pISSN 2383-899X eISSN 2234-7631
Introduction
Obesity is a disease in which body fat excessively accumulates to the extent that health is negatively affected.
1Obesity is reported to be associated with diseases including diabetes, hypertension, hypercho- lesterolemia, asthma, arthritis, heart disease, stroke, endometrial cancer, breast cancer, and colorectal cancer.
2,3According to a 2014 report by the World Health Organization (WHO), the obese popula- tion has more than doubled since 1980, and now comprises approxi- mately 13% of the global adult population (11% and 15% of the male and female populations, respectively).
3This is also the case in Korea, where the obese population is steadily increasing at a higher rate than that noted previously. In 2013, the prevalence of adult obesity, defined as body mass index (BMI) ≥ 25 kg/m
2, was approximately
32.5% (37.6% for men and 27.5% for women). Hence, a third of the Korean adult population is obese.
4Generally, BMI and waist circumference are used as indexes for obesity.
1Obesity is defined as a BMI ≥30 kg/m
2in Western coun- tries
1and ≥ 25 kg/m
2in Asia-Pacific countries
5; the latter definition is used in Korea. Additionally, waist circumference and waist-hip ra- tio are used as indexes for abdominal obesity.
6The Korean Society for the Study of Obesity gives the definition of abdominal obesity as waist circumference ≥90 cm for men and ≥85 cm for women.
7BMI does not provide information regarding body fat mass or fat-free mass
8, but recent studies have reported that metabolic syndrome is associated with body fat and fat-free masses.
9Of several body com- position measures, fat-free mass in particular has an inverse J- shaped association with all-cause mortality, and the level of fat-free
Comparison of Muscle Mass Indexes According to Protein Intake in Obese Patients
Mingyeong Heo, Yeonjoo Lee, Yongsoon Park*
Department of Food and Nutrition, Hanyang University, Seoul, Korea
Background: Protein is the most important nutrient for the muscle synthesis and maintenance of muscle mass. However, there have been no studies comparing muscle mass indexes according to protein intake in obese patients. Thus, the present study investigated the hypothesis that high protein intake positively affected muscle mass indexes in obese patients.
Methods: Male (n=50) and female (n=30) obese patients were recruited. Each sex was divided into 2 groups according to their protein intake.
Dietary intake, fat-free mass, appendicular skeletal muscle mass, appendicular skeletal muscle mass/height
2, skeletal muscle index, total bone area, bone mineral count, bone mineral density, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, inter- leukin-6, tumor necrosis factor α, smoking, alcohol, and exercise were assessed.
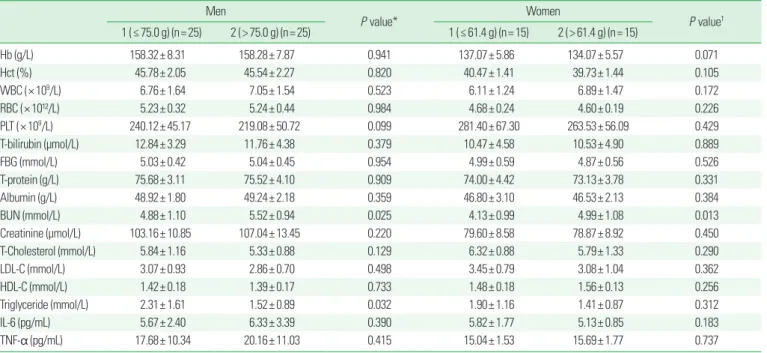
Results: Fat-free mass, appendicular skeletal muscle mass, and appendicular skeletal muscle mass/height
2were significantly higher in obese men in the higher protein intake group, while serum triglyceride was significantly lower in the same group. However, bone indexes and inflam- matory cytokines according to protein intake were not significantly different in obese men. Fat-free mass, bone indexes, muscle indexes, blood lipid profiles, and inflammatory cytokines were not significantly different according to protein intake in obese women.
Conclusion: In this population, increasing protein intake may positively affect fat-free mass, appendicular skeletal muscle mass, appendicular skeletal muscle mass/height
2, and serum triglyceride in obese men. Therefore, additional studies may be necessary to identify the appropriate level of protein intake required to prevent a decrease in muscle mass, and increase muscle mass in obese patients.
Key words: Protein intake, Muscle mass indexes, Obese patients, Fat-free mass, Appendicular skeletal muscle mass, Appendicular skeletal muscle mass/height
2*Corresponding author Yongsoon Park http://orcid.org/0000-0001-5110-5716 Department of Food and Nutrition, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Korea
Tel +82-2-2220-1205 Fax +82-2-2292-1226 E-mail yongsoon@hanyang.ac.kr Received Feb. 29, 2016 Reviewed Mar. 25, 2016 Accepted Apr. 7, 2016
Copyright © 2016 Korean Society for the Study of Obesity
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.