저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
hs-CRP level represents
the disease burden and the age
but not vulnerability of
coronary atherosclerosis
by
Se Jun Park
Major in Medicine
Department of Medical Science
The Graduate School, Ajou University
hs-CRP level representsthe disease burden and the age
but not vulnerability of coronary atherosclerosis
: a study of volumetric plaque composition by 3-vessel virtual
histology-intravascular ultrasound
by
Se Jun Park
A Dissertation Submitted to The Graduate School of Ajou
University in Partial fulfillment of The Requirements for
The Degree of Master of Medicine
Supervised by
SeungJeaTahk, M.D., Ph.D.
Major in Medicine
Department of Medical Science
The Graduate School, Ajou University
This certifies that the dissertation
of Se Jun Park is approved
.
SUPERVISORY COMMITTEE
SeungJeaTahk
SeungJeaTahk
Joon Han Shin
Joon Han Shin
So Yeon Choi
So Yeon Choi
The Graduate School, Ajou University
i
-ABSTRACT -
hs-CRP level represents the disease burden and the age but not
vulnerability of coronary atherosclerosis : a study of volumetric
plaque composition by 3-vessel virtual histology-intravascular
ultrasound
Background:hs-CRP (high sensitive C-reactive protein) has been known as a systemic
inflammatory marker of atherosclerosis and considered as one of the predictors of future cardiac events. Some reports presented hs-CRP level was associated with plaque
vulnerability but most studies were performed by assessing focal target plaque but not whole plaques from a coronary tree.
Methods: To evaluate of the relationship of plasma hs-CRP level and volumetric
plaque composition of the coronary arterial tree, we performed ‘whole vessel” virtual histology-intravascular ultrasound (VH-IVUS) in 189 vessels of 63 patients. The
components of atherosclerosis were classified as fibrous (FI), fibrous-fatty (FF), necrotic core (NC) and dense calcium (DC). Quantitative assessment of these plaque components and the presence of VH-IVUS–derived thin-cap fibroatheroma (VH-TCFA) in the coronary arterial trees were compared to hs-CRP levels in individuals. hs-CRP levels were measured before coronary angiogram and IVUS study.
Results: Forty-nine patients (77.8%) were diagnosed with acute coronary syndrome in
this population. The mean values of hs-CRP were 0.24±0.52 mg/dl (0~3.27mg/dl). The analyzed vessel length was 56.1±17.4 mm for the left anterior descending coronary artery, 51.9±19.0 mm for the left circumflex coronary artery, and 74.2±18.8 mm for the right coronary artery. The number of VH-TCFAs was 1.0±0.8 for the left anterior descending, 0.6±0.7 for the left circumflex, and 0.8±1.0 for the right coronary arteries.
ii
volume index, volume index of FF and DC. But parameters of NC and the number of VH-TCFA were not related with hs-CRP level. In multivariate analysis, the volume index of DC was most reliable factor to hs-CRP (β=3.646, CI=2.036 to 5.255, p<0.001)
Conclusions: This three-vessel VH-IVUS presented that hs-CRP were related to the
total atherosclerotic burden and the maturing (coronary calcium) but not vulnerable features (NC or VH-TCFA) of plaques in coronary arterial tree. Increased hs-CRP level as a biomarker to predict cardiovascular events might imply atherosclerosis severity of whole coronary tree but not current plaque vulnerability.
Keyword: high sensitive C-reactive protein, hs-CRP, coronary atherosclerosis, virtual histology, intravascular ultrasound, vulnerable plaque, vulnerability
iii
TABLE OF CONTENTS
ABSTRACT ···i
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ···iv
LIST OF TABLES ···v I. INTRODUCTION ··· 1
II. MATERIAL AND METHODS ··· 2
A. PATIENTS··· 2
B. LABORATORY ASSESSMENT··· 2
C. IVUS IMAGING AND ANALYSIS··· 2
D. STATISTICAL ANALYSIS··· 3
III. RESULTS··· 4
A. BASELINE CLINCAL AND VH-IVUS DATA···4
B. CORRELATION BETWEENHS-CRP AND PLAQUE COMPONENTS··· 9
IV. DISCUSSION··· 15
V. CONCLUSION··· 18
REFERENCES···19
iv
LIST OF FIGURE
Fig 1. The correlation of hs-CRP with mean plaque burden and plaque volume index ··· 10
Fig 2. The correlation of hs-CRP with mean burdens of each plaque
components.··· 11 Fig 3.The correlation of hs-CRP with volume indices of each plaque components. ··· 12
Fig 4. The correlation of hs-CRP with the number of VH-TCFA ··· 13
v
LIST OF TABLES
Table 1. Baseline clinical data··· 5
Table 2. Baseline lesion characteristics in the VH-IVUS analysis··· 6 Table 3. Volumetric data of VH-IVUS according to interquartile range of
hs-CRP··· 8
1
I. INTRODUCTION
Coronary artery disease has have high mortality and incidence, although the technology of the medicine has been developed. The atherosclerosis is most important cause for coronary artery disease, so it’s important to know the pathogenesis of that. It has known the fate of atherosclerosis is related to the characteristic and composition of the plaque according to autopsy data(David MJ, 1993).The concept of ‘Vulnerable plaque’originated from aforementioned cause, composed of large lipd core, thin fibrous cap, positive remodeling, abundant inflammatory cells and scant smooth muscle cells(Yamagishi et al., 2000). The inflammatory reaction is also key factors in determining plaque vulnerability. Inflammatory cell is considered to have a role in the loss of fibrous cap, that is related to plaque rupture and thrombosis (Shah, 2009).
During the past decade, there were remarkable developments of the evaluation for intra-coronary imaging in ex-vivo. The gray scaled intravascular ultrasound (IVUS) can show the anatomical characteristics of the plaque. But there are some limitations of IVUS in detecting vulnerable plaque and one of those is not to distinguish the elements of plaque composition exactly. The virtual histology-intravascular ultrasound (VH-IVUS)come over this one; description of the composition of the plaque as 4 color codes (Rodriguez-Granillo et al., 2005).
It is known the inflammatory reaction and the vulnerable plaque are intercorrelated by some VH studies, but that were set limits to culprit lesion, not whole coronary trees (Otake et al., 2008; Ko et al., 2012). Therefore, the purpose of this study was to find out the relationship between the plaque vulnerability and inflammation by the analaysis of 3-vessel VH-IVUS and inflammatory biomarker; high-sensitive C-reactive protein (hs-CRP).
2
II. Material and Method
A. Patients
A total of 63 patients diagnosed with ischemic heart disease (history of angina and ≥ 30% luminal narrowing on coronary angiogram by visual estimation) were enrolled into this study from September 2006 and August 2008, at a single medical center. They were performed preinterventional 3-vessel VH-IVUS. Patients with any totally occlusive vessel, severely tortuous vessel, extensively calcified lesions, severe left main coronary artery disease (diameter stenosis ≥ 50%), and hemodynamic instability were excluded. Written informed consent was obtained from all patients.
B. Laboratory assessment
Blood samples were obtained within 24 hours before coronary angiography and VH-IVUS, and then were centrifuged immediately, aliquoted and stored at -80℃ for subsequent analysis. Serum hs-CRP was measured by a latex-enhanced turbidmetry immunoassay, using a chemistry autoanalyzer (TBA 200FR, TOSHIBA Co., Tokyo, Japan).
C. IVUS imaging and analysis
VH-IVUS studies were implemented with a phased-array, 20-MHz, 2.9-F IVUS catheter (Eagle eye, Volcano Corporation, Racho Cordova, California). After intracoronary
administration of nitroglycerin (100~200 µg), the transducer was introduced as far distal as possible into each major epicardial artery, paying particular attention to identify any evident atherosclerosis. Using motorized pullback (0.5 mm/s), imaging was performed back to aorto-ostial junction. While on pulling back, the gray-scale IVUS was recorded; and the raw radiofrequency data were captured at the top of the R waves for the reconstruction of color-coded map by VH data recorder (Volcano Corporation).
An experienced analystwho was unaware of the patient’s clinical data, measured the parameters of the lumen and the media-adventitia interface by manual contour tracing. Volumetric VH-IVUS analysis was done from the most distal part where VH-IVUS plaque components were detected to the respective ostium, and volumetric data were generated with
3
using pcVH software (version 2.1, Volcano Corporation). Total plaque volume was obtained by analyzing 3 vessels in each patient (including all lesions and reference segments), and a volumetric index was calculated as total plaque volume divided by total vessel length. Mean plaque plus media (P+M) burden was calculated as the total plaque volume divided by total vessel volume X100. VH-IVUS analysis was described as 4 different color-coded tissue; green (fibous, FI), yellow-green (fibro-fatty, FF), red (necrotic core, NC), and white (dense calcium, DC). Each plaque component was measured in every recorded frame and expressed as the volume index (absolute measure) and percentages of total plaque volume.Virtual histology–intravascular ultrasound–derived thin-cap fibroatheroma (VH-TCFA) was defined as a lesion that fulfilledthe following criteria in at least 3 consecutive frames: 1) NCs ≥ 10% directly attaching to the lumen; and 2) ≥ 40%P+M burden (Rodriguez-Granillo et al., 2005). Identifying 2 separate lesions in the sameartery required a ≥ 5-mm reference segment
between them.If there was a <5-mm reference segment, they wereconsidered part of one long lesion(Garcia-Garcia et al., 2009). Similarly, identifying 2 separate VH-TCFAs required a non–VH-TCFA–containing reference segment ≥ 5 mm between them. Two experienced observers who were unaware of the patients’ clinical histories evaluated the VH-TCFA in consensus.
D.Statistical analysis
All analyses were performed using SPSS version 18.0 statistical software (SPSS Inc, Chicago, IL, USA). Categorical variables were expressed as numbers or frequencies of occurrence with comparisons using chi-square statistics or Fisher exact probability test. All continuous variables were tested by Kolmogorov-Smirnov Z test for normality analysis. Continuous data were reported as mean ± SD with comparisons using unpaired Student ttest and Pearson’s correlation analysis. If normality tests failed, the continuous values were presented as median and interquartile range and were compared by Mann-Whitney U test and Kruskal-Wallis test. Multiple linear regression analysis was performed to assess independent predictors for hs-CRP. A p value < 0.05 was considered statistically significant in this study.
4
III. Results
A. Baseline clinical and VH-IVUS data
It was shown baseline clinical characteristics on Table 1.Patients had a mean age of 59 years; 31.7% of them were ≥ 65 years of age; 65.1% were men. The study cases were composed of diabetes mellitus (DM, 27.0%), hypertension (58.7%), smoking (44.4%), dyslipidemia (20.6%) and familyhistory of premature coronary artery disease(7.9%). Clinical presentation in patients were consisted of stable angina (22.2%), unstable angina (54.0%) and acute myocardial infarction (11.1%; 3 cases: ST segment elevation myocardial
infarction, 4 cases: Non-ST segement elevation myocardial infarction).The level of hs-CRP was not different among groups seperated by clinical presentation. The value of hs-CRP was 0.24 ± 0.52 mg/dl (range: 0~3.72 mg/dl).The number of diseased vessel defined ≥ 60% of plaque burden by IVUS was 94 of whole coronary tree; left anterior descending coronary artery (LAD): 40(42.6%), left cicumflex coronary artery (LCX): 29(30.9%), right coronary artery (RCA): 25(26.6%). The analyzed vessel length was 56.1 ± 17.4 mm of LAD, 51.9 ± 19.0 mm of LCX, and 7.42 ± 18.8 mm for RCA. The number of VH-TCFA was 1.0 ± 0.8 for the left anterior descending, 0.6 ± 0.7 for the left circumflex and 0.8 ± 1.0 for the right coronary arteries (Table 2). The volume data of the total plaque was 596.7 ± 421.7 mm3;
361.8 ± 323.4 mm3 of FI, 76.0 ± 71.0 mm3 of FF, 80.3 ± 76.0 mm3 of NC and 51.6 ± 67.7
mm3 of DC. The volume index of total plaque was 3.0 ± 2.0 mm3/mm; 1.9 ± 1.6 mm3/mm of
FI, 0.4 ± 0.4 mm3/mm of FF, 0.4 ± 0.3 mm3/mm of NC and 0.3 ± 0.3 mm3/mm of DC. However volume indices of plaque components in three coronary arteries did not demonstrate any distinctive findings except for that of NC (p=0.031).
5
Table 1. Baseline clinical data (n=63)
Age (yrs) 59.6 ± 9.0 ≥ 65 yrs of age 21 (31.7%) Men 41 (65.1%) DM 17 (27.0%) Hypertension 37 (58.7%) Dyslipidemia 13 (20.6%) Smoking 28 (44.4%)
Family history of premature coronary artery disease 5 (7.9%) SBP / DBP (mmHg) 137.4 ± 17.2/ 82.9 ± 11.7 hs-CRP (mg/dl) 0.24 ± 0.52 [range: 0~3.72] Lipid profile (mg/dl) Total cholesterol 167.0 ± 36.0 Triglyceride 145.6 ± 102.2 LDL 95.8 ± 33.4 HDL 44.0 ± 9.4
Values are mean ± SD or n (%)
DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high sensitive C reactive protein; LDL, low density lipoprotein; HDL, high density lipoprotein
6
Table 2. Baseline lesion characteristics in the VH-IVUS analysis (n=63)
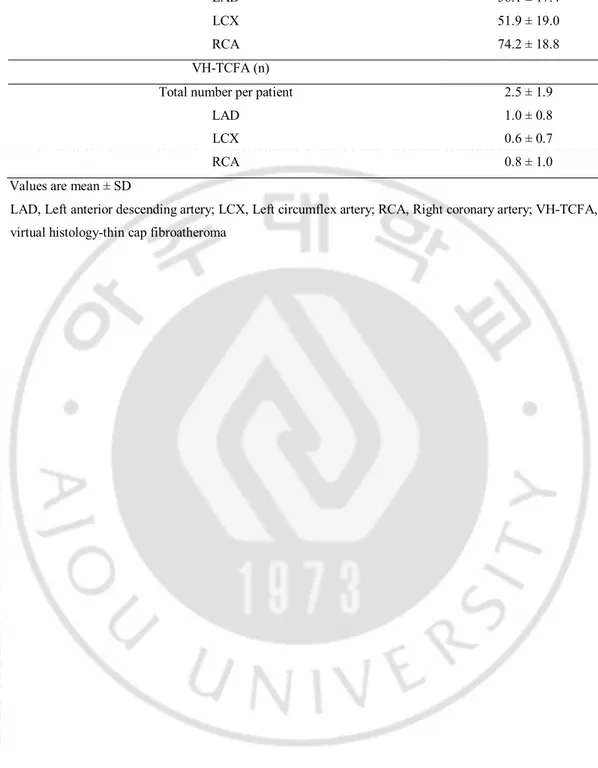
Total length of the analyzed vessel per patient (mm) 182.6 ± 45.4 LAD 56.1 ± 17.4 LCX 51.9 ± 19.0 RCA 74.2 ± 18.8 VH-TCFA (n)
Total number per patient 2.5 ± 1.9 LAD 1.0 ± 0.8 LCX 0.6 ± 0.7 RCA 0.8 ± 1.0 Values are mean ± SD
LAD, Left anterior descending artery; LCX, Left circumflex artery; RCA, Right coronary artery; VH-TCFA, virtual histology-thin cap fibroatheroma
7
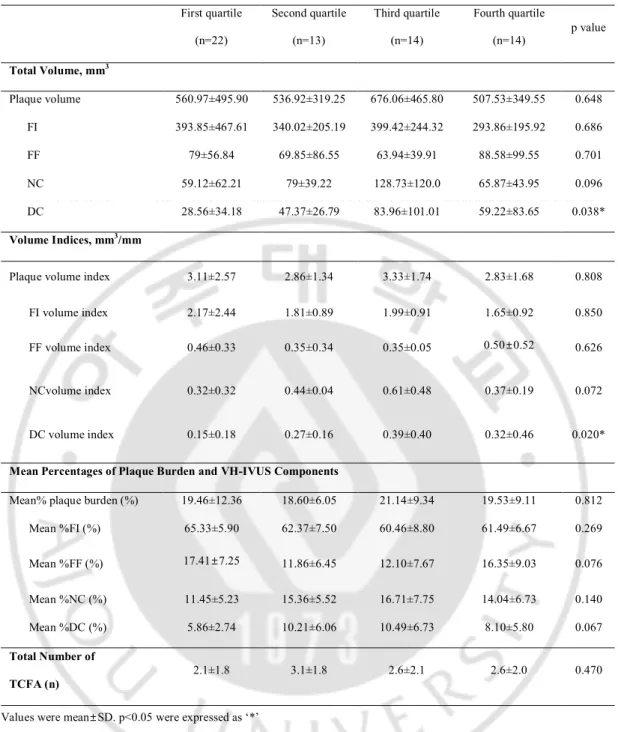
Patients were divided to 4 groups by the quartile value of hs-CRP; first quartile group: 0 ~ 0.05 mg/dl, second quartile group: 0.05 ~ 0.12 mg/dl, third quartile group: 0.12 ~ 0.22 mg/dl and fourth quartile group: ≥ 0.22 mg/dl. The base data were described in Table 3. Except for the volume and volume index of DC, there were no significant difference of volumetric data among quartile groups.
8
Table 3. Volumetric data of VH-IVUS according tointerquartile range of hs-CRP First quartile (n=22) Second quartile (n=13) Third quartile (n=14) Fourth quartile (n=14) p value Total Volume, mm3 Plaque volume 560.97±495.90 536.92±319.25 676.06±465.80 507.53±349.55 0.648 FI 393.85±467.61 340.02±205.19 399.42±244.32 293.86±195.92 0.686 FF 79±56.84 69.85±86.55 63.94±39.91 88.58±99.55 0.701 NC 59.12±62.21 79±39.22 128.73±120.0 65.87±43.95 0.096 DC 28.56±34.18 47.37±26.79 83.96±101.01 59.22±83.65 0.038* Volume Indices, mm3/mm
Plaque volume index 3.11±2.57 2.86±1.34 3.33±1.74 2.83±1.68 0.808 FI volume index 2.17±2.44 1.81±0.89 1.99±0.91 1.65±0.92 0.850 FF volume index 0.46±0.33 0.35±0.34 0.35±0.05 0.50±0.52 0.626
NCvolume index 0.32±0.32 0.44±0.04 0.61±0.48 0.37±0.19 0.072
DC volume index 0.15±0.18 0.27±0.16 0.39±0.40 0.32±0.46 0.020*
Mean Percentages of Plaque Burden and VH-IVUS Components
Mean% plaque burden (%) 19.46±12.36 18.60±6.05 21.14±9.34 19.53±9.11 0.812 Mean %FI (%) 65.33±5.90 62.37±7.50 60.46±8.80 61.49±6.67 0.269 Mean %FF (%) 17.41±7.25 11.86±6.45 12.10±7.67 16.35±9.03 0.076 Mean %NC (%) 11.45±5.23 15.36±5.52 16.71±7.75 14.04±6.73 0.140 Mean %DC (%) 5.86±2.74 10.21±6.06 10.49±6.73 8.10±5.80 0.067 Total Number of TCFA (n) 2.1±1.8 3.1±1.8 2.6±2.1 2.6±2.0 0.470
Values were mean±SD. p<0.05 were expressed as ‘*’
The quartile group were divided by hs-CRP; First quartile < 0.05 mg/dl, 0.05 mg/dl ≤Second quartile < 0.12 mg/dl, 0.12 mg/dl ≤ Third quartile < 0.22 mg/dl, Fourth quartile ≥ 0.22 mg/dl
9
B.Corrleation between hs-CRP and plaque components
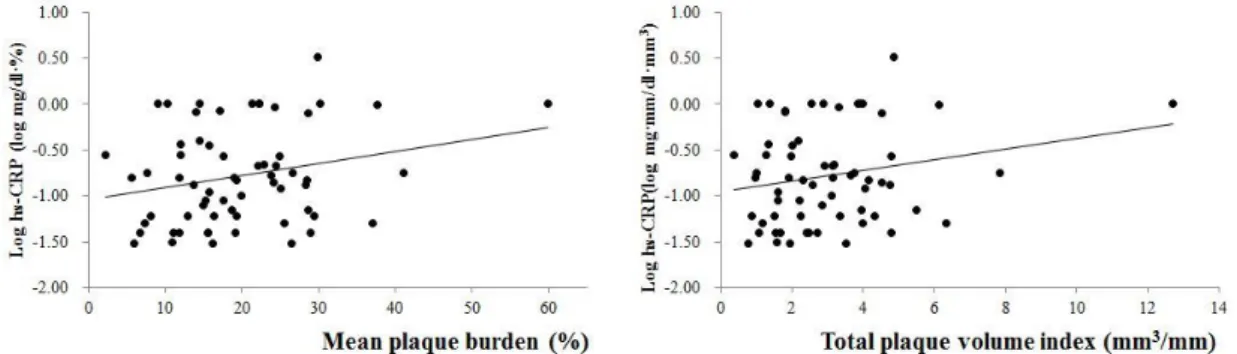
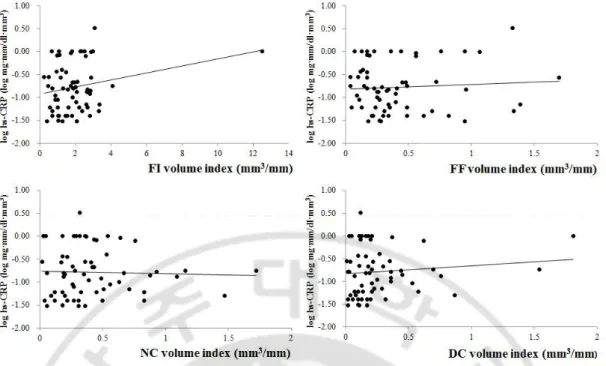
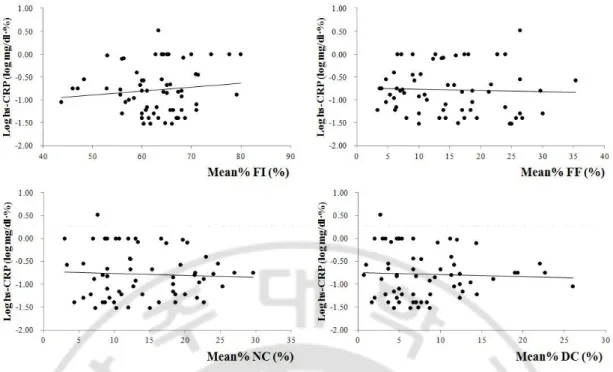
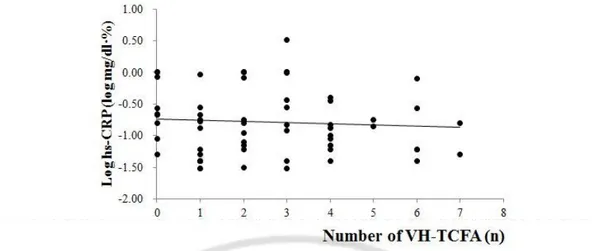
The value of hs-CRP was related to that of mean percentage of plaqueburden(r=0.332, p=0.012) and total plaque volume index (r=0.313, p=0.018)(Figure 1).On the analysis of each plaque components, the value of hs-CRP was associated the volume indicesof FF (r=0.312, p=0.018) and DC(r=0.580, p<0.001)(Figure 2). The measures of NC that were included mean percentage of burden (Mean%) and volumeindex, didnot have any correlation to that the value of hs-CRP;the Mean% and volume index of NC were (r=-0.134, p=0.321 and r=-0.027, p=0.843,respectively)(Figure 2,3).The number of VH-TCFAs didnot also show significant result with the valueof hs-CRP (r=-0.041, p=0.768)(Figure 4).
Patients in this study were divided into 3 groups based on the level of hs-CRP; the ‘Group A’ included patients with > 0.3 mg/dl of hs-CPR, the ‘Group B’ did from 0.1 mg/dl to 0.3 mg/dl and the ‘Group C’ did < 0.1 mg/dl, according to the recommendation of CDC/AHA (Pearson, 2003). There had no significant differences in the volume parameters of plaque among 3 groups (not shown in this paper)
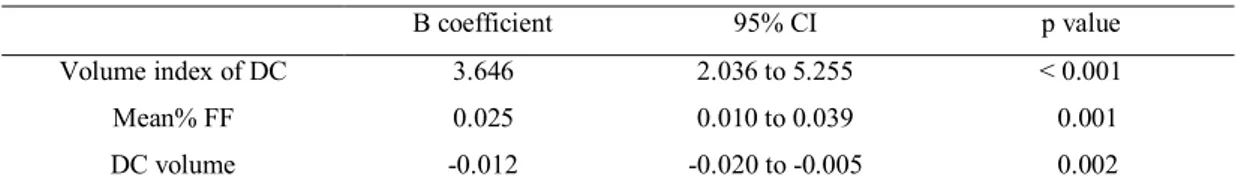
In the multivariate analysis, the volume index of DC (β=3.646, CI=2.036 to 5.255, p<0.001), Mean% FF (β=0.025, CI=0.010 to 0.039, p=0.001) and DC volume (β=-0.020, CI=-0.020 to -0.005, p=0.005) were independent predictor of hs-CRP (Table 4).
10
Fig 1. The correlation of hs-CRP with mean plaque burden and plaque volume index.
hs-CRP was correlated to mean plaque burden (r=0.332, p=0.012) and total plaque volume index (r=0.313, p=0.018) statistically. The value of hs-CRP was described by logarithmic function.
11
Fig 2. The correlation of hs-CRP with volume indices of each plaque components.
hs-CRP was correlated to volume indices of FF (r=0.312, p=0.018) and DC (r=0.580, p<0.001) statistically, although not correlated to volume index of NC (r=-0.027, p=0.843) significantly. The value of hs-CRP was described by logarithmic function.
12
Fig 3. The correlation of hs-CRP with mean burdens of each plaque components.
hs-CRP was not correlated to mean plaque burden of any plaque components. The value of hs-CRP was described by logarithmic function.
FI, fibrous; FF, fibrofatty, NC, necrotic core; DC, dense calcium; Mean%, mean percentage of burden
13
Fig 4. The correlation of hs-CRP with the number of VH-TCFA
hs-CRP was not correlated to the number of VH-TCFA (r=-0.041, p=0.768) statistically. The value of hs-CRP was described by logarithmic function.
14
Table 4. Multivariate analysis for predictors of hs-CRP
B coefficient 95% CI p value Volume index of DC 3.646 2.036 to 5.255 < 0.001 Mean% FF 0.025 0.010 to 0.039 0.001 DC volume -0.012 -0.020 to -0.005 0.002 Co-factors were as follows; total plaque volume, FI volume, FF volume, DC volume, mean plaque burden, Mean %FF, total plaque volume index, FI volume index, FF volume index, DC volume index and other risk factors of coronary atherosclerosis (age, DM, HTN, history of smoking, dyslipidemia and family history of premature coronary artery disease). Co-factors of volumetric data included multivariate analysis were selected by p value < 0.02 of correlation analysis.
15
IV. Discussion
The present study has something important as follows: 1) The hs-CRP is correlated to the volume measures of total plaque; 2) The hs-CRP has a relationship with specific components of the plaque; FF and DC. The level of hs-CRP is related the magnitude of FF and DC; the volume and volume index of that components has stastical correlation with hs-CRP; 3) Any parametersregarding NC and presense of VH-TCFAs are not related with the level of hs-CRP. 4) The volume index of DC is best independent factor for hs-CRP on multivariate analysis.
The atherosclerosis is one of important mechanism for coronary artery disease. The natural history of the atherosclerosis is related to the composition of the plaque. Previous pathological studies suggested that plaque composition detremines plaque vulnerability; large lipid core, a thin cap fibroatheroma, infiltration of inflammatory cells and less amount of smooth muscle cells (Kubo et al., 2009).
With the development of histologic studies for the plaque, there has been rapid expansion of coronary imaging, especially for vulnerable plaque in coronary arteries.One of them is IVUS; that can be possible to display structures of vessel and plaque. With advantages of IVUS, VH-IVUS adds the information about the plaque composition; that discriminates the plaque as 4 color codes, which help the analysis of plaque components in coronary artery andatheroscleorsis. In PROSPECT trial, VH-IVUS demonstrated plaque composition of the analyzed vessel affected clinical cardiac event, although that vessel had mild severity angiographically. In 697 patients in acutecoronary syndromes, nonculprit lesions associated with recurrent events were more likely characterized by 1) a plaque burden of 70% or greater or2) a minimal luminal area of 4.0 mm2 or less or 3) to have more VH-TCFAs.(Stone et al.,
2011).
Inflammation and inflammatory cells are involved in progression of the atherosclerosis. After being recruited into the atherosclerotic plaque, inflammation affect degradation of fibrous cap, expression of adhesion molecule, promoting chemotaxis to enthelial cells and etc, which were related to hs-CRP.(Pasceri et al., 2000; Pasceri et al., 2001; Pepys and
16
Hirschfield, 2003).
Based on aforementioned theories and facts, some reports advert hs-CRP was associated with the atherosclerosis, specially for plaque vulnerability. There are some evidences that confirm an association with hs-CRP and plaque vulnerability by pathologic findings (Burke et al., 2002; Ishikawa et al., 2003).Coronary computed tomography angiography also proved hs-CRP was increased as mixed calcified plaque which was represented unstable plaque, grew up(Rubin et al., 2011).It could be found similar results from VH-IVUS studies.
Culprit plaque in patients with acute coronary syndrome had much NC than those in patients without, and there was an inverse relation between hs-CRP and NC ratio (Otake et al., 2008). It can be established same connection with hs-CRP and NC in cases with stable angina (Kubo et al., 2009) and unstable angina (Ko et al., 2012). However, these studies have a concomitantfeature; they are limited in only one vessel: culprit vessel. It’s needed to be studied for whole coronary vessels forreinforcement of a connection between hs-CRP and vulnerable plaque compositions(Otake et al., 2008).
Our study was not only about culprit lesions, but also nonculprit lesions and non-target vessel and showed contrary outcomes compared to that of some reports as mentioned previously. The level of hs-CRP correlated, not to any parameters of NC and VH-TCFAs that were markers of plaque vulnerability, tothat of DC and FF.
It was known that NC was associated plaque vulnerability, which ultimately had high probability of acute cardiac events (Rodriguez-Granillo et al., 2006; Missel et al., 2008). VH-TCFAs also showed similar results; patients with acute coronary event or plaque rupture had more VH-TCFAs than them with stable angina in pathologic study, and Rodriguez – Granillo et al. and Hong et al. reportedthere were more VH-TCFAs with acute coronary syndrome than with stable angina in VH-IVUS studies.(Kolodgie et al., 2001; Rodriguez-Granillo et al., 2005; Hong et al., 2008).
Therefore, in our study, the most popularinflammatory biomarker-hs-CRP -indicated rather the burden than the vulnerability of the plaque, that was supported by our resultwhich DC was most intimate composition tohs-CRP and other evidences were as follows. Coronary calcium reflected the burden and age of the atherosclerosis;in other words, the extent of calcification was closely associated with that of atherosclerosis (Rumberger et al., 1995;
17
Schmermund et al., 1998; Schmermund et al., 1999; Falk, 2006). Because the progression of calcification affects the coronary tree in a systemic fashion, it would help
rather to identify subjects at increased risk than localized unstable plaque (Schmermund and Erbel, 2001).A few studies indicated what most importatnt thing in coronary calcificiation to determine plaque vulnerability was the character of distribution of calcium, not the absolute quantity. Fujii et alshowed that ruptured plaque had quantitatively less calcium but a larger number of small calcium deposits compared to with non-ruptured plaque(Fujii et al., 2005). The pattern of spotty calcification was a robust finding of plaque vulnerability (Ehara et al., 2004; Motoyama et al., 2007).
Additionally, coronary calcium could predict cardiac events (Pletcher et al., 2004; Church et al., 2007). The volume of DC in a 3-vessel VH-IVUS studywas also related to major adverse cardiac events (Shimizu et al., 2012).Although clinical outcomes didn’t descrbied in our study, hs-CRP and DC of VH-IVUS would be used to forecast cardiac disease, totally.
There were some limitation in our study.This study was a single center, retrospective study and based on a small patient population. The distribution of patients differentiated from clinical presentation was not even;more than a half of cases were unstable angina. This one would have affected not only the composition of plaque, but also relationship between hs-CRP and specific plaque compositions. Furthermore there are limited data on the reproducibility or the ability of VH-IVUS to predict future events because VH-IVUS have limitation in analyzing small vessel and in distinguishing from thrombus and other plaque component.
18
V. Conclusion
The hs-CRP is an inflammatory marker in the atherosclerosis and is closely related to DC and FF of a 3-vessel VH-IVUS study. This biomarker reflect the burden of plaque and the maturing of atherosclerosis, and not plaque vulnerability. Elevated hs-CRP to predict cardiovascular events can indicate atherosclerosis severity of whole coronary tree but not current plaquevulnerability.
19
REFERENCES
1. Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R: Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation 105: 2019-2023, 2002
2. Church TS, Levine BD, McGuire DK, Lamonte MJ, Fitzgerald SJ, Cheng YJ, Kimball TE, Blair SN, Gibbons LW, Nichaman MZ: Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis 190: 224-231, 2007
3. David MJ RP, Woof N, Katz DR, Mann J: Risk of thrombosis in human aherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. . Br Heart J 69: 377-381, 1993
4. Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M: Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110: 3424-3429, 2004
5. Falk E: Pathogenesis of atherosclerosis. J Am Coll Cardiol 47: C7-12, 2006
6. Fujii K, Carlier SG, Mintz GS, Takebayashi H, Yasuda T, Costa RA, Moussa I, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB: Intravascular ultrasound study of patterns of calcium in ruptured coronary plaques. Am J Cardiol 96: 352-357, 2005
7. Garcia-Garcia HM, Mintz GS, Lerman A, Vince DG, Margolis MP, van Es GA, Morel MA, Nair A, Virmani R, Burke AP, Stone GW, Serruys PW: Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention 5: 177-189, 2009
8. Hong MK, Mintz GS, Lee CW, Lee JW, Park JH, Park DW, Lee SW, Kim YH, Cheong SS, Kim JJ, Park SW, Park SJ: A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol 101: 568-572, 2008
9. Ishikawa T, Hatakeyama K, Imamura T, Date H, Shibata Y, Hikichi Y, Asada Y, Eto T: Involvement of C-reactive protein obtained by directional coronary atherectomy in plaque
20
instability and developing restenosis in patients with stable or unstable angina pectoris. Am J
Cardiol 91: 287-292, 2003
10. Ko YG, Le VC, Kim BH, Shin DH, Kim JS, Kim BK, Choi D, Jang Y, Hong MK:
Correlations between coronary plaque tissue composition assessed by virtual histology and blood levels of biomarkers for coronary artery disease. Yonsei Med J 53: 508-516, 2012 11. Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, Finn AV, Virmani R: The
thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 16: 285-292, 2001
12. Kubo T, Matsuo Y, Hayashi Y, Yamano T, Tanimoto T, Ino Y, Kitabata H, Takarada S, Hirata K, Tanaka A, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T: High-sensitivity C-reactive protein and plaque composition in patients with stable angina pectoris: a virtual histology intravascular ultrasound study. Coron Artery Dis 20: 531-535, 2009
13. Missel E, Mintz GS, Carlier SG, Sano K, Qian J, Kaple RK, Castellanos C, Dangas G, Mehran R, Moses JW, Stone GW, Leon MB: Necrotic core and its ratio to dense calcium are predictors of high-risk non-ST-elevation acute coronary syndrome. Am J Cardiol 101: 573-578, 2008
14. Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J: Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 50: 319-326, 2007
15. Otake H, Shite J, Shinke T, Watanabe S, Tanino Y, Ogasawara D, Sawada T, Hirata K, Yokoyama M: Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol 101: 1-7, 2008
16. Pasceri V, Cheng JS, Willerson JT, Yeh ET: Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation 103: 2531-2534, 2001
17. Pasceri V, Willerson JT, Yeh ET: Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 102: 2165-2168, 2000
18. Pearson TA: Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107: 499-511, 2003
21
19. Pepys MB, Hirschfield GM: C-reactive protein: a critical update. J Clin Invest 111: 1805-1812, 2003
20. Pletcher MJ, Tice JA, Pignone M, Browner WS: Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern
Med 164: 1285-1292, 2004
21. Rodriguez-Granillo GA, Garcia-Garcia HM, Mc Fadden EP, Valgimigli M, Aoki J, de Feyter P, Serruys PW: In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol 46: 2038-2042, 2005 22. Rodriguez-Granillo GA, McFadden EP, Valgimigli M, van Mieghem CA, Regar E, de Feyter
PJ, Serruys PW: Coronary plaque composition of nonculprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am
Heart J 151: 1020-1024, 2006
23. Rubin J, Chang HJ, Nasir K, Blumenthal RS, Blaha MJ, Choi EK, Chang SA, Yoon YE, Chun EJ, Choi SI, Agatston AS, Rivera JJ: Association between high-sensitivity C-reactive protein and coronary plaque subtypes assessed by 64-slice coronary computed tomography angiography in an asymptomatic population. Circ Cardiovasc Imaging 4: 201-209, 2011 24. Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS: Coronary artery
calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 92: 2157-2162, 1995
25. Schmermund A, Baumgart D, Adamzik M, Ge J, Gronemeyer D, Seibel R, Sehnert C, Gorge G, Haude M, Erbel R: Comparison of electron-beam computed tomography and
intracoronary ultrasound in detecting calcified and noncalcified plaques in patients with acute coronary syndromes and no or minimal to moderate angiographic coronary artery disease.
Am J Cardiol 81: 141-146, 1998
26. Schmermund A, Denktas AE, Rumberger JA, Christian TF, Sheedy PF, 2nd, Bailey KR, Schwartz RS: Independent and incremental value of coronary artery calcium for predicting the extent of angiographic coronary artery disease: comparison with cardiac risk factors and radionuclide perfusion imaging. J Am Coll Cardiol 34: 777-786, 1999
27. Schmermund A, Erbel R: Unstable Coronary Plaque and Its Relation to Coronary Calcium.
Circulation 104: 1682-1687, 2001
28. Shah PK: Inflammation and plaque vulnerability. Cardiovasc Drugs Ther 23: 31-40, 2009 29. Shimizu T, Maehara A, Farah T, Teles R, Lerman A, Hamm C, Templin B, Lansky A, Xu K,
22
High-Risk “Vulnerable Plaque” Characteristics, and Future Adverse Cardiac Events: The Prospect Study. Journal of the American College of Cardiology 59: E2102, 2012
30. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, Investigators P: A prospective natural-history study of coronary atherosclerosis. N Engl J
Med 364: 226-235, 2011
31. Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S, Ito K, Yasumura Y, Miyatake K: Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 35: 106-111, 2000
23 - 국문요약–
혈중 hs-CRP 와 관상동맥경화증 중등도 및 죽상반 취약성과의
연관 관계에대한 연구:
가상조직-혈관내 초음파(Virtual histology-Intravascular
ultrasound,VH-IVUS)를 통한 관상동맥 죽상반 분석
목적:hs-CRP (high sensitive C-reactive protein)는 동맥경화증의 염증 표지자로
알려져 있으며, 심혈관 질환의 예후를 예측하는 인자로도 인식되고 있다.몇몇 연구들을 통해 hs-CRP 수치가 죽상반의 취약성과 연관이 있다고 밝히고 있지만, 이들 대부분은 관상동맥 전체가 아닌 국소적인 죽상반에 대한 연구로써 그 한계점을 가지고 있다. 방법:63 명의 환자들의 총 189 개의 혈관에 대해서 가장조직-혈관내초음파를 시행하여 분석한 죽상반결과를 혈중 hs-CRP 수치와 비교 분석하였다. 가상조직-혈관내초음파는죽상반의 성분을 fibrous, fibrofatty, necrotic core, dense calicum 으로 4 개의 색을 통해 구별하였고, 죽상반의 양적 분석 결과 및 thin cap fibroatheroma(VH-TCFA)유무도 hs-CRP 와 수치와 비교하였다. 혈중 hs-CRP 는 혈관내 초음파 및 관상동맥 조영술 전에 채혈하였다.. 결과: 급성 관동맥 증후군은 전체 환자 중 49 명(77.8%)이였다.혈중 hs-CRP 수치는 평균 0.24±0.52 mg/dl (0~3.27mg/dl)이였고, 혈관의 길이는 각각 좌전하행지 56.1±17.4 mm,좌회선지 51.9±19.0 mm, 우관상동맥 74.2±18.8 mm 였다. VH-TCFAs 는 평균적으로 좌전하행지 1.0±0.8 개, 좌회선지 0.6 ±0.7 개, 우관상동맥 0.8±1.0 개가 존재하였다.혈중 hs-CRP 수치는 평균 죽상반 양, 총 죽상반 양, fibrofatty 및 dense calcium 의 양적 지수와 양의
24 상관관계를 보였다. 그러나 necrotic core 의 각종 양적 수치들과 VH-TCFA 와는 유의한 상관관계를 보이지 못했다. 다변량 분석결과 dense calcium 의 양적 지수가 hs-CPR 를 가장 잘 예측하는 인자로 분석되었다. (β=3.646, CI=2.036 to 5.255, p<0.001) 결론:이상의 결과를 통해 혈중 hs-CRP 수치는 죽상반의 취약성보다는 동맥경화증의 양적 및 질적 정도와 연관성이 있음을 알 수 있었다. 이를 통해 혈중 hs-CRP 수치를 통해 심혈관 질환의 예후를 예상할 수 있을 것으로 생각된다.
핵심어: high sensitive C-reactive protein, hs-CRP, 관상동맥경화증, 혈관내 초음파, 죽상반 취약성
hs-CRP level represents
the disease burden and the age
but not vulnerability of
coronary atherosclerosis
by
Se Jun Park
Major in Medicine
Department of Medical Science
The Graduate School, Ajou University
hs-CRP level representsthe disease burden and the age
but not vulnerability of coronary atherosclerosis
: a study of volumetric plaque composition by 3-vessel virtual
histology-intravascular ultrasound
by
Se Jun Park
A Dissertation Submitted to The Graduate School of Ajou
University in Partial fulfillment of The Requirements for
The Degree of Master of Medicine
Supervised by
SeungJeaTahk, M.D., Ph.D.
Major in Medicine
Department of Medical Science
The Graduate School, Ajou University
This certifies that the dissertation
of Se Jun Park is approved
.
SUPERVISORY COMMITTEE
Seung Jea Tahk
Seung Jea Tahk
Joon Han Shin
Joon Han Shin
So Yeon Choi
So Yeon Choi
The Graduate School, Ajou University
i
-ABSTRACT -
hs-CRP level represents the disease burden and the age but not
vulnerability of coronary atherosclerosis : a study of volumetric
plaque composition by 3-vessel virtual histology-intravascular
ultrasound
Background:hs-CRP (high sensitive C-reactive protein) has been known as a systemic
inflammatory marker of atherosclerosis and considered as one of the predictors of future cardiac events. Some reports presented hs-CRP level was associated with plaque
vulnerability but most studies were performed by assessing focal target plaque but not whole plaques from a coronary tree.
Methods: To evaluate of the relationship of plasma hs-CRP level and volumetric
plaque composition of the coronary arterial tree, we performed ‘whole vessel” virtual histology-intravascular ultrasound (VH-IVUS) in 189 vessels of 63 patients. The
components of atherosclerosis were classified as fibrous (FI), fibrous-fatty (FF), necrotic core (NC) and dense calcium (DC). Quantitative assessment of these plaque components and the presence of VH-IVUS–derived thin-cap fibroatheroma (VH-TCFA) in the coronary arterial trees were compared to hs-CRP levels in individuals. hs-CRP levels were measured before coronary angiogram and IVUS study.
Results: Forty-nine patients (77.8%) were diagnosed with acute coronary syndrome in
this population. The mean values of hs-CRP were 0.24±0.52 mg/dl (0~3.27mg/dl). The analyzed vessel length was 56.1±17.4 mm for the left anterior descending coronary artery, 51.9±19.0 mm for the left circumflex coronary artery, and 74.2±18.8 mm for the right coronary artery. The number of VH-TCFAs was 1.0±0.8 for the left anterior descending, 0.6±0.7 for the left circumflex, and 0.8±1.0 for the right coronary arteries.
ii
volume index, volume index of FF and DC. But parameters of NC and the number of VH-TCFA were not related with hs-CRP level. In multivariate analysis, the volume index of DC was most reliable factor to hs-CRP (β=3.646, CI=2.036 to 5.255, p<0.001)
Conclusions: This three-vessel VH-IVUS presented that hs-CRP were related to the
total atherosclerotic burden and the maturing (coronary calcium) but not vulnerable features (NC or VH-TCFA) of plaques in coronary arterial tree. Increased hs-CRP level as a biomarker to predict cardiovascular events might imply atherosclerosis severity of whole coronary tree but not current plaque vulnerability.
Keyword: high sensitive C-reactive protein, hs-CRP, coronary atherosclerosis, virtual histology, intravascular ultrasound, vulnerable plaque, vulnerability
iii
TABLE OF CONTENTS
ABSTRACT ··· i TABLE OF CONTENTS ··· iii LIST OF FIGURES ···iv LIST OF TABLES···vi INTRODUCTION ··· 1 II. MATERIAL AND METHODS ··· 2 A. PATIENTS··· 2 B. LABORATORY ASSESSMENT··· 2 C. IVUS IMAGING AND ANALYSIS··· 2 D. STATISTICAL ANALYSIS··· 3 III. RESULTS··· 4 A. BASELINE CLINCAL AND VH-IVUS DATA···4 B. CORRELATION BETWEENHS-CRP AND PLAQUE COMPONENTS··· 9 IV. DISCUSSION··· 15 V. CONCLUSION··· 18 REFERENCES···19 국문요약··· 23
iv
LIST OF FIGURE
Fig 1. The correlation of hs-CRP with mean plaque burden and plaque volume index ··· 10 Fig 2. The correlation of hs-CRP with mean burdens of each plaque
components.··· 11 Fig 3.The correlation of hs-CRP with volume indices of each plaque components.
··· 12 Fig 4. The correlation of hs-CRP with the number of VH-TCFA
v
LIST OF TABLES
Table 1. Baseline clinical data··· 5 Table 2. Baseline lesion characteristics in the VH-IVUS analysis··· 6 Table 3. Volumetric data of VH-IVUS according to interquartile range of
hs-CRP··· 8 Table 4. Multivariate analysis for predictors of hs-CRP··· 14
1
I. INTRODUCTION
Coronary artery disease has have high mortality and incidence, although the technology of the medicine has been developed. The atherosclerosis is most important cause for coronary artery disease, so it’s important to know the pathogenesis of that. It has known the fate of atherosclerosis is related to the characteristic and composition of the plaque according to autopsy data(David MJ, 1993).The concept of ‘Vulnerable plaque’originated from aforementioned cause, composed of large lipd core, thin fibrous cap, positive remodeling, abundant inflammatory cells and scant smooth muscle cells(Yamagishi et al., 2000). The inflammatory reaction is also key factors in determining plaque vulnerability. Inflammatory cell is considered to have a role in the loss of fibrous cap, that is related to plaque rupture and thrombosis (Shah, 2009).
During the past decade, there were remarkable developments of the evaluation for intra-coronary imaging in ex-vivo. The gray scaled intravascular ultrasound (IVUS) can show the anatomical characteristics of the plaque. But there are some limitations of IVUS in detecting vulnerable plaque and one of those is not to distinguish the elements of plaque composition exactly. The virtual histology-intravascular ultrasound (VH-IVUS)come over this one; description of the composition of the plaque as 4 color codes (Rodriguez-Granillo et al., 2005).
It is known the inflammatory reaction and the vulnerable plaque are intercorrelated by some VH studies, but that were set limits to culprit lesion, not whole coronary trees (Otake et al., 2008; Ko et al., 2012). Therefore, the purpose of this study was to find out the relationship between the plaque vulnerability and inflammation by the analaysis of 3-vessel VH-IVUS and inflammatory biomarker; high-sensitive C-reactive protein (hs-CRP).
2
II. Material and Method
A. Patients
A total of 63 patients diagnosed with ischemic heart disease (history of angina and ≥ 30% luminal narrowing on coronary angiogram by visual estimation) were enrolled into this study from September 2006 and August 2008, at a single medical center. They were performed preinterventional 3-vessel VH-IVUS. Patients with any totally occlusive vessel, severely tortuous vessel, extensively calcified lesions, severe left main coronary artery disease (diameter stenosis ≥ 50%), and hemodynamic instability were excluded. Written informed consent was obtained from all patients.
B. Laboratory assessment
Blood samples were obtained within 24 hours before coronary angiography and VH-IVUS, and then were centrifuged immediately, aliquoted and stored at -80℃ for subsequent analysis. Serum hs-CRP was measured by a latex-enhanced turbidmetry immunoassay, using a chemistry autoanalyzer (TBA 200FR, TOSHIBA Co., Tokyo, Japan).
C. IVUS imaging and analysis
VH-IVUS studies were implemented with a phased-array, 20-MHz, 2.9-F IVUS catheter (Eagle eye, Volcano Corporation, Racho Cordova, California). After intracoronary
administration of nitroglycerin (100~200 µg), the transducer was introduced as far distal as possible into each major epicardial artery, paying particular attention to identify any evident atherosclerosis. Using motorized pullback (0.5 mm/s), imaging was performed back to aorto-ostial junction. While on pulling back, the gray-scale IVUS was recorded; and the raw radiofrequency data were captured at the top of the R waves for the reconstruction of color-coded map by VH data recorder (Volcano Corporation).
An experienced analystwho was unaware of the patient’s clinical data, measured the parameters of the lumen and the media-adventitia interface by manual contour tracing. Volumetric VH-IVUS analysis was done from the most distal part where VH-IVUS plaque components were detected to the respective ostium, and volumetric data were generated with
3
using pcVH software (version 2.1, Volcano Corporation). Total plaque volume was obtained by analyzing 3 vessels in each patient (including all lesions and reference segments), and a volumetric index was calculated as total plaque volume divided by total vessel length. Mean plaque plus media (P+M) burden was calculated as the total plaque volume divided by total vessel volume X100. VH-IVUS analysis was described as 4 different color-coded tissue; green (fibous, FI), yellow-green (fibro-fatty, FF), red (necrotic core, NC), and white (dense calcium, DC). Each plaque component was measured in every recorded frame and expressed as the volume index (absolute measure) and percentages of total plaque volume.Virtual histology–intravascular ultrasound–derived thin-cap fibroatheroma (VH-TCFA) was defined as a lesion that fulfilledthe following criteria in at least 3 consecutive frames: 1) NCs ≥ 10% directly attaching to the lumen; and 2) ≥ 40%P+M burden (Rodriguez-Granillo et al., 2005). Identifying 2 separate lesions in the sameartery required a ≥ 5-mm reference segment
between them.If there was a <5-mm reference segment, they wereconsidered part of one long lesion(Garcia-Garcia et al., 2009). Similarly, identifying 2 separate VH-TCFAs required a non–VH-TCFA–containing reference segment ≥ 5 mm between them. Two experienced observers who were unaware of the patients’ clinical histories evaluated the VH-TCFA in consensus.
D.Statistical analysis
All analyses were performed using SPSS version 18.0 statistical software (SPSS Inc, Chicago, IL, USA). Categorical variables were expressed as numbers or frequencies of occurrence with comparisons using chi-square statistics or Fisher exact probability test. All continuous variables were tested by Kolmogorov-Smirnov Z test for normality analysis. Continuous data were reported as mean ± SD with comparisons using unpaired Student ttest and Pearson’s correlation analysis. If normality tests failed, the continuous values were presented as median and interquartile range and were compared by Mann-Whitney U test and Kruskal-Wallis test. Multiple linear regression analysis was performed to assess independent predictors for hs-CRP. A p value < 0.05 was considered statistically significant in this study.
4
III. Results
A. Baseline clinical and VH-IVUS data
It was shown baseline clinical characteristics on Table 1.Patients had a mean age of 59 years; 31.7% of them were ≥ 65 years of age; 65.1% were men. The study cases were composed of diabetes mellitus (DM, 27.0%), hypertension (58.7%), smoking (44.4%), dyslipidemia (20.6%) and familyhistory of premature coronary artery disease(7.9%). Clinical presentation in patients were consisted of stable angina (22.2%), unstable angina (54.0%) and acute myocardial infarction (11.1%; 3 cases: ST segment elevation myocardial
infarction, 4 cases: Non-ST segement elevation myocardial infarction).The level of hs-CRP was not different among groups seperated by clinical presentation. The value of hs-CRP was 0.24 ± 0.52 mg/dl (range: 0~3.72 mg/dl).The number of diseased vessel defined ≥ 60% of plaque burden by IVUS was 94 of whole coronary tree; left anterior descending coronary artery (LAD): 40(42.6%), left cicumflex coronary artery (LCX): 29(30.9%), right coronary artery (RCA): 25(26.6%). The analyzed vessel length was 56.1 ± 17.4 mm of LAD, 51.9 ± 19.0 mm of LCX, and 7.42 ± 18.8 mm for RCA. The number of VH-TCFA was 1.0 ± 0.8 for the left anterior descending, 0.6 ± 0.7 for the left circumflex and 0.8 ± 1.0 for the right coronary arteries (Table 2). The volume data of the total plaque was 596.7 ± 421.7 mm3;
361.8 ± 323.4 mm3 of FI, 76.0 ± 71.0 mm3 of FF, 80.3 ± 76.0 mm3 of NC and 51.6 ± 67.7
mm3 of DC. The volume index of total plaque was 3.0 ± 2.0 mm3/mm; 1.9 ± 1.6 mm3/mm of
FI, 0.4 ± 0.4 mm3/mm of FF, 0.4 ± 0.3 mm3/mm of NC and 0.3 ± 0.3 mm3/mm of DC. However volume indices of plaque components in three coronary arteries did not demonstrate any distinctive findings except for that of NC (p=0.031).
5
Table 1. Baseline clinical data (n=63)
Age (yrs) 59.6 ± 9.0 ≥ 65 yrs of age 21 (31.7%) Men 41 (65.1%) DM 17 (27.0%) Hypertension 37 (58.7%) Dyslipidemia 13 (20.6%) Smoking 28 (44.4%)
Family history of premature coronary artery disease 5 (7.9%) SBP / DBP (mmHg) 137.4 ± 17.2/ 82.9 ± 11.7 hs-CRP (mg/dl) 0.24 ± 0.52 [range: 0~3.72] Lipid profile (mg/dl) Total cholesterol 167.0 ± 36.0 Triglyceride 145.6 ± 102.2 LDL 95.8 ± 33.4 HDL 44.0 ± 9.4
Values are mean ± SD or n (%)
DM, diabetes mellitus; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high sensitive C reactive protein; LDL, low density lipoprotein; HDL, high density lipoprotein
6
Table 2. Baseline lesion characteristics in the VH-IVUS analysis (n=63)
Total length of the analyzed vessel per patient (mm) 182.6 ± 45.4 LAD 56.1 ± 17.4 LCX 51.9 ± 19.0 RCA 74.2 ± 18.8 VH-TCFA (n)
Total number per patient 2.5 ± 1.9 LAD 1.0 ± 0.8 LCX 0.6 ± 0.7 RCA 0.8 ± 1.0 Values are mean ± SD
LAD, Left anterior descending artery; LCX, Left circumflex artery; RCA, Right coronary artery; VH-TCFA, virtual histology-thin cap fibroatheroma
7
Patients were divided to 4 groups by the quartile value of hs-CRP; first quartile group: 0 ~ 0.05 mg/dl, second quartile group: 0.05 ~ 0.12 mg/dl, third quartile group: 0.12 ~ 0.22 mg/dl and fourth quartile group: ≥ 0.22 mg/dl. The base data were described in Table 3. Except for the volume and volume index of DC, there were no significant difference of volumetric data among quartile groups.
8
Table 3. Volumetric data of VH-IVUS according tointerquartile range of hs-CRP First quartile (n=22) Second quartile (n=13) Third quartile (n=14) Fourth quartile (n=14) p value Total Volume, mm3 Plaque volume 560.97±495.90 536.92±319.25 676.06±465.80 507.53±349.55 0.648 FI 393.85±467.61 340.02±205.19 399.42±244.32 293.86±195.92 0.686 FF 79±56.84 69.85±86.55 63.94±39.91 88.58±99.55 0.701 NC 59.12±62.21 79±39.22 128.73±120.0 65.87±43.95 0.096 DC 28.56±34.18 47.37±26.79 83.96±101.01 59.22±83.65 0.038* Volume Indices, mm3/mm
Plaque volume index 3.11±2.57 2.86±1.34 3.33±1.74 2.83±1.68 0.808 FI volume index 2.17±2.44 1.81±0.89 1.99±0.91 1.65±0.92 0.850 FF volume index 0.46±0.33 0.35±0.34 0.35±0.05 0.50±0.52 0.626
NCvolume index 0.32±0.32 0.44±0.04 0.61±0.48 0.37±0.19 0.072
DC volume index 0.15±0.18 0.27±0.16 0.39±0.40 0.32±0.46 0.020*
Mean Percentages of Plaque Burden and VH-IVUS Components
Mean% plaque burden (%) 19.46±12.36 18.60±6.05 21.14±9.34 19.53±9.11 0.812 Mean %FI (%) 65.33±5.90 62.37±7.50 60.46±8.80 61.49±6.67 0.269 Mean %FF (%) 17.41±7.25 11.86±6.45 12.10±7.67 16.35±9.03 0.076 Mean %NC (%) 11.45±5.23 15.36±5.52 16.71±7.75 14.04±6.73 0.140 Mean %DC (%) 5.86±2.74 10.21±6.06 10.49±6.73 8.10±5.80 0.067 Total Number of TCFA (n) 2.1±1.8 3.1±1.8 2.6±2.1 2.6±2.0 0.470
Values were mean±SD. p<0.05 were expressed as ‘*’
The quartile group were divided by hs-CRP; First quartile < 0.05 mg/dl, 0.05 mg/dl ≤Second quartile < 0.12 mg/dl, 0.12 mg/dl ≤ Third quartile < 0.22 mg/dl, Fourth quartile ≥ 0.22 mg/dl
9
B.Corrleation between hs-CRP and plaque components
The value of hs-CRP was related to that of mean percentage of plaqueburden(r=0.332, p=0.012) and total plaque volume index (r=0.313, p=0.018)(Figure 1).On the analysis of each plaque components, the value of hs-CRP was associated the volume indicesof FF (r=0.312, p=0.018) and DC(r=0.580, p<0.001)(Figure 2). The measures of NC that were included mean percentage of burden (Mean%) and volumeindex, didnot have any correlation to that the value of hs-CRP;the Mean% and volume index of NC were (r=-0.134, p=0.321 and r=-0.027, p=0.843,respectively)(Figure 2,3).The number of VH-TCFAs didnot also show significant result with the valueof hs-CRP (r=-0.041, p=0.768)(Figure 4).
Patients in this study were divided into 3 groups based on the level of hs-CRP; the ‘Group A’ included patients with > 0.3 mg/dl of hs-CPR, the ‘Group B’ did from 0.1 mg/dl to 0.3 mg/dl and the ‘Group C’ did < 0.1 mg/dl, according to the recommendation of CDC/AHA (Pearson, 2003). There had no significant differences in the volume parameters of plaque among 3 groups (not shown in this paper)
In the multivariate analysis, the volume index of DC (β=3.646, CI=2.036 to 5.255, p<0.001), Mean% FF (β=0.025, CI=0.010 to 0.039, p=0.001) and DC volume (β=-0.020, CI=-0.020 to -0.005, p=0.005) were independent predictor of hs-CRP (Table 4).
10
Fig 1. The correlation of hs-CRP with mean plaque burden and plaque volume index.
hs-CRP was correlated to mean plaque burden (r=0.332, p=0.012) and total plaque volume index (r=0.313, p=0.018) statistically. The value of hs-CRP was described by logarithmic function.
11
Fig 2. The correlation of hs-CRP with volume indices of each plaque components.
hs-CRP was correlated to volume indices of FF (r=0.312, p=0.018) and DC (r=0.580, p<0.001) statistically, although not correlated to volume index of NC (r=-0.027, p=0.843) significantly. The value of hs-CRP was described by logarithmic function.
12
Fig 3. The correlation of hs-CRP with mean burdens of each plaque components.
hs-CRP was not correlated to mean plaque burden of any plaque components. The value of hs-CRP was described by logarithmic function.
FI, fibrous; FF, fibrofatty, NC, necrotic core; DC, dense calcium; Mean%, mean percentage of burden
13
Fig 4. The correlation of hs-CRP with the number of VH-TCFA
hs-CRP was not correlated to the number of VH-TCFA (r=-0.041, p=0.768) statistically. The value of hs-CRP was described by logarithmic function.
14
Table 4. Multivariate analysis for predictors of hs-CRP
B coefficient 95% CI p value Volume index of DC 3.646 2.036 to 5.255 < 0.001 Mean% FF 0.025 0.010 to 0.039 0.001 DC volume -0.012 -0.020 to -0.005 0.002 Co-factors were as follows; total plaque volume, FI volume, FF volume, DC volume, mean plaque burden, Mean %FF, total plaque volume index, FI volume index, FF volume index, DC volume index and other risk factors of coronary atherosclerosis (age, DM, HTN, history of smoking, dyslipidemia and family history of premature coronary artery disease). Co-factors of volumetric data included multivariate analysis were selected by p value < 0.02 of correlation analysis.
15
IV. Discussion
The present study has something important as follows: 1) The hs-CRP is correlated to the volume measures of total plaque; 2) The hs-CRP has a relationship with specific components of the plaque; FF and DC. The level of hs-CRP is related the magnitude of FF and DC; the volume and volume index of that components has stastical correlation with hs-CRP; 3) Any parametersregarding NC and presense of VH-TCFAs are not related with the level of hs-CRP. 4) The volume index of DC is best independent factor for hs-CRP on multivariate analysis.
The atherosclerosis is one of important mechanism for coronary artery disease. The natural history of the atherosclerosis is related to the composition of the plaque. Previous pathological studies suggested that plaque composition detremines plaque vulnerability; large lipid core, a thin cap fibroatheroma, infiltration of inflammatory cells and less amount of smooth muscle cells (Kubo et al., 2009).
With the development of histologic studies for the plaque, there has been rapid expansion of coronary imaging, especially for vulnerable plaque in coronary arteries.One of them is IVUS; that can be possible to display structures of vessel and plaque. With advantages of IVUS, VH-IVUS adds the information about the plaque composition; that discriminates the plaque as 4 color codes, which help the analysis of plaque components in coronary artery andatheroscleorsis. In PROSPECT trial, VH-IVUS demonstrated plaque composition of the analyzed vessel affected clinical cardiac event, although that vessel had mild severity angiographically. In 697 patients in acutecoronary syndromes, nonculprit lesions associated with recurrent events were more likely characterized by 1) a plaque burden of 70% or greater or2) a minimal luminal area of 4.0 mm2 or less or 3) to have more VH-TCFAs.(Stone et al.,
2011).
Inflammation and inflammatory cells are involved in progression of the atherosclerosis. After being recruited into the atherosclerotic plaque, inflammation affect degradation of fibrous cap, expression of adhesion molecule, promoting chemotaxis to enthelial cells and etc, which were related to hs-CRP.(Pasceri et al., 2000; Pasceri et al., 2001; Pepys and
16
Hirschfield, 2003).
Based on aforementioned theories and facts, some reports advert hs-CRP was associated with the atherosclerosis, specially for plaque vulnerability. There are some evidences that confirm an association with hs-CRP and plaque vulnerability by pathologic findings (Burke et al., 2002; Ishikawa et al., 2003).Coronary computed tomography angiography also proved hs-CRP was increased as mixed calcified plaque which was represented unstable plaque, grew up(Rubin et al., 2011).It could be found similar results from VH-IVUS studies.
Culprit plaque in patients with acute coronary syndrome had much NC than those in patients without, and there was an inverse relation between hs-CRP and NC ratio (Otake et al., 2008). It can be established same connection with hs-CRP and NC in cases with stable angina (Kubo et al., 2009) and unstable angina (Ko et al., 2012). However, these studies have a concomitantfeature; they are limited in only one vessel: culprit vessel. It’s needed to be studied for whole coronary vessels forreinforcement of a connection between hs-CRP and vulnerable plaque compositions(Otake et al., 2008).
Our study was not only about culprit lesions, but also nonculprit lesions and non-target vessel and showed contrary outcomes compared to that of some reports as mentioned previously. The level of hs-CRP correlated, not to any parameters of NC and VH-TCFAs that were markers of plaque vulnerability, tothat of DC and FF.
It was known that NC was associated plaque vulnerability, which ultimately had high probability of acute cardiac events (Rodriguez-Granillo et al., 2006; Missel et al., 2008). VH-TCFAs also showed similar results; patients with acute coronary event or plaque rupture had more VH-TCFAs than them with stable angina in pathologic study, and Rodriguez – Granillo et al. and Hong et al. reportedthere were more VH-TCFAs with acute coronary syndrome than with stable angina in VH-IVUS studies.(Kolodgie et al., 2001; Rodriguez-Granillo et al., 2005; Hong et al., 2008).
Therefore, in our study, the most popularinflammatory biomarker-hs-CRP -indicated rather the burden than the vulnerability of the plaque, that was supported by our resultwhich DC was most intimate composition tohs-CRP and other evidences were as follows. Coronary calcium reflected the burden and age of the atherosclerosis;in other words, the extent of calcification was closely associated with that of atherosclerosis (Rumberger et al., 1995;
17
Schmermund et al., 1998; Schmermund et al., 1999; Falk, 2006). Because the progression of calcification affects the coronary tree in a systemic fashion, it would help
rather to identify subjects at increased risk than localized unstable plaque (Schmermund and Erbel, 2001).A few studies indicated what most importatnt thing in coronary calcificiation to determine plaque vulnerability was the character of distribution of calcium, not the absolute quantity. Fujii et alshowed that ruptured plaque had quantitatively less calcium but a larger number of small calcium deposits compared to with non-ruptured plaque(Fujii et al., 2005). The pattern of spotty calcification was a robust finding of plaque vulnerability (Ehara et al., 2004; Motoyama et al., 2007).
Additionally, coronary calcium could predict cardiac events (Pletcher et al., 2004; Church et al., 2007). The volume of DC in a 3-vessel VH-IVUS studywas also related to major adverse cardiac events (Shimizu et al., 2012).Although clinical outcomes didn’t descrbied in our study, hs-CRP and DC of VH-IVUS would be used to forecast cardiac disease, totally.
There were some limitation in our study.This study was a single center, retrospective study and based on a small patient population. The distribution of patients differentiated from clinical presentation was not even;more than a half of cases were unstable angina. This one would have affected not only the composition of plaque, but also relationship between hs-CRP and specific plaque compositions. Furthermore there are limited data on the reproducibility or the ability of VH-IVUS to predict future events because VH-IVUS have limitation in analyzing small vessel and in distinguishing from thrombus and other plaque component.
18
V. Conclusion
The hs-CRP is an inflammatory marker in the atherosclerosis and is closely related to DC and FF of a 3-vessel VH-IVUS study. This biomarker reflect the burden of plaque and the maturing of atherosclerosis, and not plaque vulnerability. Elevated hs-CRP to predict cardiovascular events can indicate atherosclerosis severity of whole coronary tree but not current plaquevulnerability.
19
REFERENCES
1. Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R: Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation 105: 2019-2023, 2002
2. Church TS, Levine BD, McGuire DK, Lamonte MJ, Fitzgerald SJ, Cheng YJ, Kimball TE, Blair SN, Gibbons LW, Nichaman MZ: Coronary artery calcium score, risk factors, and incident coronary heart disease events. Atherosclerosis 190: 224-231, 2007
3. David MJ RP, Woof N, Katz DR, Mann J: Risk of thrombosis in human aherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. . Br Heart J 69: 377-381, 1993
4. Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M: Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110: 3424-3429, 2004
5. Falk E: Pathogenesis of atherosclerosis. J Am Coll Cardiol 47: C7-12, 2006
6. Fujii K, Carlier SG, Mintz GS, Takebayashi H, Yasuda T, Costa RA, Moussa I, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW, Leon MB: Intravascular ultrasound study of patterns of calcium in ruptured coronary plaques. Am J Cardiol 96: 352-357, 2005
7. Garcia-Garcia HM, Mintz GS, Lerman A, Vince DG, Margolis MP, van Es GA, Morel MA, Nair A, Virmani R, Burke AP, Stone GW, Serruys PW: Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention 5: 177-189, 2009
8. Hong MK, Mintz GS, Lee CW, Lee JW, Park JH, Park DW, Lee SW, Kim YH, Cheong SS, Kim JJ, Park SW, Park SJ: A three-vessel virtual histology intravascular ultrasound analysis of frequency and distribution of thin-cap fibroatheromas in patients with acute coronary syndrome or stable angina pectoris. Am J Cardiol 101: 568-572, 2008
9. Ishikawa T, Hatakeyama K, Imamura T, Date H, Shibata Y, Hikichi Y, Asada Y, Eto T: Involvement of C-reactive protein obtained by directional coronary atherectomy in plaque