies due to the effects of long-term UV exposure. Photodynamic therapy (PDT) is a therapeutic modality for cutaneous malignant and premalignant condition. Recently, several clinical studies reported that the PDT has photorejuvenation effects on the aged skin. In the previous report, which is examined direct effect of PDT on fibroblast (FB), PDT induced increase of matrix metalloproteinase (MMP)-3 and collagen type Iα through prolonged activation of extracellular signal–regulated kinase (ERK). Increased MMP-3 may induce breakage of old collagen and allow new collagen synthesis.
Purpose: In this study, I investigated molecular mechanism of photorejuvenation after PDT focused on interaction between keratinocyte (KC), FB, and melanocyte (MC).
Methods: The “low level PDT” condition was used for PDT therapy to the KC, FB, and MC. FBs were stimulated with the KC-conditioned medium taken after PDT and MCs were stimulated with the KC-conditioned medium or FB-conditioned medium taken after PDT. Various kinds of cytokines in the supernatants of KC and FB were evaluated by enzyme-linked immunosorbent assay (ELISA). The mRNA level of MMPs, transforming growth factor (TGF)-β and collagen type Iα in the FB were determined by real-time polymerase
ii
chain reaction (PCR). The melanin content and dopa oxidase activity of the MC were also evaluated.
Results: After stimulating FB with KC-conditioned medium, the mRNA of MMP-1 was decreased and the mRNA of collagen type Iα were increased compare to control. However, MMP-3 and TGF-β were not significantly changed. Among the FB stimulating cytokines, a significant elevation of interleukin (IL)-1α, IL-6, tumor necrosis factor (TNF)-α level in KC supernatants was noted after PDT compared with the untreated controls. TGF-β were not significantly altered after PDT. After PDT, significantly reduced melanin content and dopa oxidase activity were noted compared to the control group after 24 and 48h in MC. In addition, MC treated with KC-conditioned medium taken after PDT showed a significant reduction of melanin content and dopa oxidase activity compared with the control groups. Among the well known MC stimulating cytokines from KC, the level of stem cell factor (SCF) in KC-conditioned medium was significantly decreased after PDT, whereas endothelin (ET)-1 and granulocyte-macrophage colony stimulating factors (GM-CSF) were not significantly altered. MC treated with FB-conditioned medium taken after PDT showed a significant reduction of melanin content and dopa oxidase activity compared with control groups. Among the MC stimulating cytokines from FB, the hepatocyte growth factor (HGF) in FB-conditioned medium was significantly decreased after PDT compared to the untreated control. The SCF and basic fibroblast growth factor (bFGF) were not changed after PDT. Conclusion: PDT induced new collagen synthesis may be mediated not only by direct effect of PDT on FB but also by indirect effect of PDT on FB through cytokines from KC. In addition, PDT has inhibitory effect of melanogenesis in the human MC. This inhibitory
iii
effect of melanogenesis thought to be regulated indirectly by KC and FB as well as directly by PDT. Decreased secretion of SCF from KC and HGF from FB may have crucial role which induce inhibition of melanogenesis of MC after PDT.
iv
TABLE OF CONTENTS
ABSTRACT... i
TABLE OF CONTENTS ... iv
LIST OF FIGURES ... vii
I. INTRODUCTION ... 1
II. MATERIAL AND METHODS ... 4
A. MATERIALS ... 4
1. Cell culture ... 4
2. Reagents ... 5
B. METHODS... 5
1. PDT procedure ... 5
2. Stimulation of fibroblasts with keratinocyte-conditioned medium ... 6
3. Stimulation of melanocytes with keratinocyte-conditioned medium or fibroblast-conditioned medium... 6
4. Assessment of cell viability ... 6
5. Western blot analysis ... 6
6. Melanin content assay ... 7
v
8. Enzyme-linked immunosorbent assay (ELISA) ... 7
9. Real-time polymerase chain reaction (PCR) ... 8
10. Statistical analysis ... 8
III. RESULTS ... 9
A. The determination of the optimal “low level-PDT” condition of keratinocytes and melanocytes which induces activation of ERK ... 9
B. Indirect effect of photodynamic therapy on the fibroblasts through keratinocytes-fibroblasts interaction ... 13
C. Changes of the amount of fibroblast stimulating cytokines secreted by keratinocytes after photodynamic therapy ... 15
D. Direct effect of photodynamic therapy on the melanocytes ... 17
E. Indirect effect of photodynamic therapy on the melanocytes through keratinocyte-melanocyte interaction... 19
F. Changes in the amount of melanocytes stimulating cytokines secreted by keratinocytes after photodynamic therapy ... 21
G. Indirect effect of photodynamic therapy on the melanocytes through fibroblast-melanocyte interaction... 23
H. Changes in the amount of melanocytes stimulating cytokines secreted by fibroblasts after photodynamic therapy ... 25
vi
V. CONCLUSION ... 34 REFERENCES ... 35 국문요약 ... 39
vii
LIST OF FIGURES
Fig.1. The determination of the optimal “low level-PDT” condition of keratinocytes and HaCaT cell which induced activation of ERK ... 11
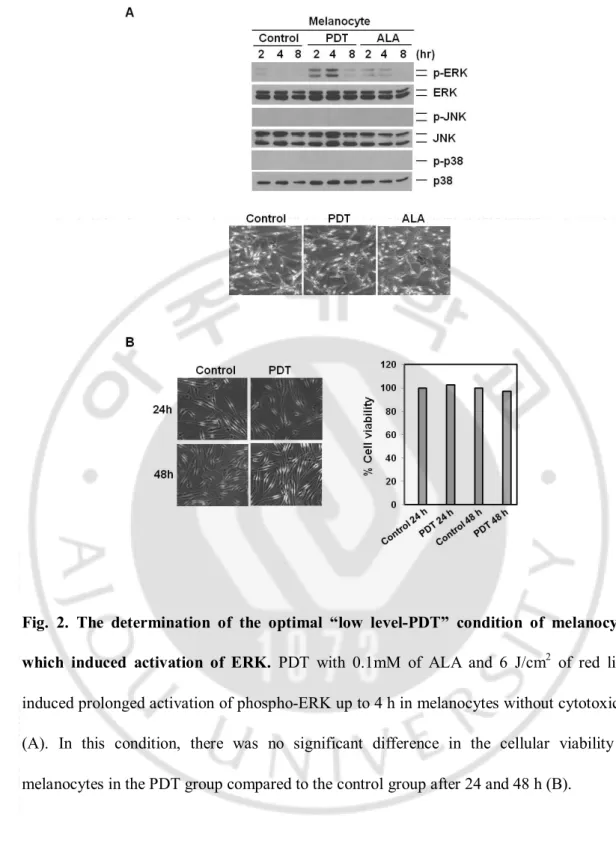
Fig.2. The determination of the optimal “low level-PDT” condition of melanocytes which induced activation of ERK ... 12
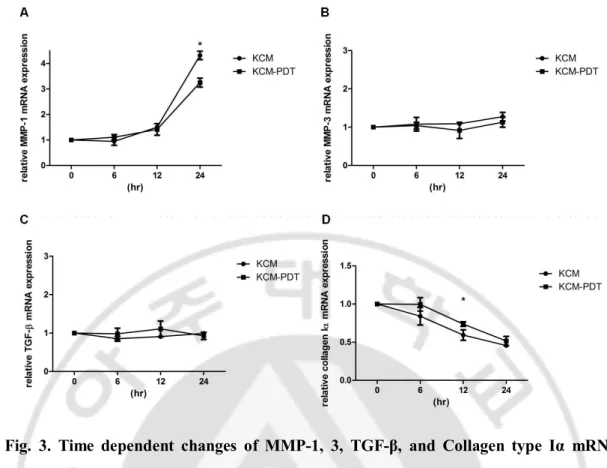
Fig.3. Time dependent changes of MMP-1, 3, TGF-β, and Collagen type Iα mRNA levels in fibroblastsafter stimulation with keratinocyte-conditioned medium taken after photodynamic therapy... 14
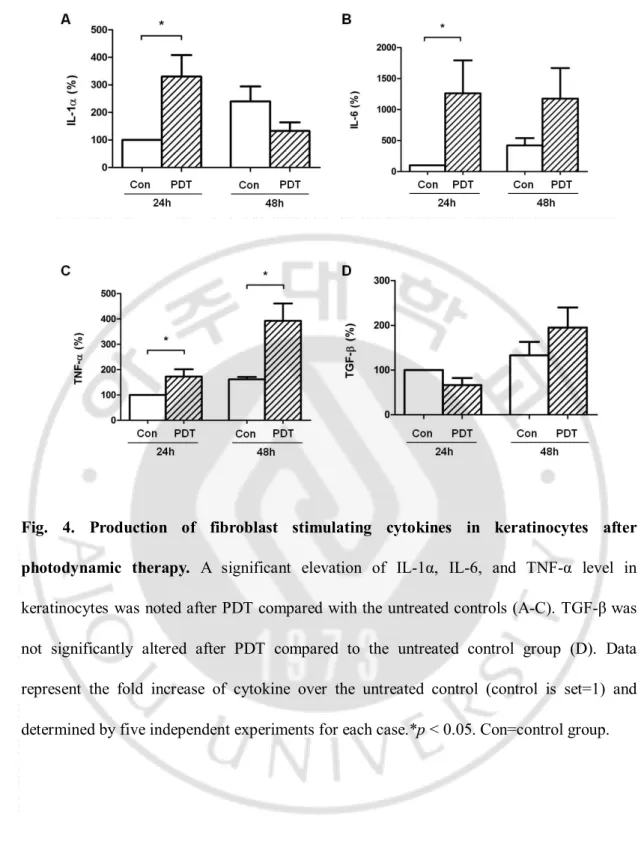
Fig.4. Production of fibroblast stimulating cytokines in keratinocytes after photodynamic therapy... 16
Fig.5. Reduced melanin content and dopa oxidase activity in melanocytes after photodynamic therapy ... 18
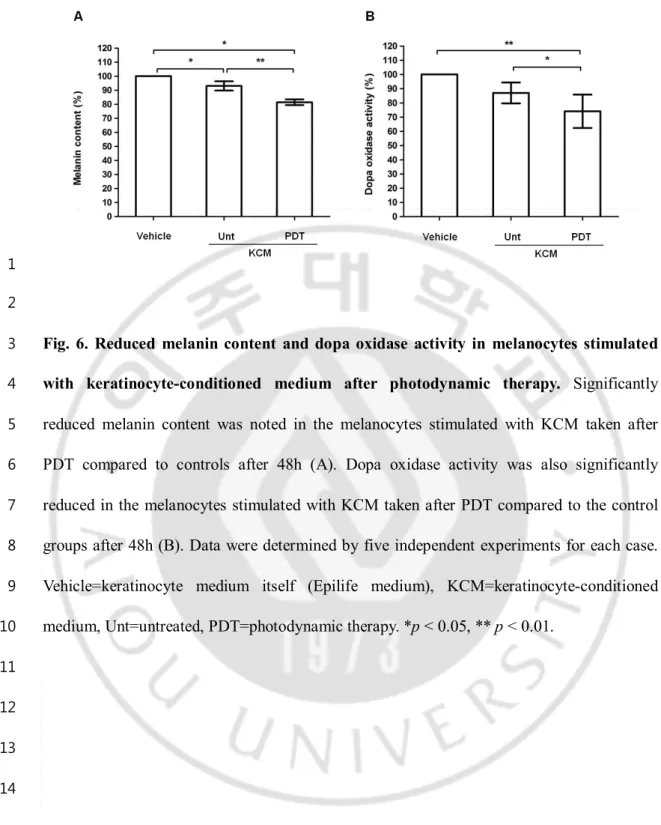
Fig.6. Reduced melanin content and dopa oxidase activity in melanocytes stimulated with keratinocyte-conditioned medium after photodynamic therapy ... 20
viii
therapy... 22
Fig.8. Reduced melanin content and dopa oxidase activity in melanocytes stimulated with fibroblast-conditioned medium after photodynamic therapy ... 24
Fig.9. Production of melanocytes stimulating cytokines in fibroblasts after photodynamic therapy... 26
Fig.10. A schematic diagram of low level photodynamic therapy induced photorejuvenation ... 33
- 1 -
I. INTRODUCTION
12
The photoaging is the effect of long-term UV exposure and sun damage superimposed
3
on intrinsically aged skin (Rabe et al., 2006). This process results in alteration of the skin
4
such as wrinkles, mottled pigmentation, telangiectasia, and malignancies of the skin. The
5
most notable change of the photoaging is wrinkles due to reduced dermal collagen and
6
dyspigmentation caused by increased melanogenesis.
7
Photodynamic therapy (PDT) is a therapeutic modality for cutaneous malignant and
8
premalignant condition. Topical PDT using 5-aminolevulinic acid (ALA) is based on the
9
photosensitization of the diseased tissue by ALA-induced porphyrins and subsequent
10
irradiation with light of specific wavelength. The excitation of the photosensitizer results in
11
the generation of reactive oxygen species (ROS) and it mediate cellular effects resulting in
12
cytotoxic reaction to the targeted cells (Brackett and Gollnick, 2011).
13
Recently, several clinical studies reported that the PDT has photorejuvenation effects on
14
the aged skin (Dover et al., 2005; Gold et al., 2006; Kohl et al., 2010). In clinical practice,
15
physicians also can notice photorejuvenated skin of elderly patients who had PDT to treat of
16
malignant or premalignant disease of skin. Peripheral skin of target lesion of PDT showed
17
improvement of wrinkles and mottled pigmentation. However, despite of several reports
18
about photorejuvenation effects of PDT, it remains controversial for the treatment of
19
photoaging skin because of limited data about its basic molecular mechanisms.
20
Recently, several reports were introduced about molecular mechanisms of increased
21
collagen synthesis after PDT in human skin and human dermal fibroblast (FB) (Park et al.,
- 2 -
2010; Jang et al., 2013). In human skin, the authors reported increased expressions of type I
1
and III procollagen, increased total collagen volume in the dermis and reduced expressions
2
of matrix metalloproteinase (MMP)-1, MMP-3, and MMP-12 after PDT by
3
immunohistochemical stain (Park et al., 2010). In recent study, the condition of “low-level
4
PDT” which induced activation of extracellular signal–regulated kinase (ERK) in human
5
dermal FB without cytotoxic effects was introduced; ALA concentration of 0.1mM,
6
incubation time of 30 minutes, and 3 J/cm2 of irradiation. After this “low-level PDT”, FB
7
proliferation and increased secretion of the MMP-3 was noted mediated by prolonged
8
activation of ERK. Increasing MMP-3 expression may trigger destruction of old extracellular
9
matrix and FB proliferation may also lead to production of new collagen synthesis (Jang et
10
al., 2013).
11
Contrast to the studies about effect of PDT to FB, there are few data about molecular
12
mechanism of PDT to melanocyte (MC) which is induces inhibition of melanin synthesis. In
13
one study, light-emitting diodes (LED) irradiation at wavelengths of 830, 850, and 940 nm
14
effectively reduces melanogenesis, without any cytotoxic effects (Kim et al., 2012).
15
In photoaging response, there are cross-talks between cells, for example, keratinocyte
16
(KC), FB, and MC. UV induce various cytokines in KC such as tumor necrosis factor
(TNF)-17
α, interleukin (IL)-1, IL-6 and transforming growth factor (TGF)-β. These cytokines regulate
18
MMPs and result in UV-mediated down-regulation of collagen synthesis in the dermal FB
19
(Karrer et al., 2004; Rabe et al., 2006). UV irradiation is also known to stimulate KC and FB
20
to induce numerous cytokines that is responsible for the proliferations and activation of MC;
21
stem cell factor (SCF), prostaglandin (PG), endothelin (ET) and granulocyte-macrophage
- 3 -
colony stimulating factors (GM-CSF) in the KC; SCF, hepatocyte growth factor (HGF) and
1
basic fibroblast growth factor (bFGF) in the FB, respectively (Imokawa, 2004; Tanaka et al.,
2
2010).
3
The PDT can affect FB and MC directly and indirectly. Because KC exists on the outer
4
most surface of the skin, PDT will affect KC firstly and then cytokines from the KC may
5
influence other cells around KC, for example, FB and MC. Therefore, interaction among
6
these three major cells of the skin, which are KC, FB and MC, is important to understand the
7
mechanisms of photorejuvenation induced by PDT.
8
In this study, I investigated the molecular mechanism of photorejuvenation after PDT,
9
focused on interaction between KC, FB, and MC.
- 4 -
II. MATERIALS AND METHODS
1 2 A. Materials 3 1. Cell culture 4Primary normal human KC was cultured as previously described (Pincelli et al., 1994).
5
Skin specimens obtained from repeat Caesarean section deliveries and circumcisions were
6
used for the cultures. The cells were suspended in Epilife medium (Cascade Biologics) with
7
human keratinocytes growth supplement (Cascade Biologics) at 37oC in an incubator
8
containing 5% CO2. Cells at passage 3-10th were used for experiments.
9
The HaCaT cells (human keratinocyte cell line) were cultured in DMEM (Gibco)
10
supplemented with 10% fetal bovine serum (Gibco) and 100 U/ml of penicillin/streptomycin
11
(Gibco) at 37oC in an incubator containing 5% CO 2.
12
Primary normal human dermal FB was cultured as previously described (Palmetshofer
13
et al., 1995). Skin specimens obtained from circumcisions were used for the cultures. FBs
14
were cultured in DMEM supplemented with 10% fetal bovine serum, 2mM glutamine and
15
100 U/ml of penicillin/streptomycin (Gibco) at 37oC in an incubator containing 5% CO 2. FB
16
were subcultured by trypsinization and used between the 5th and 20th passages.
17
To culture the MC, I purchased human neonatal epidermal MC (Cascade Biologics,
18
Portland, OR, USA) and maintained according to the manufacturer’s instructions. Briefly,
19
the cells were suspended in Medium 254 (Cascade Biologics) supplemented with bovine
20
pituitary extract, fetal bovine serum (FBS), bovine insulin, hydrocortisone, bFGF, bovine
21
transferrin, heparin and phorbol myristate acetate (all of supplements from Cascade
- 5 -
Biologics) at 37oC in an incubator containing 5% CO
2. Cells at passage 3-6th were used for
1 experiments. 2 3 2. Reagents 4
Anti-phospho-c-Jun N-terminal kinase (JNK), anti-phospho-ERK, anti-phospho-p38,
5
anti-ERK, and anti-p38 antibodies were purchased from Cell Signaling (Beverly, MA, USA).
6
Anti-JNK antibody was purchased from Invitrogen (Carlsbad, CA, USA). Anti-MMP-1 and
7
3 antibodies were purchased from Epitomics (Burlingame, CA, USA). Antitubulin and
anti-8
collagen type I antibodies were purchased from Abcam (Cambridge, MA, USA).
Anti-TGF-9
β antibody was purchased from Gene Tex (San Antonio, TX, USA). ALA was purchased
10
from Sigma. (St. Luis, MO, USA).
11 12 B. Methods 13 1. PDT procedure 14
PDT procedure was similar to the previous study (Jang et al., 2013). Briefly, cells were
15
plated in 60 mm dish. After the growth medium was removed, serum-free medium
16
containing 0.1mM of ALA was added, and cells were allowed to take up ALA for 30 minutes.
17
The medium containing ALA was removed; the cells were rinsed and then submerged with
18
phosphate-buffered saline. Irradiation of the cells within monolayer culture was performed
19
using an incoherent light source with 3 or 6 J/cm2 (Omnilux revive, 633nm,
20
Phototherapeutics, Montgomeryville, PA).
21 22
- 6 -
2. Stimulation of fibroblasts with keratinocyte-conditioned medium
1
The FBs were stimulated with the KC-conditioned medium taken 48 h after PDT. After
2
stimulation, cell pellet were taken at 6, 12, and 24h to determine mRNA level of MMPs,
3
TGF- β, and collagen type I.
4 5
3. Stimulation of melanocytes with keratinocyte-conditioned medium or
fibroblast-6
conditioned medium
7
The MCs were stimulated with the KC-conditioned medium or FB-conditioned medium
8
taken 24 h after PDT. The cell pellets were taken at 48h after stimulation to determine
9
melanin content and dopa oxidase activity.
10 11
4. Assessment of cell viability
12
Cell viability was determined using the tetrazolium dye colorimetric test (MTT assay).
13
The MTT absorbance was then read at 570 nm. Using a phase-contrast microscope, the
14
representative images were taken.
15 16
5. Western blot analysis
17
Upon PDT, cells were lysed in M2 buffer (20mM Tris at pH 7, 0.5% NP-40, 250mM
18
NaCl, 3mM EDTA, 3mM EGTA, 2mM DTT, 0.5mM PMSF, 20mM b-glycerol phosphate,
19
1mM sodium vanadate, and 1 mg/ml leupeptin). Equal amounts of cell extracts were
20
resolved by 12% SDS-PAGE and analyzed by western blotting and visualized by enhanced
21
chemiluminescence (Amersham, Buckinghamshire, UK)
- 7 - 1
6. Melanin content assay
2
Melanin content was measured according to the method of Tsuboi et al. (Tsuboi et al.,
3
1998) with a slight modification. MC was cultured in a 60-mm culture dish at a density of
4
5.0×105 cells. Twenty-four hours after PDT or stimulation with KC-conditioned
medium/FB-5
conditioned medium, cells were solubilized in 1 M NaOH and the relative melanin content
6
was measured at 490 nm using an enzyme-linked immunosorbent assay reader. The
7
absorbance was compared with a standard curve of synthetic melanin (Sigma).
8 9
7. Dopa oxidase activity assay
10
Dopa oxidase activity was determined by the method described by Tomita et al. (Tomita
11
et al., 1992) with slight modification. The enzyme induction was assayed
12
spectrophotometrically using L-DOPA as the substrate. MCs were solubilized with 1% SDS
13
+ 1% Tween20 in PBS PH 6.8 and 1 mM L-DOPA (Sigma) and incubated at 37oC for 90
14
minutes. The absorbance at 490 nm was measured to calculate the enzyme activity.
15 16
8. Enzyme-linked immunosorbent assay (ELISA)
17
KC and FB were cultured at a density of 3.0×105 cells/well in 6 well plates. The
18
secretion levels of cytokines including IL-1α, IL-6, TNF-α, TGF-β, SCF, ET-1, GM-CSF,
19
HGF, and bFGF in the conditioned medium were measured by ELISA kits (R&D Systems,
20
Minneapolis, MN, USA) at 24 and 48 h after PDT. The secretion levels are expressed as
21
pg/ml.
- 8 - 1
9. Real-time polymerase chain reaction (PCR)
2
Total RNA was isolated from cells using the RNeasy® mini kit. cDNA production for
3
mRNA was performed using the Superscript® III First-Strand (Invitrogen, Carlsbad, CA,
4
USA) according to the manufacturer’s instructions. The obtained cDNA was analyzed by
5
real-time PCR using the ABI Prism 7000 Sequence Detection System (Applied Biosystems,
6
Foster, CA, USA) according to the manufacturer’s protocol and the 2–∆∆Ct method.
7
Normalization was performed using glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
8
for mRNA normalization by calculating ∆Ct = (∆Ctsample − ∆CtGAPDH). The calibrator sample
9
(∆Ct calibration) was assigned from the control group. Relative mRNA levels were
10
calculated by the expression 2–∆∆Ct, where ∆∆Ct= (∆Ct
sample − ∆Ctcalibration). Primers and
11
internal probes for MMP-1, MMP-3, TGF-β and collagen type Iα were purchased as assays
12
on demand primer-probe sets (Applied Biosystems, Foster, CA, USA).
13 14
10. Statistical analysis
15
All Data are expressed as the mean ± standard deviation. To compare two groups, a
16
Mann-Whitney U test (nonparametric) was used. The statistics program SPSS 11.0 (SPSS,
17
Inc., Chicago, IL, USA) was used for the analysis. A p-value of <0.05 was considered
18
statistically significant.
19 20
- 9 -
III. RESULTS
12
A. The determination of the optimal “low level-PDT” condition of keratinocytes and
3
melanocytes which induces activation of ERK
4
Previously, “low-level PDT” condition of FB (0.1mM of ALA, 3 J/cm2 of red light)
5
which induced prolonged activation of ERK was determined (Jang et al., 2013). However,
6
same condition of “low-level PDT” did not induce the activation of ERK in KC and MC
7
(data not shown). Therefore, I increased the dose of red light to obtain proper condition of
8
low-level PDT in KC and MC which induces activation of ERK similar to FB. PDT with
9
0.1mM of ALA and 6 J/cm2 of red light resulted in prolonged activation of phospho-ERK up
10
to 8 h in KC and up to 4h in HaCaT cell compare to control group and ALA alone group.
11
Cytotoxicity to KC and HaCaT cell was not observed (Fig. 1A and 1B). In this condition,
12
there was no significant change in the proliferation of KC and HaCaT cell in the PDT group
13
compared to the control group after 24 and 48 h (Fig. 1C). PDT with same condition (0.1mM
14
of ALA and 6 J/cm2 of red light) also induced activation of phospho-ERK up to 4 h in MC
15
compare to control group and ALA alone group. However, the protein of phospho-p38 and
16
phospho-JNK was not detected in all three groups. Cytotoxicity to MC was not observed
17
(Fig. 2A). In this condition, there was a no significant change in the cellular viability of MC
18
in the PDT group compared to control group after 24 and 48h (Fig. 2B). Because KC and
19
MC exists in the epidermis, KC and MC will receive more lights than FB in the dermis
20
during clinical application of PDT. Therefore, KC and MC may need higher dose of red light
21
than FB due to this structural position of these cells in the skin. Throughout this study, this
- 10 -
“low level PDT” condition was used; 0.1mM of ALA and 6 J/cm2 of red light to KC and MC;
1
0.1mM of ALA, 3 J/cm2 of red light to FB.
2 3
- 11 - 1
2
Fig. 1. The determination of the optimal “low level-PDT” condition of keratinocytes
3
and HaCaT cell which induced activation of ERK. PDT with 0.1mM of ALA and 6 J/cm2
4
of red light induced prolonged activation of phospho-ERK up to 8 h in KC and up to 4h in
5
HaCaT cell. Cytotoxicity to KC and HaCaT cell was not observed (A and B). In this
6
condition, there was no significant change in the proliferation of KC and HaCaT cell in the
7
PDT group compared to the control group after 24 and 48 h (C).
8 9
- 12 - 1
2
Fig. 2. The determination of the optimal “low level-PDT” condition of melanocytes
3
which induced activation of ERK. PDT with 0.1mM of ALA and 6 J/cm2 of red light
4
induced prolonged activation of phospho-ERK up to 4 h in melanocytes without cytotoxicity
5
(A). In this condition, there was no significant difference in the cellular viability of
6
melanocytes in the PDT group compared to the control group after 24 and 48 h (B).
7 8 9
- 13 -
B. Indirect effect of photodynamic therapy on the fibroblasts through
keratinocytes-1
fibroblasts interaction
2
In photoaging process, increased secretion of inflammatory cytokines such as TGF-β
3
induces expression of MMP-1 and MMP-3 which results in degradation of collagen I (Shin
4
et al., 2005). In previous report about photorejuvenation mechanism of PDT, the authors
5
examined direct effect of PDT on FB. In that report, PDT induced increase of MMP-3 and
6
collagen type Iα through prolonged activation of ERK. Increased MMP-3 may induce
7
breakage of old collagen and allow new collagen synthesis (Jang et al., 2013). In the present
8
study, the indirect effect of PDT on FB through KC-FB interaction was examined by using
9
KC-conditioned medium. The changes in MMP-1, MMP-3, TGF-β and collagen type Iα was
10
evaluated by real-time PCR after stimulation with KC-conditionied medium. FB were
11
stimulated with KC-conditioned medium (taken 48 h after PDT) for up to 24 h. FB were also
12
stimulated with supernatants of untreated KC to evaluate as controls. MMP-1 mRNA
13
significantly decreased in PDT group compared with control at 24 h (Fig. 3A). The mRNA
14
of MMP-3 has a tendency to decrease in PDT group compare to control up to 24 h. However,
15
it was not statistically significant (Fig. 3B). Although it was not significant, TGF-β mRNA
16
level has a tendency to increase after PDT at 6 and 12h (Fig. 3C). The mRNA of collagen
17
type Iα is significantly increased in PDT group compare to control at 12 h (Fig. 3D).
18 19
- 14 - 1
Fig. 3. Time dependent changes of MMP-1, 3, TGF-β, and Collagen type Iα mRNA
2
levels in fibroblasts after stimulation with keratinocyte-conditioned medium taken after
3
photodynamic therapy. MMP-1 mRNA significantly decreased in PDT group compared
4
with control at 24 h (A). MMP- 3 mRNA has a tendency to decrease in PDT group compared
5
with control at 6, 12 and 24h (B). TGF-β mRNA level has a tendency to increase after PDT
6
at 6 and 12h (C). Collagen Iα mRNA level is significantly increased after PDT at 12 h (D).
7
Data represent the fold increase of mRNA over the untreated control (control is set=1) and
8
determined by four independent experiments for each case. KCM= fibroblasts stimulated
9
with keratinocyte-conditioned medium without PDT, KCM-PDT= fibroblasts stimulated
10
with keratinocyte-conditioned medium taken after PDT. *p < 0.05.
11 12
- 15 -
C. Changes of the amount of fibroblast stimulating cytokines secreted by keratinocytes
1
after photodynamic therapy
2
Cytokine of KC after PDT (0.1mM of ALA, 6 J/cm2 of red light) was determined by
3
ELISA in KC supernatants. The level of cytokines including IL-1α, IL-6, TNF-α and TGF-β
4
were evaluated 24 and 48 h after PDT. A significant elevation of IL-1α, IL-6, and TNF-α
5
level in KC was noted after PDT compared with the untreated controls (Fig. 4). TGF-β was
6
not significantly altered after PDT compared to the untreated control group.
7
It is possible that an elevated IL-1α, IL-6, and TNF-α may increase expression of
8
MMPs which induce degradation of old damaged collagen fibers. This phenomenon may
9
lead the FB to initiate formation of new collagen fibers to replace them.
10 11
- 16 - 1
Fig. 4. Production of fibroblast stimulating cytokines in keratinocytes after
2
photodynamic therapy. A significant elevation of IL-1α, IL-6, and TNF-α level in
3
keratinocytes was noted after PDT compared with the untreated controls (A-C). TGF-β was
4
not significantly altered after PDT compared to the untreated control group (D). Data
5
represent the fold increase of cytokine over the untreated control (control is set=1) and
6
determined by five independent experiments for each case.*p < 0.05. Con=control group.
7 8 9
- 17 -
D. Direct effect of photodynamic therapy on the melanocytes
1
The melanin content and dopa oxidase activity in MC after PDT was examined to
2
determine the direct effect of PDT on the MC. After PDT, significantly reduced melanin
3
content was noted in PDT group compared to control group after 24 and 48h. Reduced
4
melanin content was also visualized in cell pellets (Fig. 5A). The cell number was not
5
changed by PDT. In addition, dopa oxidase activity was significantly decreased in PDT
6
group compared to the untreated control after 24 and 48h (Fig. 5B).
7 8
- 18 - 1
Fig. 5. Reduced melanin content and dopa oxidase activity in melanocytes after
2
photodynamic therapy. After PDT, significantly reduced melanin content was noted in PDT
3
group compared to the control group after 24 and 48h (A). Dopa oxidase activity was also
4
significantly reduced in PDT group compared to the untreated control after 24 and 48h (B).
5
*p < 0.05, **p < 0.01.
6 7 8
- 19 -
E. Indirect effect of photodynamic therapy on the melanocytes through
keratinocyte-1
melanocyte interaction
2
The indirect effect of PDT on MC through KC-MC interaction was examined using
3
KC-conditioned medium. The changes of melanin content and dopa oxidase activity were
4
evaluated in MC after stimulation with KC-conditioned medium (taken 24 h after PDT) for
5
48 h. As controls, MC was also stimulated with vehicle (keratinocyte medium itself; Epilife
6
medium) or untreated conditioned medium. MC stimulated with PDT treated
KC-7
conditioned medium showed a significant reduction of melanin content and dopa oxidase
8
activity compared to the control groups (Fig. 6).
9 10
- 20 - 1
2
Fig. 6. Reduced melanin content and dopa oxidase activity in melanocytes stimulated
3
with keratinocyte-conditioned medium after photodynamic therapy. Significantly
4
reduced melanin content was noted in the melanocytes stimulated with KCM taken after
5
PDT compared to controls after 48h (A). Dopa oxidase activity was also significantly
6
reduced in the melanocytes stimulated with KCM taken after PDT compared to the control
7
groups after 48h (B). Data were determined by five independent experiments for each case.
8
Vehicle=keratinocyte medium itself (Epilife medium), KCM=keratinocyte-conditioned
9
medium, Unt=untreated, PDT=photodynamic therapy. *p < 0.05, ** p < 0.01.
10 11 12 13 14
- 21 -
F. Changes in the amount of melanocytes stimulating cytokines secreted by
1
keratinocytes after photodynamic therapy
2
The MC stimulating cytokines secreted by KC after PDT was determined by ELISA in
3
KC supernatants. The level of cytokines including SCF, ET-1 and GM-CSF were evaluated
4
at 24 and 48 h after PDT. The level of SCF in KC-conditioned medium was significantly
5
decreased at 24 and 48 h after PDT compared with the untreated controls (Fig. 7A). ET-1 and
6
GM-CSF were not significantly altered after PDT compared to the untreated control group
7
(Fig. 7B and 7C). Therefore, decreased secretion of SCF in KC may play crucial role in
8
inhibiting melanin synthesis in MC after PDT.
9 10
- 22 - 1
2
Fig. 7. Production of melanocytes stimulating cytokines in keratinocytes after
3
photodynamic therapy. Significantly reduced level of SCF in keratinocytes was noted at 24
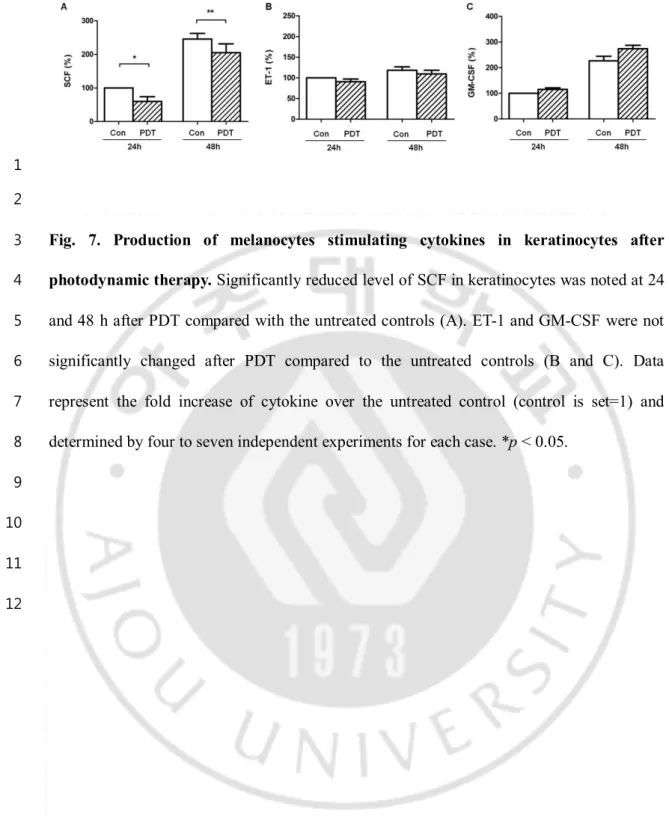
4
and 48 h after PDT compared with the untreated controls (A). ET-1 and GM-CSF were not
5
significantly changed after PDT compared to the untreated controls (B and C). Data
6
represent the fold increase of cytokine over the untreated control (control is set=1) and
7
determined by four to seven independent experiments for each case. *p < 0.05.
8 9 10 11 12
- 23 -
G. Indirect effect of photodynamic therapy on the melanocytes through
fibroblast-1
melanocyte interaction
2
The indirect effect of PDT on MC through FB-MC interaction was evaluated by using
3
FB-conditioned medium. The level of melanin content and dopa oxidase activity were
4
examined in MC after stimulation with FB-conditioned medium (taken 24 h after PDT) for
5
48 h. As controls, MC was also stimulated with vehicle (1:1 mixture of fibroblast medium
6
and melanocyte medium) or untreated FB-conditioned medium. Contrast to result E, 1:1
7
mixture of FB medium and MC medium was used instead of FB medium alone, because MC
8
was not growing well in FB medium. MC stimulated with PDT treated FB-conditioned
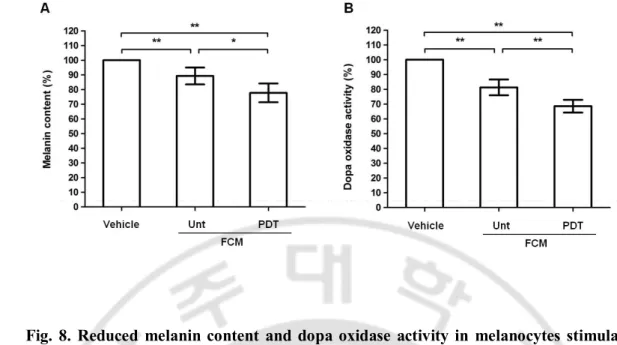
9
medium showed a significant reduction of melanin content and dopa oxidase activity
10
compared with the control groups (Fig. 8).
11 12
- 24 - 1
2
Fig. 8. Reduced melanin content and dopa oxidase activity in melanocytes stimulated
3
with fibroblast-conditioned medium after photodynamic therapy. Significantly reduced
4
melanin content was noted in the melanocytes stimulated with FCM taken after PDT
5
compared to the controls after 48h (A). Dopa oxidase activity was also significantly reduced
6
in the melanocytes stimulated with FCM taken after PDT compared with the controls after
7
48h (B). Data were determined by five independent experiments for each case. Vehicle=1:1
8
mixture of fibroblast medium and melanocyte medium, FCM=fibroblast-conditioned
9
medium, Unt=untreated, PDT=photodynamic therapy. *p < 0.05, ** p < 0.01.
10 11
- 25 -
H. Changes in the amount of melanocytes stimulating cytokines secreted by fibroblasts
1
after photodynamic therapy
2
The MC stimulating cytokines secreted by FB after PDT was determined by ELISA in
3
FB supernatants. The level of cytokines including SCF, HGF and bFGF were evaluated at 24
4
and 48 h after PDT. The HGF in FB-conditioned medium was significant decreased at 48 h
5
after PDT compared to the untreated control (Fig. 9A). The SCF was not significantly altered
6
after PDT compared to the untreated control (Fig. 9B). The bFGF, detected at very low level,
7
also showed no significant change after PDT compared with the untreated control (Fig. 9C).
8
Therefore, decreased secretion of HGF in FB may play a valuable part in inhibiting melanin
9
synthesis in MC after PDT.
10 11
- 26 - 1
2
Fig. 9. Production of melanocytes stimulating cytokines in fibroblasts after
3
photodynamic therapy. Significantly reduced level of HGF in fibroblast was noted at 48 h
4
after PDT compared to the untreated control (A). SCF and bFGF were not significantly
5
changed after PDT compared to the untreated control (B and C). Data represent the fold
6
increase of cytokine over the untreated control (control is set=1) and determined by three to
7
six independent experiments for each case.*p < 0.05.
8 9 10 11 12 13
- 27 -
IV. DISCUSSION
12
The results of this study show that FB was affected by PDT indirectly through cytokines
3
from KC. The PDT also has inhibitory effect of melanogenesis in the human MC. This
4
inhibitory effect of melanogenesis thought to be regulated indirectly by cytokines from KC
5
and FB as well as directly by PDT (Fig. 10).
6
In the previous report, the investigators demonstrated direct effect of PDT on FB which
7
is result of photorejuvenation effect such as increased collagen fiber. They suggested that the
8
PDT induced increase of MMP-3 and collagen type Iα through prolonged activation of ERK
9
(Jang et al., 2013). Increased level of MMP-3 may induce destruction of old damaged
10
collagen and then induce new collagen synthesis. In the present study, the indirect effect of
11
PDT on FB mediated by cytokines from KC was evaluated. After stimulation of FB with
12
KC-conditioned medium taken after PDT, The mRNA level of MMP-1 was decreased and
13
the mRNA level of collagen type Iα was increased in FB. However, mRNA level of MMP-3
14
and TGF-β were not changed significantly. According to these data, PDT significantly
15
affected FB indirectly through KC-conditioned medium. Among the FB stimulating
16
cytokines, IL-1α, IL-6, and TNF-α were elevated in the KC-conditioned medium after PDT.
17
Although it was not significant, TGF- β was also increased at 48 h in the KC-conditioned
18
medium after PDT. IL-1α was increased at 24 h and rapidly decreased at 48 h after PDT. In
19
photoaging process, increased secretion of inflammatory cytokines induces expression of
20
MMPs which results in degradation of collagen fibers in the dermis and makes wrinkles
21
(Shin et al., 2005). In the present results, although IL-1α, IL-6, and TNF-α were increased in
- 28 -
KC-conditioned medium after PDT, mRNA of MMP-1 was decreased and mRNA of
1
collagen Iα in FB was increased after stimulation with KC-conditioned medium taken after
2
PDT. Similar context with previous reports (Jang et al., 2013), there may be possible
3
explanation for these inconsistent results. At initial phase of PDT, secretion of inflammatory
4
cytokines may be increased to induce MMPs and degradation the old collagen fiber.
5
Breakage of old damaged collagen may allow induction of new collagen synthesis. At later
6
phase of PDT, inflammatory cytokines and MMPs may be decreased than initial phase which
7
allow accelerated synthesis of new collagen.
8
In the result of this study, although mRNA level of collagen Iα in FB was significantly
9
increased at 12 h after stimulation with KC-conditioned medium taken after PDT, it became
10
almost equal at 24 h in PDT group and control group (Fig 3D). The similar finding was also
11
detected in the result of TGF- β (Fig 3C). Although it was not significant, the mRNA level of
12
TGF-β in PDT group was rather become lower than the control group. It may be due to the
13
reduced effect of PDT after 12 h. In previous result, the authors also presented that rapid and
14
prolonged activation of phospho-ERK in FB up to 8 hours after PDT using western blot
15
analysis (Jang et al., 2013). Therefore, if FB was stimulated with KC-conditioned medium
16
taken after PDT in multiple times every 12 h, more prolonged and more increased mRNA
17
level of collagen Iα in FB could be noted. Based on these results, we suggested that PDT
18
induced new collagen synthesis may be mediated not only by direct effect of PDT on FB but
19
also by indirect effect of PDT on FB through cytokines from KC.
20
As we mentioned above, PDT has been known to improve photoaged skin by induction
21
of new collagen synthesis in human FB (Park et al., 2010; Jang et al., 2013). However,
- 29 -
inhibition of melanogenesis of human MC by PDT has rarely been reported. According to
1
this study, it was clearly demonstrated that PDT inhibited melanogenesis not only by direct
2
effect to MC but also by indirect effect of PDT through cytokines from KC and FB.
3
In the present study, melanogenesis was reduced in the MC after PDT without
4
influencing cell viability. According to the figure 5A, the melanin content was increased at
5
48h compared with 24h in both control and PDT group. It may be due to stabilization of the
6
MC at 48h than 24h. It may also caused by the increase of culture time and the consequent
7
increase in cell number.
8
Melanogenesis was regulated by various cytokines through interactions between
KC-9
MC and FB-MC. SCF, ET-1 and GM-CSF are representative cytokines secreted by KC and
10
stimulated MC (Imokawa, 2004). According to this study result, among above cytokines,
11
SCF was reduced in the KC-conditioned medium after PDT. Therefore, SCF is thought to be
12
a major cytokine that induces inhibitory effect of melanogenesis in the MC after PDT. There
13
are several signaling pathways regulating melanogenesis of human MC. SCF/c-kit pathway
14
is one of the major melanogenesis pathway in human MC. SCF lead to activation of
15
mitogen-activated protein kinase (MAPK) cascade by binding to its corresponding receptor
16
c-kit. MAPK activates microphthalmia-associated tramscription factor (MITF) and result in
17
transcription of the melanogenic enzymes such as tyrosinase, tyrosinase-related protein-1
18
(TRP-1) and TRP-2 (Imokawa et al., 2000). Therefore, decreased secretion of SCF by KC
19
after PDT may lead to inhibition of melanogenesis through this SCF/c-kit pathway in MC.
20
Future studies should be focused on the downstream cascade of SCF/c-kit pathway in MC
21
after stimulating with KC-conditioned medium after PDT.
- 30 -
In the present result, SCF was detected at lower level in KC-conditioned medium
1
compared to the previous report (Fig 7A) (Okazaki et al., 2005). However, in seven
2
independent experiments, the data showed similar result that the SCF level was significantly
3
decreased after stimulation with KC-conditioned medium taken after PDT. If the experiment
4
performed after activation of KC using such as UV irradiation, the level of SCF might be
5
detected at increased level and degree of decline after PDT also would be increased.
6
Therefore PDT therapy after pretreatment of the cells with UV irradiation can be another
7
meaningful experiment to investigate restoring effect of PDT in photo-damaged cell.
8
HGF, SCF and bFGF are well known cytokines secreted from FB and stimulated MC
9
(Imokawa, 2004). Among these cytokines, HGF was decreased in the FB-conditioned
10
medium after PDT in this study. Therefore, HGF thought to be a main cytokine from FB
11
induced inhibitory effect of melanogenesis in the MC after PDT. HGF have been known as a
12
mitogens for human MC in vitro (Imokawa, 2004). In the previous study, stimulation of MC
13
with 10 nM of HGF induced proliferation of cultured human MC (Matsumoto et al., 1991).
14
However, in the present study, there was no significant proliferation of MC after PDT
15
compared to the control. In dermatofibroma, a benign dermal tumor characterized by
16
proliferation of FB-like cells, hyperpigmentation in the overlying epidermis is supposed to
17
be mediated by high expression of HGF of dermal FB (Shishido et al., 2001). The high
18
expression of HGF in the dermis of dermatofibroma was detected at both the gene and the
19
protein levels (Imokawa, 2004). To date, precise mechanism of HGF in enhancement of
20
melanogenesis in human MC is not fully demonstrated. However, much evidence support a
21
distinct role of HGF in stimulating epidermal MC. Taken together with these data, decreased
- 31 -
secretion of HGF from FB after PDT thought to induce inhibition of melanogenesis in the
1
MC in our study.
2
According to the results showing reduced melanin content and dopa oxidase activity in
3
MC after stimulation with KC-conditioned medium taken after PDT, the melanin content and
4
dopa oxidase activity also reduced in MC stimulated with KC-conditioned medium without
5
PDT compared to the MC stimulated with KC medium itself (Fig 6). In addition, the melanin
6
content and dopa oxidase activity also reduced in MC stimulated with FB-conditioned
7
medium without PDT compared to the MC stimulated with 1:1 mixture of FB medium and
8
MC medium (Fig 8). It may be caused by growth factors in supplements of KC and FB
9
medium. The various kinds of growth factors, such as human epidermal growth factor,
10
bovine insulin, hydrocortisone, bovine pituitary extract, bovine transferrin and FBS, exist in
11
the supplement of KC and FB medium. These growth factors in media, which is able to
12
stimulate MC to synthesis of melanin, may be used up during culture of KC or FB. Therefore,
13
decreased level of growth factors in KC-conditioned medium and FB-conditioned medium
14
may result in reduced melanin content and dopa oxidase activity in MC stimulated with
KC-15
conditioned medium without PDT or FB-conditioned medium without PDT.
16
Contrast to our study, Karrer et al. suggested that KC stimulated by PDT produced
17
proinflammatory cytokines such as IL-1a and TNF-a, which influenced FB to produce
18
increased amounts of MMP-1 and MMP-3. They suggested that this paracrine induction of
19
collagen-degrading MMPs results in the reduction of collagen volume after PDT (Karrer et
20
al., 2004). However, they used higher light energy in PDT procedure compare to this study;
21
100 µM/L of ALA and 24 J /cm2 of red light. We used “low-level PDT” (0.1 mM /L of ALA
- 32 -
and 3 J /cm2 of red light) to induce activation of FB without cytotoxic effects. Therefore,
1
“low-level PDT” used in this study, can be an explanation about different result compare
2
with previous studies (Karrer et al., 2003, 2004).
3
In the similar context, the different results of other studies examined PDT effect in
4
melanogenesis can be explained. There are a few studies that demonstrated PDT induced
5
hyperpigmentation in vivo (Monfrecola et al., 2002; Juzeniene et al., 2008). To begin with,
6
in vivo study might differ from in vitro study. Also, “low-level PDT” may induce different
7
result compare to previous studies which used conventional parameter of PDT. In future
8
study, if we apply “low-level PDT” to the human skin in vivo, we may be able to observe
9
inhibition of melanogenesis.
10 11
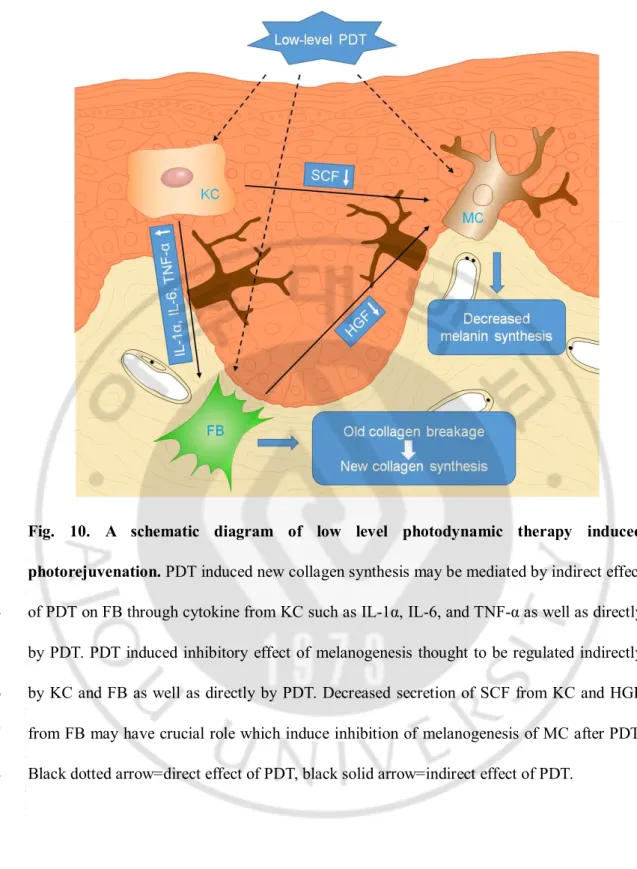
- 33 - 1
Fig. 10. A schematic diagram of low level photodynamic therapy induced
2
photorejuvenation. PDT induced new collagen synthesis may be mediated by indirect effect
3
of PDT on FB through cytokine from KC such as IL-1α, IL-6, and TNF-α as well as directly
4
by PDT. PDT induced inhibitory effect of melanogenesis thought to be regulated indirectly
5
by KC and FB as well as directly by PDT. Decreased secretion of SCF from KC and HGF
6
from FB may have crucial role which induce inhibition of melanogenesis of MC after PDT.
7
Black dotted arrow=direct effect of PDT, black solid arrow=indirect effect of PDT.
- 34 -
V. CONCLUSION
12
Based on above results, PDT induced new collagen synthesis may be mediated by
3
indirect effect of PDT on FB through cytokine from KC such as IL-1α, IL-6, and TNF-α. In
4
addition, PDT has inhibitory effect of melanogenesis in the human MC. This inhibitory
5
effect of melanogenesis thought to be regulated indirectly by KC and FB as well as directly
6
by PDT. Decreased secretion of SCF from KC and HGF from FB may have crucial role
7
which induce inhibition of melanogenesis of MC after PDT.
8 9 10
- 35 -
REFERENCES
12
1. Brackett CM, Gollnick SO: Photodynamic therapy enhancement of anti-tumor
3
immunity. Photochem Photobiol Sci 10: 649-652, 2011
4
2. Dover JS, Bhatia AC, Stewart B, Arndt KA: Topical 5-aminolevulinic acid combined
5
with intense pulsed light in the treatment of photoaging. Arch Dermatol 141:
1247-6
1252, 2005
7
3. Gold MH, Bradshaw VL, Boring MM, Bridges TM, Biron JA: Split-face comparison
8
of photodynamic therapy with 5-aminolevulinic acid and intense pulsed light versus
9
intense pulsed light alone for photodamage. Dermatol Surg 32: 795-801; discussion
10
801-793, 2006
11
4. Imokawa G: Autocrine and paracrine regulation of melanocytes in human skin and
12
in pigmentary disorders. Pigment Cell Res 17: 96-110, 2004
13
5. Imokawa G, Kobayasi T, Miyagishi M: Intracellular signaling mechanisms leading
14
to synergistic effects of endothelin-1 and stem cell factor on proliferation of cultured
15
human melanocytes. Cross-talk via trans-activation of the tyrosine kinase c-kit
16
receptor. J Biol Chem 275: 33321-33328, 2000
17
6. Jang YH, Koo GB, Kim JY, Kim YS, Kim YC: Prolonged Activation of ERK
18
Contributes to the Photorejuvenation Effect in Photodynamic Therapy in Human
19
Dermal Fibroblasts. J Invest Dermatol, 2013
20
7. Juzeniene A, Nielsen KP, Zhao L, Ryzhikov GA, Biryulina MS, Stamnes JJ, Stamnes
21
K, Moan J: Changes in human skin after topical PDT with hexyl aminolevulinate.
- 36 -
Photodiagnosis Photodyn Ther 5: 176-181, 2008
1
8. Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM: Influence of
5-2
aminolevulinic acid and red light on collagen metabolism of human dermal
3
fibroblasts. J Invest Dermatol 120: 325-331, 2003
4
9. Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM:
Keratinocyte-5
derived cytokines after photodynamic therapy and their paracrine induction of
6
matrix metalloproteinases in fibroblasts. Br J Dermatol 151: 776-783, 2004
7
10. Kim JM, Kim NH, Tian YS, Lee AY: Light-emitting diodes at 830 and 850 nm
8
inhibit melanin synthesis in vitro. Acta Derm Venereol 92: 675-680, 2012
9
11. Kohl E, Torezan LA, Landthaler M, Szeimies RM: Aesthetic effects of topical
10
photodynamic therapy. J Eur Acad Dermatol Venereol 24: 1261-1269, 2010
11
12. Matsumoto K, Tajima H, Nakamura T: Hepatocyte growth factor is a potent
12
stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res
13
Commun 176: 45-51, 1991
14
13. Monfrecola G, Procaccini EM, D'Onofrio D, Roberti G, Liuzzi R, Staibano S,
15
Manco A, De Rosa G, Santoianni P: Hyperpigmentation induced by topical
5-16
aminolaevulinic acid plus visible light. J Photochem Photobiol B 68: 147-155, 2002
17
14. Okazaki M, Yoshimura K, Uchida G, Harii K: Correlation between age and the
18
secretions of melanocyte-stimulating cytokines in cultured keratinocytes and
19
fibroblasts. Br J Dermatol 153 Suppl 2: 23-29, 2005
20
15. Palmetshofer A, Zechner D, Luger TA, Barta A: Splicing variants of the human
21
growth hormone mRNA: detection in pituitary, mononuclear cells and dermal
- 37 -
fibroblasts. Mol Cell Endocrinol 113: 225-234, 1995
1
16. Park MY, Sohn S, Lee ES, Kim YC: Photorejuvenation induced by 5-aminolevulinic
2
acid photodynamic therapy in patients with actinic keratosis: a histologic analysis. J
3
Am Acad Dermatol 62: 85-95, 2010
4
17. Pincelli C, Sevignani C, Manfredini R, Grande A, Fantini F, Bracci-Laudiero L, Aloe
5
L, Ferrari S, Cossarizza A, Giannetti A: Expression and function of nerve growth
6
factor and nerve growth factor receptor on cultured keratinocytes. J Invest Dermatol
7
103: 13-18, 1994
8
18. Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN: Photoaging:
9
mechanisms and repair. J Am Acad Dermatol 55: 1-19, 2006
10
19. Shin MH, Rhie GE, Kim YK, Park CH, Cho KH, Kim KH, Eun HC, Chung JH:
11
H2O2 accumulation by catalase reduction changes MAP kinase signaling in aged
12
human skin in vivo. J Invest Dermatol 125: 221-229, 2005
13
20. Shishido E, Kadono S, Manaka I, Kawashima M, Imokawa G: The mechanism of
14
epidermal hyperpigmentation in dermatofibroma is associated with stem cell factor
15
and hepatocyte growth factor expression. J Invest Dermatol 117: 627-633, 2001
16
21. Tanaka K, Asamitsu K, Uranishi H, Iddamalgoda A, Ito K, Kojima H, Okamoto T:
17
Protecting skin photoaging by NF-kappaB inhibitor. Curr Drug Metab 11: 431-435,
18
2010
19
22. Tomita Y, Maeda K, Tagami H: Melanocyte-stimulating properties of arachidonic
20
acid metabolites: possible role in postinflammatory pigmentation. Pigment Cell Res
21
5: 357-361, 1992
- 38 -
23. Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y: Enhanced melanogenesis induced by
1
tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron
2
capture therapy for amelanotic melanoma. Pigment Cell Res 11: 275-282, 1998
3 4 5
- 39 - - 국문요약 - – 1 2
광역동치료에 의한 광회춘 효과의 분자적 기전
(섬유아세포-3각질세포-멜라닌세포 간의 상호작용에 의한 면역조절 효과를
4중심으로)
5 6 배경: 피부의 광노화란 장기간에 걸친 자외선 노출에 의해 피부에 주름, 색소침 7 착, 모세혈관확장, 피부암 등이 발생하는 것을 말한다. 광역동치료는 피부암과 8 전암병변을 치료하기 위해 사용하는 방법으로 최근 몇몇 임상 연구에 따르면 광 9 역동치료가 노화된 피부의 광회춘효과를 주는 것으로 보고되고 있다. 섬유아세포 10 에 대한 광역동치료의 직접적 효과를 연구한 이전 연구를 보면 광역동치료에 의 11 해 섬유아세포 내에서 지속적으로 ERK이 활성화 되고 이것은 MMP-3와 12collagen type Iα의 증가를 유도하는 것으로 보고 되었다. 증가된 MMP-3는
13 오래된 collagen을 분해하고 이것은 새로운 collagen 형성을 유도할 것으로 생 14 각된다. 15 목적: 이 연구에서 저자는 광역동치료에 의한 광회춘 효과의 분자적 기전에 대해 16 각질세포, 섬유아세포, 멜라닌세포 간의 상호작용을 중심으로 알아보고자 한다. 17 방법: 각질세포, 섬유아세포, 멜라닌세포에 대한 광역동 치료 시에“저용량 광역 18 동치료”조건을 사용하였다. 섬유아세포는 광역동치료를 한 각질세포 배양액으로 19 자극했고, 멜라닌세포는 광역동치료를 한 각질세포 배양액 또는 섬유아세포 배양 20 액으로 자극하였다. 각질세포와 섬유아세포 상층액에 있는 다양한 종류의 싸이토 21 카인을 효소면역측정법(ELISA)으로 측정하였다. 섬유아세포 내 MMP, TGF-β, 22
- 40 -
collagen type Iα의 mRNA를 실시간 중합효소연쇄반응(real-time PCR)로 측
1
정하였다. 멜라닌세포의 멜라닌 함유량과 dopa oxidase의 활성도도 측정하였다.
2
결과: 섬유아세포를 각질세포배양액으로 자극한 후 섬유아세포 내 MMP-1의
3
mRNA가 대조군에 비해 감소하였고 collagen type Iα의 mRNA는 대조군에 비
4 해 증가하였다. 그러나 MMP-3와 TGF-β 는 의미 있는 변화가 없었다. 섬유 5 아세포를 자극하는 싸이토카인 중에서 IL-1α, IL-6, TNF-α 가 각질세포에 6 광역동치료한 후 각질세포 배양액에서 대조군에 비해 증가되어 있었다. TGF-β 7 는 광역동 치료 후 각질세포 배양액에서 변화가 없었다. 광역동치료 후 멜라닌세 8 포 내 멜라닌 함유량과 dopa oxidase 활성도가 24시간과 48시간에 대조군에 9 비해 감소하는 것으로 나타났다. 또한 멜라닌세포를 광역동치료한 각질세포 배양 10 액으로 자극한 후에도 멜라닌 함유량과 dopa oxidase 활성도가 대조군에 비해 11 감소하는 것으로 나타났다. 각질세포에서 분비되어 멜라닌세포를 자극하는 것으 12 로 알려진 싸이토카인들 중에서 SCF가 광역동 치료 후 각질세포 배양 상층액에 13 서 의미있게 감소되어 있는 것으로 측정되었고 ET-1과 GM-CSF는 변화가 없 14 었다. 멜라닌세포를 광역동치료한 섬유아세포 배양액으로 자극한 후에 역시 멜라 15 닌 함유량과 dopa oxidase 활성도가 대조군에 비해 감소하는 것으로 나타났다. 16 섬유아세포에서 분비되어 멜라닌세포를 자극하는 것으로 알려진 싸이토카인들 17 중에서 HGF가 광역동 치료 후 각질세포 배양 상층액에서 의미있게 감소되어 있 18 는 것으로 측정되었고 SCF와 bFGF는 변화가 없었다. 19 결론: 광역동치료는 새로운 collagen 생성을 유도하는데 이는 광역동치료가 섬유 20 아세포에 미치는 직접적인 영향뿐 아니라 각질세포에서 분비되는 싸이토카인에 21 의한 광역동치료의 섬유아세포에 대한 간접적인 영향에 의해서도 발생하는 것으 22
- 41 - 로 생각된다. 본 저자는 또한 광역동치료가 멜라닌세포에서 멜라닌 형성을 억제 1 한다고 제안한다. 이 멜라닌 형성 억제 효과는 광역동치료가 멜라닌세포에 미치 2 는 직접적인 영향뿐 아니라 각질세포와 섬유아세포에 의한 광역동치료의 멜라닌 3 세포에 대한 간접적인 영향에 의해서 발생하는 것으로 생각된다. 각질세포에서 4 분비되는 SCF와 섬유아세포에서 분비되는 HGF가 광역동치료 후 멜라닌세포의 5 멜라닌 형성 억제를 유도하는 주요 싸이토카인으로 보인다. 6 7 핵심어: 광역동 치료, 광회춘, 각질세포, 섬유아세포, 멜라닌세포 8 9