Silibinin Induces Apoptotic Cell Death Via ROS-dependent Mitochondrial Pathway in Human Glioma Cells
전체 글
수치
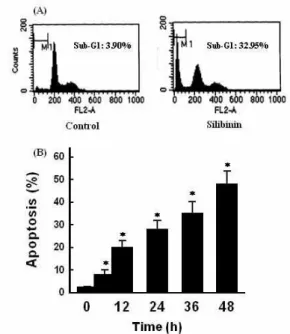
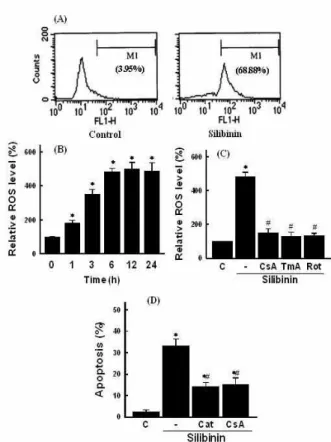
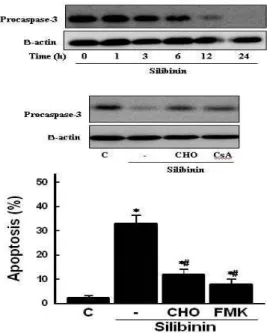
관련 문서
These results suggest that the bilobalide inhibits the cell proliferation and induces the apoptotic cell death in FaDu human pharyngeal squamous cell carcinoma via both
Taken together, these results suggested that latex containing the ficin inhibited the cell growth and induced apoptosis by caspase and Bcl-2 family signaling pathway in
We found spiropyran derivatives have photoswitching properties in both fluorescence and singlet oxygen generation in aqueous solutions and cells, and
Oxidative injury and inflamma- tory periodontal disease: The challenge of anti-oxidants to free radicals and reactive oxygen species.. The role of oxygen and
In vitro cell migration in APE or JAG1 siRNA-treated M059K cell line Fig,11 Down-regulation of JAG1 induces S phase arrest in
We determined the nucleotide sequences of the mitochondrial DNA (mtDNA) control region using cloning and sequencing, and obtained the complete sequence from the cattle bones
이 를 위해 cell proliferation assay (MTT), cytochrome C ELISA assay, caspase―3 enzyme activity assay, fluorescence microscopy에 의한 apoptosis 관찰,
Glutamate excitotoxicity induced by excessive activation of NMDA receptor causes various damage to cells, which leads to cell death.. In previous studies, increased ROS