저작자표시 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이차적 저작물을 작성할 수 있습니다. l 이 저작물을 영리 목적으로 이용할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다.
Doctoral Thesis in Philosophy
Inflammation induced by Fc region of
internalizing anti-DNA antibody
Graduate School of Ajou University
Department of Biomedical Sciences
Inflammation induced by Fc region of
internalizing anti-DNA antibody
Myung-Hee Kwon, Advisor
I submit this thesis as the
Doctoral thesis in Philosophy
August 2018
Graduate School of Ajou University
Department of Biomedical Sciences
ABSTRACT
Conventionally, the extracellular effector functions of antibody are mediated by the Fc regions of immunoglobulin (Ig) molecules. Recently, however, intracellular effector functions of antibodies have been reported. After the antibody-virus complex enters the cytosol, the complex can be detected by an intracellular Fc receptor (tripartite motif-containing 21, TRIM21), leading to the degradation of the virus and activation of transcription factors related to the immune response. A subset of anti-DNA IgG autoantibodies termed internalizing IgGs naturally enter living cells without the formation of immune complexes. We investigated the cytokine response to internalizing antibody, chimeric 3D8 IgG. Treatment of 3D8 IgG and 3D8 single-chain variable fragment-Fc (scFv-Fc) induced production of the pro-inflammatory cytokines interleukin-8 (IL-8) and tumor necrosis factor alpha (TNF-α) in human monocytes. In contrast, 3D8 scFv composed of only variable domains did not. The 3D8 IgG N434D mutant that was incapable of binding to the TRIM21 displayed increased cytokine production compared to 3D8 IgG. In addition, the production of 3D8 IgG-induced cytokines was greater in TRIM21-knockdown THP-1 cells than in control cells, indicating that cytokine signaling is not mediated by TRIM21. 3D8 IgG is involved in the de-ubiquitination of TRIM21 and consequently decreases the production of pro-inflammatory cytokines. Unlike the DNA/anti-DNA antibody immune complex, 3D8 IgG was internalized into the cell via clathrin-mediated endocytosis using heparin sulfate proteoglycan (HSPG) and chondroitin sulfate proteoglycan (CSPG) surface receptors. The results demonstrate that the 3D8 IgG internalizing antibody induces the production of IL-8 and TNF-α in Fc-dependent manner, but not depending on DNA-binding activity by the variable region of the intracellular Fc receptor. TRIM21 negatively regulates the immune response by 3D8 IgG. We suggest that an as-yet unknown Fc-interacting molecule
induces the secretion of pro-inflammatory cytokines. The present data provide a new insight into the pathogenesis of autoimmune diseases.
Anti-DNA antibody, TRIM21, Internalizing antibody, Endocytosis receptor, Systemic lupus erythematosus (SLE)
TABLE OF CONTENTS
ABSTRACT --- ⅰ
TABLES OF CONTENTS --- ⅲ
LIST OF FIGURES --- ⅵ
Ⅰ
. INTRODUCTION --- 1
Ⅱ
. METERIAL AND METHOD --- 7
A. Antibodies and reagents --- 7
B. Cell culture --- 8
C. Lentiviral vector construction --- 8
D. Establishment of stable cell lines --- 9
E. Preparation of 3D8 antibodies and proteins --- 9
F. Enzyme-Linked Immunosorbent Assay (ELISA) --- 10
G. Confocal microscopy --- 11
I. Western blotting --- 13
J. Immunoprecipitation (IP) --- 13
K. Flow cytometry --- 14
L. Luciferase reporter assay --- 15
Ⅲ
. RESULTS --- 16
A. Production of pro-inflammatory cytokines is induced by 3D8 IgG in various cells -16 B. Internalizing anti-DNA antibody, 3D8 IgG promotes the production of pro-inflammatory cytokines, IL-8 and TNF-α in THP-1 cells --- 19
C. Fc region of the internalized anti-DNA antibody mediates secretion of IL-8 and TNF-α from THP-1 cells --- 22
D. 3D8 IgG variants are internalized into the THP-1 and CD14+ monocytes --- 26
E. Production of pro-inflammatory cytokines by internalized IgG is not through signaling pathways via intracellular TLR9 --- 28
F. 3D8 scFv binds to HSPG and CSPG on the cell membrane --- 32
G. Both transmembrane and glycosylphosphatidylinositol (GPI)-anchored forms of HSPGs function as endocytic receptors for 3D8 scFv --- 36
H.Both transmembrane and GPI-anchored forms of CSPGs participate as endocytic receptors for 3D8 scFv --- 41
I. HS and CS chains, but not core proteins, are responsible for endocytosis
of 3D8 scFv --- 46
J. Production of IL-8 and TNF-α by internalizing IgG is not mediated by TRIM21 signaling pathway --- 49
K. 3D8 IgG promotes de-ubiquitination of TRIM21 and negatively regulates IL-8 and TNF-α production --- 53
L. IL-8 and TNF-α response to internalizing IgG are produced by NF-κB activation and MAPK-signaling --- 58
Ⅳ
. DISCUSSION --- 61
Ⅴ
. CONCLUSION --- 67
Ⅵ
. REFERENCE --- 68
LIST OF FIGURES
Fig. 1. Production of pro-inflammatory cytokines is induced by 3D8 IgG
in various cells --- 17
Fig. 2. 3D8 IgG internalizing anti-DNA antibody promotes production
of IL-8 and TNF-α pro-inflammatory cytokines in THP-1 cell --- 20 Fig. 3. Fc region of the internalized IgG mediates the secretion of IL-8 and
TNF-α from THP-1 cells --- 23 Fig. 4. 3D8 IgG variants are internalized into the THP-1 and CD14+
monocytes --- 27 Fig. 5. Production of pro-inflammatory cytokines by internalized IgG is not
through signaling pathways via intracellular TLR9 --- 29 Fig. 6. 3D8 scFv binds to HSPG and CSPG on the cell membrane --- 33 Fig. 7. Both transmembrane and GPI-anchored forms of HSPGs
participate as endocytic receptors for 3D8 scFv --- 37 Fig. 8. Both transmembrane and GPI-anchored forms of CSPGs
participate as endocytic receptors for 3D8 scFv --- 42 Fig. 9. HS and CS chains, but not core proteins, are responsible for
Fig. 10. Production of IL-8 and TNF-α by internalizing IgG is not
mediated by signaling function of TRIM21 --- 50 Fig. 11. 3D8 IgG promotes de-ubiquitination of TRIM21 and negatively
regulates IL-8 and TNF-α production --- 55 Fig. 12. IL-8 and TNF-α response to internalizing IgG are produced by
NF-κB activation and MAPK-signaling --- 59
Fig. 13. The proposed inflammation mechanism by internalizing
Ⅰ. INTRODUCTION
All antibodies have a common structure of two identical heavy chains (H) and two identical light chains (L). Interaction of each H-L or H-H chains is via a disulfide bond between the corresponding cysteine residues to form a closely associated heterodimer. Antibodies have two functional regions: a variable region (V) and a constant (C) region The V region is the most important domain for binding to antigens. Specifically, the loop of the V region of heavy chain (VH) chain and light
chain (VL) comprises the complementarity determining region (CDR), which confers
antigen specificity. The constant region consists of three or four domains depending on the antibody isotype, and the Fc region of the constant region mediates its specific function during the response to the antigen. (Schroeder and Cavacini, 2010).
Antibodies generated by activated B cells protect the body against pathogens. They can neutralize pathogens by binding to and blocking receptors that the particular pathogen uses to enter the cells. Antibodies also opsonize the pathogen by binding and recruiting phagocytic cells such as macrophages, and initiate the complement cascade, that destroy the pathogen membranes. In addition, antibodies can cooperate with the cellular branch of the immune system by binding and recruiting the activities of cytotoxic cells, including natural killer (NK) cells and granulocytes, which are lymphoid and myeloid members of the innate immune system, respectively, in a process called antibody-dependent cell-mediated cytotoxicity (ADCC) (Schroeder and Cavacini, 2010). Antibody effector functions are mostly in the extracellular environment including the cell surface. Only the extracellular functions of antibodies have been elucidated because antibodies cannot actively enter cells.
Recently, it has been reported that the formation of an immune complex involving an antigen and the specific pathogens induces an immune response when the complex enters the cytosol of cells. In the cell interior, the complex interacts with
the intracellular Fc receptor, tripartite motif-containing 21 (TRIM21). (Mallery et al., 2010). It recognizes the incoming antibody-pathogen complex, leading to two different events. First, the incoming antibody-pathogen complex recognized by TRIM21 is directed to the proteasome-degradation pathway with the assistance of the valosin-containing protein/p97 protein (VCP/p97), resulting in pathogen neutralization (antibody-dependent intracellular neutralization, ADIN). (Hauler et al., 2012). TRIM21 also elicits immune responses, such as the production of pro-inflammatory cytokines and chemokines that include interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), interferon-beta (IFN-β), chemokine (C-C motif) ligand 5 (CCL5), and C-X-C motif chemokine 10 (CXCL10), and activation of nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), and the antiviral system mediated by IFN regulatory factors (IRFs). These responses occur upon the generation of the K63-linked ubiquitin chains that activate transforming growth factor beta-activated kinase 1 (TAK1). Interestingly, TRIM21-mediated signal transduction is induced only when the antigen-coated antibody (immune complex, IC) is internalized into the cytosol, but the free form of the antibody is not induced when it is delivered into the cytosol via transfection method (McEwan et al., 2013). In addition, the 9D11-TAT antibody, which targets the intracellular Hepatitis B virus X protein (HBx), is internalized into cells through the endocytosis pathway, which in turn stimulates the TRIM21-mediated immune response. (Zhang et al., 2018).
TRIM21, also known as Ro52 or RNF81, is an autoantigen that is present in sera of patients with autoimmune diseases including systemic lupus erythematosus (SLE). (Ben-Chetrit et al., 1988). TRIM21 is the Fc receptor but not structurally related to all known other Fc receptors. TRIM21 is also an Fc receptor that is structurally unrelated to all other known Fc receptors. TRIM21 also shares a RING domain with E3 ligase activity, B-box, and coiled-coil domain. Approximately half the TRIM family members including TRIM21 possess the PRYSPRY domain at the C-terminus, which has ligand specificity and function (Hatakeyama, 2011).
Especially, the PRYSPRY domain strongly interacts with the CH2-CH3 domain in
the IgG Fc region with subnanomolar binding affinity (Rhodes and Trowsdale, 2007). The function of TRIM21 in the immune response is associated with the ubiquitination of IRFs 3, 7, and 8 that are activated in response to stimulation of Toll-like receptors (TLRs) (Kong et al., 2007; Higgs et al., 2008; Higgs et al., 2010).
SLE is a chronic autoimmune inflammatory disease with various presentations that involve multiple organs. (Ronnblom and Elkon, 2010). The well-known pathogenesis mechanism of SLE is a dysfunction of immune response induced by genetic factors or autoantibody production due to loss of tolerance to autoantigens (Oelke and Richardson, 2002).
Anti-DNA autoantibodies are of biomedical interest because they are associated with autoimmune diseases and are naturally present in healthy humans, but preferentially found in patients with SLE patients. They constitute a subgroup of antinuclear antibodies that bind single-stranded DNA, double-stranded DNA and Z-DNA (Hahn, 1998; Jang and Stollar, 2003). The titer of anti-Z-DNA antibody was found to be high in SLE patients, and the pathogenesis of autoimmune disease by anti-DNA antibody has been studied (Dubrovskaya et al., 2003). Although the exact production mechanism of anti-DNA antibody is still unknown, it is likely that extracellular DNA is one cause of an immune response against dsDNA. There is an evidence that dead or dying cells are one major source of this extracellular DNA (Su and Pisetsky, 2009). Upon release of nuclear proteins and chromatin, antigen presenting cells, such as dendritic cells and macrophages, display these antigens to T helper cells. Although the details of this process are still controversial, evidence shows that to produce an immune response, DNA must activate an antigen presenting cell to produce type 1 interferons. This cytokine serves to induce maturation of plasmacytoid dendritic cells (pDCs) so that they can display their antigens to T helper cells. The mechanism in which eukaryotic DNA activates these cells is still as yet unclear. The T helper cells then activate B cells, which are also in the presence of
these antigens, causing the production of anti-DNA antibody (Rekvig and Nossent, 2003; Graham and Utz, 2005; Su and Pisetsky, 2009).
Anti-DNA antibody induces inflammation by the formation of an immune complex with DNA (DNA-IC) released from dead cells (Bave et al., 2003; Honda et al., 2006; Kawai and Akira, 2007; Henault et al., 2016). Immune system abnormalities, such as the overproduction of cytokines are crucial in the onset or progression of SLE. In plasmacytoid dendritic cells (pDCs), DNA-ICs are internalized via phagocytosis mediated by Fcγ receptors, and then recognized by TLR9, which promotes the production of pro-inflammatory cytokines and type I IFN. DNA-ICs also induce the production of IL-1β via activation of the NLRP3 inflammasome in human monocytes (Shin et al., 2013). The free form of anti-DNA antibodies elicits immune signaling using different mechanisms. For example, IL-6 is secreted by binding to annexin II of internalized anti-DNA antibody in human mesangial cells. Production of IL-2 is induced by interaction with phosphoglycerate kinase 1 (PGK-1) in Jurkat cells, and binding to TLR4 activates the NLRP3 inflammasome, resulting in secretion of IL-1β and IL-17A in monocytes/macrophages from SLE patients (Luan et al., 2006; Yung et al., 2010; Zhang et al., 2016). Inflammation by anti-DNA antibodies was promoted by interaction with autoantigens such as self-DNA, annexin II, PGK-1 and TLR4, which bind to variable regions of the antibody. However, these observations are not conclusive evidence that the antibodies are internalized into the cytosol of cells.
A subset of anti-DNA antibodies termed internalizing anti-DNA antibody can spontaneously enter cells without antigens, such as DNA and pathogens (Zack et al., 1996; Ternynck et al., 1998). For example, monoclonal anti-DNA antibody, H7, derived from lupus-prone mice can cross the cellular membrane by myosin 1 expressed on cell surface, and monoclonal anti-DNA antibody F14.6 is internalized into the cell via calreticulin, as an endocytosis receptor (Yanase et al., 1997; Seddiki et al., 2001). In addition, 3E10, the anti-DNA single-chain variable fragment (scFv)
penetrates into living cells with specific nuclear localization (Hansen et al., 2007). 9D7 monoclonal anti-DNA antibody penetrates into Jurkat cells through electrostatic interaction of arginine residues with the negatively charged sulfated polysaccharides on the cell surface (Song et al., 2008).
The internalizing ability of the anti-DNA might be attributable to the high number of positively-charged amino acids present in the CDR regions of the variable region (Foster et al., 1994; Zack et al., 1996; Yanase et al., 1997; Ternynck et al., 1998). Cell penetrating peptides (CPPs) such as Tat, Rev, and R9 peptides, comprise
a high proportion of the positively-charged amino acids, and enter the cell using electrostatic interaction with negatively-charged surface receptors including heparin sulfate proteoglycan (HSPG) and chondroitin sulfate proteoglycan (CSPG), which are core proteins covalently linked with glycosaminoglycans (GAGs) (Zack et al., 1996; Avrameas et al., 2001; Foged and Nielsen, 2008). HSPG and CSPG are highly negatively-charged chains due to the presence of acidic sugar residues. Especially, HSPG and CSPG act as receptors for various ligands and function in cellular signaling. (Bernfield et al., 1999; Rabenstein, 2002; Purushothaman et al., 2012; Pantazaka and Papadimitriou, 2014).
The TRIM21-mediated immune response associated with ADIN is promoted when the antigen-pathogen complex is internalized into the cell in a process mediated by the Fc region of the antibody. The prowess of TRIM21 is limited because it only recognizes the antibody associated with the complex. Internalizing anti-DNA antibodies that internalize into cells without antigen participation are involved in the production of cytokines. However, the inflammatory mechanism mediated by intracellular Fc receptors remains unsolved.
We investigated the Fc region of internalizing anti-DNA antibody that mediates the production of pro-inflammatory cytokines in human monocytes. 3D8 IgG is a monoclonal anti-DNA antibody that originated from an autoimmune-prone MRL-lpr/lpr mouse. 3D8 IgG can enter the cell cytosol of various cell lines in the
absence of antigens (Jang et al., 2009; Lee et al., 2013). We used the chimeric 3D8 IgG as an internalizing model antibody, in which variable region, derived from a mouse were fused a human IgG1 backbone. The cytosol internalization of the 3D8 scFv composed of only the variable region was mediated by HSPGs and CSPGs on the cell membrane. 3D8 IgG and 3D8 scFv-Fc induced the production of the pro-inflammatory cytokines IL-8 and TNF-α in THP-1 cells and primary monocytes via Fc region-dependent pathway. The 3D8 scFv negative control was used to verify that the Fc region of IgG alone was responsible for the cytokine response, independent of the variable regions. The TRIM21 binding mutant 3D8 IgG N434D mutant was used to investigate the involvement of TRIM21 in inflammation. 3D8 IgG-mediated inflammation used an alternative pathway that was different from the TRIM21-mediated immune response in THP-1 cells.
The collective results demonstrate the existence of a potent cellular mechanism that allows human monocytes to stimulate pro-inflammatory cytokine production by detecting a free form of IgG molecule in the cytosol.
Ⅱ. MATERIALS AND METHOD
A. Antibodies and reagents
We used the following antibodies : TRIM21(cat# sc-25351), Ubiqitin (cat# sc-8017), HA (cat# sc7392), Caveolin-1 (cat# sc-53564), Glypican-3 (cat# sc-65443) and GAPDH (cat# sc-32233) from Santa Cruz ; Flag (cat# F1804), Polyclonal human IgG (cat# I8640) and polyclonal rabbit IgG (cat# I5006) from Sigma-Aldrich ; Human IgG-Fc (cat# ab97221), Human kappa chain (cat# 134083), Chondroitin sulfate (cat# ab11570), His (cat# ab9108), CD44 (cat# ab6124) and GFP (cat# ab1218) from Abcam ; β-actin (cat# A300-491A) from Bethyl Laboratories ; p38 (cat# 9212), p-p38 (cat# 9211), ERK (cat# 4695), p-ERK (cat# 9101), JNK (cat# 9252) and p-JNK (cat# 9251) from Cell Signaling ; Heparan sulfate (cat# H1890) from US Biological ; Syndecan-2 (cat# bs-1904R) from Bioss ; Brevican (cat# H00063827-B01P) from Abnova ; Polyclonal rabbit α-3D8 that we made. The HRP-conjugated α-mouse IgG (cat# 7076) secondary antibody was purchase from Cell Signaling and HRP-conjugated α-rabbit IgG (cat# 6120) and α-goat IgG (cat# 81-1620) were purchase from Zymed.
The following antibodies were used as secondary antibodies in immunofluorescence analysis : Dylight550-α-Human IgG Fc (cat# ab97004) from Abcam ; TRITC-α-mouse IgG (cat# sc-3796) from Santa Cruz ; TRITC-α-rabbit IgG (cat# T6778) from Sigma ; Alexa Fluor 488-α-mouse IgM/µ chain-specific antibody (cat# A21042), Alexa Fluor 488-α-mouse IgG + IgM (cat# A10680) and Alexa Fluor 647-α-rabbit IgG (cat# A21244) from Invitrogen.
We use the following reagents : 5z-7-oxozeaenol (cat# O9890), Chlorpromazine (cat# C8138), MβCD (cat# C4555), Cytochalasin D (cat# C8273), SP600125 (cat# S5567), Heparinase III (cat# H8891) and Chondroitinase ABC (cat# C2905), non-enzymatic cell dissociation solution (cat# C5914) from Sigma ; IKK
inhibitor VII (cat# 401486), PD98059 (cat# 513000) and SB202190 (cat# 559388) from Calbiochem ; Human Fc blocker (cat# 564219) from BD Biosciences ; TLR9 inhibitor (tlrl-ttag151) and control inhibitor (tlrl-ttagc) from InvivoGen ; DNase Ⅰ (cat# M0303) from New England Biolabs.
B. Cell culture
A549, HeLa, U87MG, MEF, HEK293T and HEK293TN cells were grown in DMEM (Welgene); THP-1, Jurkat, DC2.4, RAW 264.7 and BV2 cells were maintained in RPMI 1640 (Welgene). Both DMEM and RPMI 1640 were supplemented with 10% FBS and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin, Welgene). To maintain the TRIM21 shRNA and NF-KB luciferase reporter THP-1 cells, puromycin (2 µg/ml) was added to RPMI 1640. All cells were cultured at 37 ℃ in a humidified 5% CO2 incubator. HEK293F cells were maintained
in Freestyle 293F expression medium (cat# 12338018, Invitrogen) at 37℃ in a humidified 8% CO2 incubator in air on an orbital shaker platform rotating at 135 rpm.
PBMCs were isolated from the healthy donor’s blood samples by density-gradient centrifugation using Ficoll-paque (GE Healthcare). Subsequently, CD14+ monocytes were isolated from PBMC by Magnisort using human CD14 positive selection kit (cat# 8802-6834-74, Invitrogen).
C. Lentiviral vector construction
Silencing of endogenous TRIM21 mRNA was carried out using shRNA. The TRIM21 shRNA plasmids were expressed under the pSIH vector (cat# SI500A-1, System Biosciences) for establishment of stable cell line. The TRIM21 shRNA plasmids were constructed by synthesizing two cDNA oligonucleotides baring the target sequence (GCAGCACGCTTGACAATGA), loop sequence
(CTTCCTGTCAGA) and terminator sequence (TTTTT), annealing and ligating them into the pSIH vector (McEwan et al., 2012). For transduction with lentiviral particle encoding luciferase gene under the control of an NF-κB responsive promoter, pGreenFire1-NF-κB-EF1α-puro vector (cat# TR012PA-P, System Biosciences) was purchased.
D. Establishment of stable cell lines
For establishment of stable TRIM21 knockdown and NF-κB reporter THP-1 cells, HEK293TN cells were co-transfected with lentiviral expression (shTRIM2THP-1 and luciferase) vector and lentiviral packing vector (cat# LV500A-1, System Biosciences) mixture by transfection reagent, LipoJet (cat# SL100468, SignaGen Labatory). 3 days after transfection, collect the medium and then adding to culture medium of THP-1 cells. 3 days post transduction, puromycin (2 µg/ml, Gibco) was added to culture medium to select the transduced THP-1 cells.
E. Preparation of 3D8 antibodies and proteins
FreeStyle HEK293F cells (cat# R79007, Thermo Fisher) that that have been adapted to serum-free suspension culture were used as the host cells for protein expression. The cells (100 ml) at a concentration of 2 × 106 cells/ml were were
transiently transfected with the KV10 plasmids encoding 3D8 antibody derivatives (3D8 IgG wild type, 3D8 IgG N434D, 3D8 IgE, 3D8 scFv-Fc, and 3D8 scFv) using polyethylenimine (PEI, 4 µg/ml) reagent with an average molecular weight of 25 kDa (cat# 23966-2, Polyscience) in a 500 ml flask (cat# 431145, Corning), and then culture supernatants were harvested after 7 days transfection. Culture supernatants were clarified by filtration using a 0.45 µm cellulose acetate filter (Sartorius). Protein A affinity chromatography (cat# 171279, GE Healthcare) was used to purify 3D8
IgG and scFv-Fc, and protein L agarose affinity chromatography (cat# 175478, GE Healthcare) was used to purify 3D8 IgG N434D, 3D8 IgE and 3D8 scFv. 3D8 scFv was also purified from Escherichia coli. BL21(DE3) pLysE cells (Novagen) were transformed with pIg20-3D8 scFv expression vector, in which the variable domains are linked by a (G4S)3 linker after the Pho A leader signal sequence. The transformed
cells were grown at 37 ℃ to an A600 of ~0.8 in 1-liter of LB medium containing 100 µg/ml ampicillin and 25 µg/ml chloramphenicol, and then induced for 18 h at 23 ℃ by adding 0.5 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG, Calbiochem). The protein-induced cells were harvested and the culture supernatants were purified by the protein L affinity column. All eluted proteins were dialyzed to PBS with pH 7.4 and sterilized by filtration using a cellulose acetate membrane filter, and all proteins were loaded on 4-20% gradient acrylamide gels under reducing condition and visualized with coomassie blue. The protein concentration was adjusted based on the molar extinction coefficient at 280 nm, which was calculated from the amino acid sequence.
F. Enzyme-Linked Immunosorbent Assay (ELISA)
To characterize cytokine profiles, cells were seeded at 1 x 105 cells in
48-well plate and treated with 10 µM 3D8 IgG for 24 h and 48 h in transfection optimized medium (TOM, cat# TR004-01, Welgene). The culture supernatants were harvested and assayed for inflammatory cytokines using Human and Mouse Inflammatory Cytokines Multi-Analyte ELISArray Kits (cat# MEH-004A, MEM-004A, Qiagen,). THP-1 cells grown at a density of 1 x 105 cells in 48-well plates
were incubated with TOM, and CD14+ monocytes seeded at a density of 5 x 104
cells per FACS tube were incubated with RPMI 1640. After treatment of 3D8 proteins or polyclonal human IgG to cells, culture supernatants were harvested. Prior to 3D8 IgG treatment, 0.5 µM 5z-7-oxozeaenol, 200 nM IKK inhibitor VII, 10 µM
SB202190, 50 µM PD98059, 20 µM SP600125, 10 µg/ml chlorpromazine, 5 mM MβCD, 1 µg/ml Cytochalasin D, 5 µg/ml human Fc blocker, 5 µM TLR9 inhibitor, 5 µM control inhibitor, 2.5-10 µg/ml genomic DNA, 1 U/ml DNaseⅠ and DNase Ⅰ reaction buffer were incubated for 1 h. Human genomic DNA was purified from THP-1 cells using PurelinkTM genomic DNA mini kit (Invitrogen). IL-8 and TNF-α
in culture supernatants was measured with IL-8 ELISA kit and TNF-α ELISA kit (Biolegend).
G. Confocal microscopy
To observe the internalization of 3D8 proteins in the THP-1 cells and CD14+ monocytes, poly-L-lysine (Sigma) was coated on glass coverslips in 24-well plates the day before use. After cells were attached to poly-L-lysine coated coverslips for 3 h at 37 ℃, cells were incubated with the 5 µM proteins for 6 h at 37 ℃, fixed by 4% paraformaldehyde (PFA) for 10 min at room temperature (RT). Cells were permeabilized with Perm-buffer (1% BSA, 0.1% saponin, 0.1% sodium azide in PBS) for 10 min at RT, followed by the incubation with dylight 550-α-human IgG-Fc for 1 hr at 4℃ for detecting 3D8 IgG wt, 3D8 IgG N434D, 3D8 scFv-Fc and polyclonal human IgGs. For detecting 3D8 scFv, cells were incubated with the polyclonal rabbit α-3D8 scFv for 1 hr at 4℃, followed by TRITC-α-rabbit IgG for 1 hr at 4℃. Each incubation step was followed by three washes with cold phosphate-buffered saline (PBS, pH 7.2). Nuclei were stained with Hoechst 33342 (cat# 62249, Thermo Fisher Scientific) for the last 10 min of incubation at RT. Cells on coverslips were mounted in Vectashield anti-fade mounting medium (Vector Labs).
To detect binding of 3D8 scFv to HSPG or CSPG on the cell membrane, 3D8 scFv and HW6 scFv were incubated with HeLa cells for 1 h at 4 °C. If necessary, 10 mIU/ml heparinase III or 100 mIU/ml chondroitinase ABC were pre-treated to HeLa cells for 1 h at 37 °C. After fixation, a combination of rabbit α-3D8 IgG and
mouse α-HS IgM antibodies, or rabbit α-3D8 IgG and mouse α-CS IgM antibodies was used as the primary antibodies, and then the mixture of TRITC-conjugated α-rabbit IgG and Alexa Fluor 488-conjugated α-mouse IgM was used as the secondary antibodies.
To observe the co-localization of 3D8 scFv, HSPG and CSPG, HeLa cells were transfected with plasmids encoding HSPG and CSPG. At 18 h post-transfection, 10 µM 3D8 scFv was incubated with transfected cells for 1 h at 4 °C or for 6 h at 37 °C. After fixation and permeabilization, HeLa cells, which were transfected with plasmids encoding SDC2-GFP, SDC2ΔTM-GFP, GPC3-nfGPI-GFP, CD44-GFP, CD44ΔTM-GFP, or BCAN-ΔGPI-GFP were incubated with α-3D8 IgG, followed by TRITC-goat α-rabbit IgG. HeLa cells which were transfected with plasmids expressing HA-GPC3 and HA-BCAN, were incubated with a mixture of α-3D8 scFv and α-HA antibodies, followed by mixture of TRITC-goat α-rabbit IgG and Alexa Fluor 488-goat α-mouse IgG antibodies.
All samples were observed by confocal microscopy using Zeiss LSM 710 laser confocal microscope.
H. Quantitative RT-PCR (qRT-PCR)
THP-1 cells grown at a density of 1 x 106 cells per well in 12-well plates
were incubated in TOM with 5 µM 3D8 proteins and human IgG for 6 h, then total RNA was extracted from THP-1 cells using RNeasy Mini Kit (cat# 74104, QIAGEN) and cDNA was prepared with PrimeScript™ RT reagent Kit (cat# RR037A, Takara). qRT-PCR was done on 7500 real-time PCR system (Appleid Biosystems) using the SYBR Premix Ex™ Taq II (cat# RR920A, Takara). The following primers for human IL-8, TNF-α and β-actin were used:
IL-8 : 5’-CTGATTTCTGCAGCTCTGTG-3’ (forward), 5’- GGGTGGAAAGGTTTGGAGTATG-3’ (backward)
TNF-α : 5’-CTGCTGCACTTTGGAGTGAT-3’ (forward), 5’-AGATGATCTGACTGCCTGGG-3’ (backward) β-actin : 5’-CATGTACGTTGCTATCCAGGC-3’ (forward),
5’-CTCCTTAATGTCACGCACGAT-3’ (backward).
Relative expression was quantified by the change-in-threshold (2−∆∆CT) method.
I. Western blotting
THP-1 cells were lysed in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton-X100) containing a protease inhibitor cocktail (cat# 04693116001, Roche). Protein concentration in the cell lysates was measured using a BCA Protein assay kit (cat# 23227, Thermo Fisher Scientific). Equivalent amounts of protein (20 µg) were separated on 4-20% gradient acrylamide gels under reducing condition and transferred to PVDF membranes (Millipore Corp.). Transferred proteins were subjected to immunoblotting with antibodies specific for TRIM21, β-actin, p38, p-p38, ERK, p-ERK, JNK and p-JNK followed by reaction with appropriate secondary antibodies of HRP-conjugated horse anti-mouse IgG, HRP- α-rabbit IgG antibody. The signals were detected by ECL (cat# RPN2106, GE Healthcare).
J. Immunoprecipitation (IP)
To examine that the 3D8 IgG interacts HSPG and CSPG, HeLa cells were transfected with plasmids encoding SDC2-GFP, GPC3, CD44-GFP and HA-BCAN. After transfection, 3D8 IgG was treated to cells for 6 h at 37℃. cell lysates were prepared using an IP kit (cat# 26148, Thermo Fisher Scientific), according to the manufacturer’s instructions. Lysates were immunoprecipitated with GFP, α-GPC3, α-CD44 or α-HA antibodies. If necessary prior to treatment of 3D8 scFv,
transfected cells were detached with non-enzymatic cell dissociation solution and incubated with 500 mIU/ml heparinase III or 500 mIU/ml chondroitinase ABC for 2 h at 37 °C. Eluted IP samples were resolved on 4–20% gradient SDS-PAGE gels, followed by immunoblotting of proteins with antibodies specific for SDC2, GPC3, CD44, HA and His tag.
To investigate interaction between 3D8 IgG and TRIM21, THP-1 cells were incubated with 5 µM 3D8 IgG and 3D8 IgG-N434D. At 6 h post-treatment, cell lysates were prepared using an IP kit. Cell lysates were subjected to IP with protein A/G coupled to a resin, and the eluted IP samples were resolved on 4–20% gradient SDS-PAGE gels, followed by immunoblotting of proteins with antibodies specific for TRIM21, IgG-Fc, or Ig kappa chain.
K. Flow cytometry
THP-1 cells were pre-incubated with Fc blocker for 30 minutes at 37℃, prior to treatment of 3D8 IgG for 1 h at 4℃, fixed by 4% paraformaldehyde (PFA) for 10 min at room temperature (RT). Cells were incubated with α-human kappa chain antibody and FITC-rabbit α-goat IgG for staining, diluted in surface buffer (0.5% BSA and 2 mM EDTA in PBS, pH 7.4).
To detected expression of HSPG and CSPGs on the cell surface, HeLa and HEK293T cells were detached with non-enzymatic cell dissociation solution, and then heparinase Ⅲ (10 mIU/ml) or chondroitinase ABC (100 mIU/ml) was treated for 1 h at 37℃ as needed. Cells were fixed with 4% PFA for 10 min at RT, followed by incubation with primary antibodies against HS, CS, SDC2, GPC3, CD44 an BCAN for 1 h at 4℃. Alexa Fluor 488-α-mouse IgM/µ chain-specific antibody was used as a secondary antibody for detecting α-HS and α-CS antibodies and Alexa Fluor 488-α-mouse IgG + IgM and Alexa Fluor 647-α-rabbit IgG was used to detect α-SDC2, GPC3, CD44 and BCAN antibodies. After pre-incubation with the enzymes,
cells were treated with 10 µM 3D8 scFv for 1 h at 4℃ to detect binding of 3D8 scFv to HSPG and CSPG. For detecting 3D8 scFv, α-3D8 antibody and TRITC-α-rabbit IgG were used.
All samples were analyzed by flow cytometry using a FACSCanto Ⅱ (BD Biosciences)
L. Luciferase reporter assay
NF-κB reporter THP-1 cells were seeded at 3 × 105 cells in 24-well plate
and incubated in TOM with 5 µM proteins for 24 h. If necessary, 5z-7-oxozeaenol (0.5 µM) was pre-incubated for 1 hr. Cells were then assayed for firefly luciferase activity using a luciferase assay system (cat# E1500, Promega). Luciferase activities are presented as fold induction relative to the normalized luciferase activity in the transduced THP-1 cells only, which was taken as 1.0.
Ⅲ. Results
A. Production of pro-inflammatory cytokines is induced by 3D8 IgG in various cells.
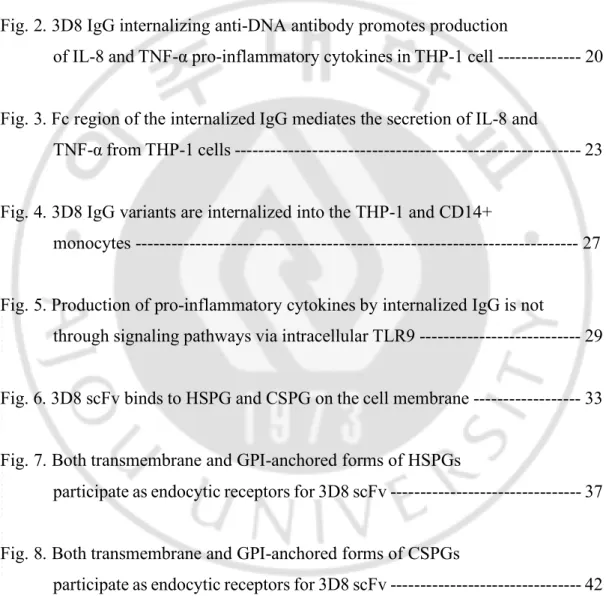
To screen the cytokine production profiles resulting from the treatment of internalizing anti-DNA antibody, chimeric 3D8 IgG was purified from HEK293F cells. 3D8 IgG was treated to five immune cell types (THP-1 human monocytic leukemia cells, Jurkat human T lymphoblastoid cells, DC2.4 mouse dendritic cells, RAW 264.7 mouse macrophages, and BV2 mouse microglia cells) (Fig. 1A) and five non-immune cell types (A549 human lung epithelial cells, HEK293T human embryonic kidney cells, U87MG human glioblastoma cells, HeLa human cervix carcinoma epithelial cells, and MEF mouse embryonic fibroblasts) (Fig. 1B). The production of cytokines was quantified by enzyme-linked immunosorbant assay (ELISA) to detect pro-inflammatory cytokines. The screening identified the marked secretion of the IL-8 and TNF-α inflammatory cytokines from THP-1 cells.
A
IL-1 A IL-1 B IL-2IL-4IL-6IL-8IL-10IL-12 IL-1 7A IFN-γ TNF-α GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm THP-1 PBS 3D8 IgG 24 h 3D8 IgG 48 h IL-1 A IL-1 B IL-2IL-4IL-6IL-10IL-12 IL-1 7A IFN-γ TNF-α G-C SF GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Absorbans at 450 nm RAW 264.7 IL-1 A IL-1 B IL-2IL-4IL-6IL-8IL-10IL-12 IL-1 7A IFN-γ TNF-α GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm Jurkat IL-1 A IL-1 B IL-2IL-4IL-6IL-10IL-12 IL-1 7A IFN-γ TNF-α G-C SF GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm BV2 IL-1 A IL-1 B IL-2IL-4IL-6IL-10IL-12 IL-1 7A IFN-γ TNF-α G-C SF GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 Absorbans at 450 nm DC2.4Fig. 1. Production of pro-inflammatory cytokines is induced by 3D8 IgG in various cells. Measurement of the production of cytokines related to inflammation
following treatment with 10 µM 3D8 IgG for 24 or 48 h in (A) immune cells (THP-1, Jurkat, DC2.4, RAW 264.7, and BV2) and (B) non-immune cells (A549, HEK293T, U87MG, HeLa, and MEF).
IL-1 A IL-1 B IL-2IL-4IL-6IL-8IL-10IL-12 IL-1 7A IFN-γ TNF-α GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm A549 PBS 3D8 IgG 24 h 3D8 IgG 48 h IL-1 A IL-1 B IL-2IL-4IL-6IL-8IL-10IL-12 IL-1 7A IFN-γ TNF-α GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm HeLa IL-1 A IL-1 B IL-2IL-4IL-6IL-8IL-10IL-12 IL-1 7A IFN-γ TNF-α GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm HEK293T IL-1 A IL-1 B IL-2IL-4IL-6IL-10IL-12 IL-1 7A IFN-γ TNF-α G-C SF GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm MEF IL-1 A IL-1 B IL-2IL-4IL-6IL-8IL-10IL-12 IL-1 7A IFN-γ TNF-α GM-C SF 0.0 0.5 1.0 1.5 2.0 2.5 Absorbans at 450 nm U87MG
B
B. Internalizing anti-DNA antibody, 3D8 IgG promotes the production of pro-inflammatory cytokines, IL-8 and TNF-α in THP-1 cells.
To investigate the time- and dose-dependent production of IL-8 and TNF-α in THP-1 cells, the 3D8 IgG incubation times of up to 6 h were used (Fig. 2A) and the dose of 3D8 IgG was increased by up to 10 µM (Fig. 2B). Time- and dose-dependent secretion of both cytokines was evident. The optimum treatment conditions of the antibody were set at 5 µM and 6 h. We next investigated the cytokine production in primary human monocytes. CD14+ monocyte cells isolated from the peripheral blood mononuclear cells (PBMCs) of a healthy donor were treated with 5 µM 3D8 IgG for 6 h (Fig. 2C). IL-8 and TNF-α were also secreted by 3D8 IgG, indicating that treatment of 3D8 IgG triggers the production of these pro-inflammatory cytokines in THP-1 and primary monocytes.
0 0.5 1 2 4 6 0 500 1000 1500 2000 Time (h) IL-8 (pg/ml) * ** *** * *** 0 0.5 1 2 4 6 0 100 200 300 400 500 Time (h) TNF-α (pg/ml) n.s. *** *** *** *** 0 0.625 1.25 2.5 5 10 0 1000 2000 3000 4000 IL-8 (pg/ml) 3D8 IgG (µM) *** *** *** *** ** 0 0.625 1.25 2.5 5 10 0 200 400 600 TNF-α (pg/ml) 3D8 IgG (µM) *** *** *** *** ***
B
A
PBS 3D8 IgG 0 200 400 600 800 1000 IL-8 (pg/ml) *** PBS 3D8 IgG 0 200 400 600 800 TNF-α (pg/ml) ***C
Fig. 2. 3D8 IgG internalizing anti-DNA antibody promotes production of IL-8 and TNF-α pro-inflammatory cytokines in THP-1 cells. (A, B) THP-1 cells were
treated with 5 µM 3D8 IgG for the indicated times at 37°C (A), or were treated with various concentrations of 3D8 IgG for 6 h at 37°C (B). (C) Cytokines secreted from in human primary CD14+ monocytes after treatment with 5 µM 3D8 IgG were
analyzed by ELISA. Data are expressed as the mean ± standard error of three independent experiments. All P-values were calculated using a two-tailed Student’s t-test (ns: not significant; *P<0.05; **P<0.01; ***P<0.001 versus negative control).
C. Fc region of internalized anti-DNA antibody mediates secretion of IL-8 and TNF-α from THP-1 cells.
To determine the portion of the internalized 3D8 IgG that is responsible for the production of cytokines in THP-1 cells, we purified the different variants of the 3D8 antibody including 3D8 scFv, which comprised of VH and VL, and 3D8
scFv-Fc fusion protein (Fig. 3A). As expected, 3D8 IgG was detected at 50 kDa and 25 kDa, whereas 3D8 scFv was detected at 27 kDa on reducing SDS-PAGE; 3D8 scFv-Fc was detected at 52 kDa (Fig. 3B). When THP-1 cells were treated with 3D8 IgG, 3D8 scFv, 3D8 scFv-Fc, and polyclonal human IgG incapable of internalizing, the secretion of IL-8 and TNF-α was observed in cells treated with 3D8 IgG and 3D8 scFv-Fc, but not in 3D8 scFv and polyclonal human IgG (Fig. 3C). The expressions of the mRNAs were also correlated (Fig. 3D). 3D8 scFv, which did not induce cytokine production, is a protein that is depleted in the constant region, indicating that the immune response is due to the constant region rather than the variable region. Moreover, the 3D8 scFv fusion protein that lacked the CH1 domain of the constant
region induced cytokine production, demonstrating that the Fc region and the CH2
-CH3 domain in the constant region are involved in the induction of inflammatory
responses. To investigate whether the production of cytokines is also induced by other subtypes, 3D8 IgE was treated to THP-1 cells, and the result was observed for IL-8 and TNF production (Fig. 3E). The collective results demonstrated that production of IL-8 and TNF-α is induced by the Fc region of 3D8 anti-DNA antibody.
D
C
PBS Huma n IgG 3D8 sc Fv 3D8 I gG 0 10 20 30 40 *** Re la ti v e IL -8 m RNA le v e l PBS Huma n IgG 3D8 scFv3D 8 IgG 0 1 2 3 4 5 ** Re la ti v e T NF -a m R N A le ve lA
Onl y cel l PBS Huma n IgG 3D8 scFv 3D8 scFv -Fc 3D8 I gG 0 1000 2000 3000 4000 5000 IL -8 ( p g /m l) ****** Onl y cel l PBS Huma n IgG 3D8 scFv 3D8 scFv -Fc 3D8 I gG 0 100 200 300 400 T NF -a ( p g /m l) *** *** VL VH < scFv > < scFv-Fc > < IgG > CH1 CH2 CH3B
Ig G ( 50 kD a + 25 kD a) scF v -F (5 2 kD a ) scF v (2 5 kD a)D
C
PBS Huma n IgG 3D8 sc Fv 3D8 I gG 0 10 20 30 40 *** Re la ti v e IL -8 m RNA le v e l PBS Huma n IgG 3D8 scFv3D 8 IgG 0 1 2 3 4 5 ** Re la ti v e T NF -a m R N A le ve lA
Onl y cel l PBS Huma n IgG 3D8 scFv 3D8 scFv -Fc 3D8 I gG 0 1000 2000 3000 4000 5000 IL -8 ( p g /m l) ****** Onl y cel l PBS Huma n IgG 3D8 scFv 3D8 scFv -Fc 3D8 I gG 0 100 200 300 400 T NF -a ( p g /m l) *** *** VL VH < scFv > < scFv-Fc > < IgG > CH1 CH2 CH3B
Ig G ( 50 kD a + 25 kD a) scF v -F (5 2 kD a ) scF v (2 5 kD a)E
Fig. 3. Fc region of internalized anti-DNA antibody mediates secretion of IL-8 and TNF-α from THP-1. (A) Schematic representation of the 3D8 IgG, 3D8
scFv-Fc and 3D8 scFv. (B) SDS-PAGE analysis of purified 3D8 variants. (C) Cells were treated with 5 µM 3D8 variants and polyclonal human IgG for 6 h at 37°C. The level of IL-8 and TNF-α in culture supernatants were quantitatively measured by ELISA. (D) Quantitative RT-PCR for the mRNA level of IL-8 and TNF-α from cells treated with 5 µM 3D8 antibody for 6 h at 37°C. (**P<0.01; ***P<0.001 versus treatment with 3D8 scFv)(E) Measurement of cytokines production by treatment of 5 µM 3D8 IgG and IgE using ELISA. Data are expressed as the mean ± standard error of three independent experiments. All P-values were calculated using a two-tailed Student’s t-test.
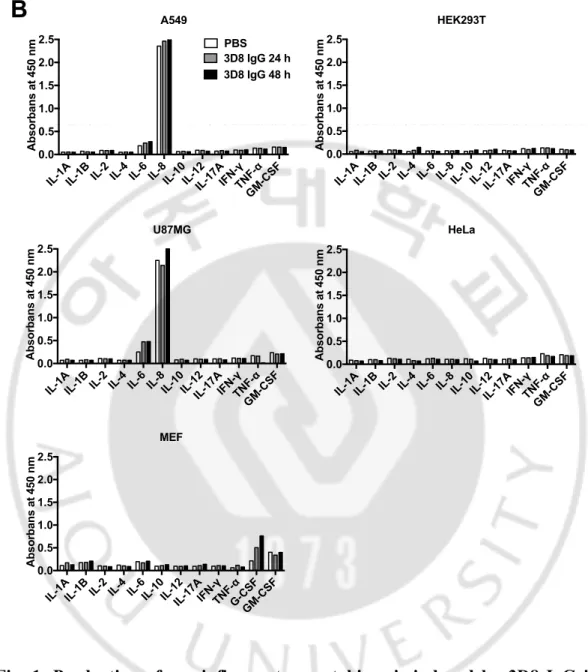
D. 3D8 IgG variants are internalized into the THP-1 and CD14+ monocytes.
Confocal microscopy examination of the internalization of the antibodies revealed that all formats of the internalizing 3D8 antibody localized in the cytosol of THP-1 cells. In contrast, polyclonal human IgG used as a negative control localized on the cell surface, likely due to capture by Fcγ receptors (FcγR) expressed on THP-1 cells (Fig. 4A). Confocal microscopy also showed that internalizing 3D8 IgG is localized at an area considered as the cytosol of CD14+ monocytes (Fig. 4B), indicating the production of IL-8 and TNF-α by the internalization of free 3D8 IgG in THP-1 cells and primary CD14+ monocyte cells.
Fig. 4. 3D8 IgG variants are internalized into THP-1 and CD14+ monocytes. (A,
B) Confocal microscopy for intracellular localization of 3D8 variants and polyclonal human IgG. THP-1 Cells (A) and CD14+ monocytes (B) were incubated with 5 µM 3D8 proteins and human IgG (hIg) 6 h at 37°C. After fixation and permeabilization, cells were incubated with dylight550 goat α-human IgG Fc for detecting 3D8 IgG, 3D8 scFv-Fc and human IgG, with α-3D8 IgG and TRITC-α-rabbit IgG to detect 3D8 scFv. The bar denotes 5 µm.
A
B
hIG scFv scFv-Fc IgG No proteinTHP-1
E. Production of pro-inflammatory cytokines by internalized IgG is not through signaling pathways via intracellular TLR9.
Pathogenesis due to anti-DNA antibodies involves the formation of DNA-IC released from dead cells and their ensuing phagocytosis by plasmacytoid dendritic cells (pDCs) through Fcγ receptors. The complex is recognized by intracellular TLR9, leading to the production of type I interferon. To investigate whether the immune response by 3D8 IgG is related or unrelated to the recognition of TLR9, we first compared the endocytosis mechanism of DNA-IC and 3D8 IgG using pharmacological inhibitors to interfere with the individual endocytic pathways (Fig. 5A). Chlorpromazine (CPZ) was used to inhibit clathrin-dependent endocytosis, methyl-β-cyclodextrin (MβCD) to inhibit caveolae/lipid raft endocytosis, and cytochalasin D (CytoD) to inhibit phagocytosis. THP-1 cells were pre-treated with these inhibitors for 30 min at 37°C, prior to the incubation with 5 µM 3D8 IgG. CPZ inhibited the production of IL-8 and TNF-α, but MβCD and CytoD did not, suggesting that 3D8 IgG is internalized via clathrin-dependent endocytosis. We next examined whether the production of cytokines was stimulated by TLR9 or Fcγ receptors signaling, which recognize DNA-ICs. In THP-1 cells pre-incubated of TLR9 inhibitor (Fig. 5B) or human Fc blocker (Fig. 5D), the levels of IL-8 and TNF-α were not affected by TLR9 inhibitor but were increased by Fc blocker. And, we confirmed that binding of 3D8 IgG to FcγR was decreased by Fc blocker by flow cytometry (Fig. 5C). The increase of cytokines reflected the increased amount of antibody that entered the cells as the 3D8 IgG partially captured by the FcγR became free. When cells were treated with DNase I and genomic DNA extracted from THP-1 cells prior to incubation with 3D8 IgG, no significant changes in the production levels of IL-8 and TNF-α were detected (Fig. 5E). These results indicated that the production of IL-8 and TNF-α by internalizing IgG is induced in a different from the TLR9-mediated pathway.
A
B
No i nhibi tor Chlor proma zine CDMb Cytochala sin D 0 1000 2000 3000 4000 5000 ****** *** IL -8 ( p g /m l) No inh ibito r Chlorpromazi ne CDb M Cyto cha lasin D 0 100 200 300 400 500 T NF -a ( p g /m l) *** n.s Cont rol inh ibitor TLR 9 inhibito r 0 500 1000 1500 2000 n.s . IL -8 ( p g /m l) Cont rol inh ibitor TLR 9 inhibito r 0 50 100 150 200 250 n.s. T NF -a (p g /m l)mean fluorescence intensity (MFI) 0 100 200 300 400 3D8 IgG Fc blocker + + + -0 2000 4000 6000 ** 3D8 IgG Fc blocker + _ ++ IL -8 ( p g /m l) 0 200 400 600 800 ** 3D8 IgG Fc blocker + _ + + T NF -a (p g /m l)
C
D
E
DNas e 1 DNA (5ug /ml) 3D8 IgGDNas e 1 DNA (2.5 ug/m l) DNA (5ug /ml) DNA (10ug /ml) 0 500 1000 1500 2000 2500 DNase I DNA (µg/ml) 3D8 IgG + _ 5 _ _ + + 5 + 10 + _ + + _ _ _ _ _ _ 2.5 n.s. IL -8 ( p g /m l) DNase 1 DNA (5ug/ml) 3D8 IgGDNase 1 DNA (2.5ug/m l)DNA (5ug/ml)DNA (10ug/ml ) 0 200 400 600 800 DNase I DNA (µg/ml) 3D8 IgG + _ 5 _ _ + + 5 + 10 + _ + + _ _ _ _ _ _ 2.5 T NF -a ( p g /m l) n.s.
Fig. 5. Production of inflammatory cytokines by internalized IgG is not through signaling pathways via intracellular TLR9. (A-B, D-E) Prior to treatment with
3D8 IgG for 6 h, THP-1 cells were treated with endocytosis inhibitors chlorpromazine (CPZ), methyl-β-cyclodextrin (MβCD), and cytochalasin D (CytoD) (A), TLR9 inhibitor (B), Fc blocker (D), or DNase1 and genomic DNA (0-10 µg/ml) extracted from THP-1 cells (E). Production of IL-8 and TNF-α in culture supernatants was measured by ELISA. (C) Inhibition of 3D8 IgG binding to FcγR by Fc blocker was observed by flow cytometry. 3D8 IgG was treated with THP-1 at 4℃ for 1 hour, and 3D8 IgG bound to the surface was detected using α-kappa antibody. All P-values were calculated using a two-tailed Student’s t test. Statistical significance is indicated on the graphs (ns: not significant; *P<0.05; **P<0.01; ***P<0.001).
F. 3D8 scFv binds to HSPG and CSPG on the cell membrane.
Phagocytosis of DNA-ICs is mediated by FcγR in pDCs. 3D8 IgG is internalized via the clathrin-mediated pathway but endocytic receptors is unknown. Therefore, we next investigated the endocytic receptors of 3D8 IgG. CPPs can penetrate the cell membrane in various living cells. The numerous positively-charged amino acids that comprise CPPs interact with GAGs, such as HS and CS, which are negatively charged when they are internalized into cells. Assuming that 3D8 IgG, which contains many amino acids that are positively-charged in CDRs of the variable region similar to CPPs, is internalized via the negatively-charged surface receptors, we first confirmed the expression level of the negatively-charged proteoglycans, HSPGs and CSPGs, in HeLa cells (Fig. 6A). Confocal microscopy revealed the binding of 3D8 scFv, which possesses internalizing ability, to HSPGs and CSPGs on the cell membranes. There was no binding of the negative control, HW6 scFv specific for human TRAIL receptor 2 did not bind. 3D8 scFv was incubated at 4 °C for 1 h in HeLa cells to inhibit endocytosis and the HS or CS chains (Fig. 6B). 3D8 scFv co-localized with HS and CS chain on the cell surface. To confirm the interaction between 3D8 scFv and GAGs, 10 mIU/ml of heparinase III and 100 mIU/ml of chondroitinase ABC were used to pre-treat HeLa cells to reduce the HS and CS chains on the cell surface (Fig. 6C). Treatment of heparinase III, chondroitinase ABC, and a mixture of both enzymes reduced the binding of 3D8 scFv by 60%, 15%, and 78%, respectively (Fig. 6D). Confocal microscopy revealed the decreased binding of 3D8 scFv to the cell membrane after treatment with GAG-degrading enzymes. The findings were corroborated by flow cytometry (Fig. 6E). The collective results indicated that 3D8 scFv binds to both HS and CS chains on the cell membrane.
A
B
Only c ells No he p III Hep II I 0 1000 2000 3000 M FI (c el l s u rf ac e HS ) Only c ells No ch on ABC Chon ABC 0 500 1000 1500 2000 MF I (c ell s u rf ac e CS ) 0 20000 40000 60000 80000 3D8 scFv Hep III Chon ABC +--
+ + + + + + +-MF I (3 D8 o n c el l s u rf ac e)
C
D
Control HSPG CSPG HSPG 3D8 Merge CSPG 3D8 Merge HW6 HSPG MergeFig. 6. 3D8 scFv binds to HSPG and CSPG on the cell membrane. (A)
Examination of the endogenous expression levels of HSPGs and CSPGs in HeLa cells by flow cytometry. (B) Detection of binding between 3D8 scFv and HSPG/CSPG by confocal microscopy. 3D8 scFv and HW6 scFv (10 µM) were incubated with HeLa cells for 1 h at 4 °C, followed by detection using a combination of rabbit α-3D8 scFv antibody and mouse α-HS IgM antibody, or rabbit α-3D8 scFv antibody and mouse α-CS IgM antibody. The mixture of TRITC-conjugated α-rabbit IgG and Alexa Fluor 488-conjugated α-mouse IgM was used as secondary antibody. (C-E) Detection of HS and CS chains (C) and 3D8 scFv on the cell surface (D) using heparinase III (10 mIU/ml) and chondroitinase ABC (100 mIU/ml) by flow cytometry (C, D) and confocal microscopy (E). Nuclei were stained with Hoechst 33342 (blue). The bar denotes 10 µm.
G. Both transmembrane and glycosylphosphatidylinositol (GPI)-anchored forms of HSPGs function as endocytic receptors for 3D8 scFv.
Transmembrane and GPI-anchored HSPGs are expressed on the cell surface. We assessed whether 3D8 IgG can be internalized into the cells via HSPG, regardless of the structure type, using the SDC2 transmembrane receptor and GPC3 GPI-anchored receptor. HeLa cells were transfected with plasmids encoding GFP-SDC2 and HA-GPC3, to express SDC2 and GPC3 on the cell surface, and with plasmids encoding SDC2ΔTM-GFP and GPC3-nfGPI-GFP, to enable intracellular expression (Fig 7A). After transfection, cells were treated with 3D8 scFv for 1 h at 4 °C to inhibit endocytosis, or for 6 h at 37°C to permit internalization (Fig. 7B-E). When endocytosis was inhibited at 4°C, 3D8 scFv mostly co-localized with SDC2 and GPC3 on the cell surface (Fig. 7B, D). However, 3D8 scFv did not co-localize with the SDC2ΔTM and GPC3-nfGPI. When 3D8 scFv was incubated for 6 h at 37 °C, it co-localized with SDC2 and GPC3 expressed the on the cell membrane, whereas 3D8 scFv did not co-localize with intracellularly expressed SDC2ΔTM and GPC3-nfGPI (Fig. 7C, E). Since 3D8 scFv is internalized into cells with receptors expressed on the cell surface, intracellularly expressed SDC2 and GPC3 were evident as red and green fluorescence.
The endogenous expression levels of SDC2 and GPC3 in HeLa and HEK293T cells were examined before confirming whether HSPG and 3D8 scFv physically interacted. Endogenous SDC2 was highly expressed in both HeLa and HEK293T cells, but endogenous GPC3 was not expressed in both cells (Fig. 7F, H). SDC2-GFP and HA-GPC3 overexpressing HeLa and HEK293T cells were incubated with 3D8 scFv for 6 h and then subjected to immunoprecipitation with GFP and α-GPC3 antibodies, respectively (Fig. G, I). 3D8 scFv, SDC2 and α-GPC3 that were pulled-down in the lysates were detected by western blot, indicating that 3D8 scFv interacts with transmembrane SDC2 and GPI-anchored GPC3.
B
D
C
E
A
37℃ 6 h 4℃ 1 h GFP-SDC2 3D8 Merge SDC2ΔTM-GFP 3D8 Merge 4℃ 1 h 37℃ 6 h HA-GPC3 3D8 Merge 4℃ 1 h 37℃ 6 h GPC3-nfGPI-GFP 3D8 Merge 4℃ 1 h 37℃ 6 h SDC2-GFP HA-GPC3 SDC2ΔTM-GFP GPC3-nfGPI-GFP 612 aa HA 1 GPI 1 821 aa GFP 200 400 600 145 201 GFP 1 393 aa 200 1 442 aa GFP TMCD SPF
G
HeLa
Control GPC3 Control SDC2H
HEK293 Control SDC2 Control GPC3I
Fig. 7. Both transmembrane and GPI-anchored forms of HSPGs participate as endocytic receptors for 3D8 scFv. (A) Schematic diagrams of SD2 and GPC3
expression vectors for cell surface or intracellular expression. Abbreviations are: SP, signal peptide; Δ, deletion; TM, transmembrane domain; CD, cytoplasmic domain; GPI, GPI anchor; GFP, green fluorescent protein; HA, HA tag. (B-E) Confocal microscopy detection of the co-localization between 3D8 scFv, SDC2, and GPC3. HeLa cells were transfected with SDC2-GFP (B) and HA-GPC3 (D) vectors expressed on the cell surface, and the SDC2ΔTM-GFP (C) and GPC3-nfGPI-GFP (E) vectors expressed intracellularly (nf indicates non-functional). After transfection, 3D8 scFv was used to treat HeLa cells for 1 h at 4 °C or 6 h at 37 °C, and SDC2, GPC3, and 3D8 scFv were detected using the appropriate antibodies. Nuclei were stained with Hoechst 33342 (blue). The bar denotes 10 µm. (F, H) Flow cytometry examination of the cell surface expression of endogenous SDC2 (F) and GPC3 (H) in HeLa and HEK293T cells. (G, I) SDC2-GFP (G) and HA-GPC3 (I) overexpressing HeLa and HEK293T cells were incubated with 5 µM 3D8 scFv for 6 h at 37 °C. Cell lysates were immunoprecipitated using α-GFP antibody and the immunoprecipitates were detected by western blot using SDC2, GPC3, and α-His antibodies. Asterisks denote endogenous SDC2.
H. Both transmembrane and GPI-anchored forms of CSPGs participate as endocytic receptors for 3D8 scFv.
We investigated whether the distinct transmembrane and GPI-anchored CSPGs play a role as endocytosis receptors for 3D8 scFv. First, we transfected cells with plasmids encoding CD44-GFP and HA-BCAN, expressed on cell surface, and CD44ΔTM-GFP and BCANΔGPI-GFP expressed intracellularly. After transfection, the cells were treated with 3D8 scFv for 1 h at 4 °C to inhibit endocytosis or for 6 h at 37 °C. When endocytosis was inhibited, 3D8 scFv mostly co-localized with CD44 and BCAN on the cell surface. However, 3D8 scFv did not co-localize with either CD44ΔTM or BCANΔGPI. When cells were incubated with 3D8 scFv for 6 h at 37 °C, 3D8 scFv co-localized with SDC2 and GPC3 expressed on the cell membrane, but not with intracellular expressed CD44ΔTM and BCANΔGPI, respectively. Since 3D8 IgG is internalized into cells with surface-expressed receptors, intracellularly expressed CD44 and BCAN were observed as red and green fluorescence, respectively.
The endogenous expression levels of CD44 and BCAN in HeLa and HEK293T cells were examined before confirming whether CSPG and 3D8 scFv physically interacted. Endogenous CD44 was expressed only on HeLa cells, however, endogenous BCAN was not expressed in both cells (Fig. 8F, H). CD44-GFP and HA-BCAN overexpressed HeLa and HEK293T cells were incubated with 3D8 scFv for 6 h, and then lysates were immunoprecipitated with α-CD44 and α-HA antibodies, respectively (Fig. 8G, I). In pull-downed lysates, 3D8 scFv, CD44 and BCAN were detected by western blot, indicating that 3D8 scFv interacts with transmembrane CD44 and GPI-anchored BCAN.
B
D
C
E
A
CD44-GFP 3D8 Merge 4℃ 1 h 37℃ 6 h CD44ΔTM-GFP 3D8 Merge 4℃ 1 h 37℃ 6 h HA-BCAN 3D8 Merge 4℃ 1 h 37℃ 6 h BCANΔGPI-GFP 3D8 Merge 4℃ 1 h 37℃ 6 hF
G
HeLa
Control
CD44
Control
BCAN
H
HEK293
Control
CD44 Control BCAN
Fig. 8. Both transmembrane and GPI-anchored forms of CSPGs participate as endocytic receptors for 3D8 scFv. (A) Schematic diagrams of CD44 and BCAN
expression vectors for cell surface or intracellular expression. Abbreviations are: SP, signal peptide; Δ, deletion; TM, transmembrane domain; CD, cytoplasmic domain; GPI, GPI anchor; GFP, green fluorescent protein; HA, HA tag. (B-E) Confocal microscopy detection of the co-localization of 3D8 scFv, CD44, and BCAN. HeLa cells were transfected with CD44-GFP (B) and HA-BCAN (D) vectors expressed on the cell surface, and CD44ΔTM-GFP (C) and BCAN-ΔGPI-GFP (E) vectors expressed intracellularly. After transfection, 3D8 scFv was used to treat HeLa cells for 1 h at 4 °C or 6 h at 37 °C, and CD44, BCAN, and 3D8 scFv were detected using the appropriate antibodies. Nuclei were stained with Hoechst 33342 (blue). The bar denotes 10 µm. (F, H) Flow cytometry examination of cell surface expression of endogenous CD44 (F) and BCAN (H) in HeLa and HEK293T cells. (G, I) CD44-GFP (G) and HA-BCAN (I) overexpressing HeLa and HEK293T cells were incubated with 5 µM 3D8 scFv for 6 h at 37 °C. Cell lysates were immunoprecipitated by α-CD44 and α-HA antibodies, and then detected by western blot using α-CD44 and α-HA antibodies. Asterisks denote endogenous CD44.
I. HS and CS chains, but not core proteins, are responsible for endocytosis of 3D8 scFv.
We assessed the interaction between 3D8 scFv and the HSPG/CSPG core proteins of the receptor for endocytosis. GAG-degrading enzymes were used to investigate whether HS and CS chains, but not the core proteins, interacted with 3D8 scFv to cause endocytosis (Fig. 9). SDC2, GPC3, CD44, and BCAN overexpressing cells were pre-treated with heparinase III and chondroitinase ABC, respectively, for 2 h at 37°C. 3D8 scFv was used to treat GAG-degraded HeLa cells for 6 h at 37°C, followed by immunoprecipitation with α-GFP, α-GPC3, α-CD44, and α-HA antibodies. Uptake of 3D8 scFv into GAG chain-degraded HeLa cells was significantly reduced compared to enzyme-untreated HeLa cells. The results confirmed the involvement of HS and CS chains in the endocytosis of 3D8 scFv.
Fig. 9. HS and CS chains, but not core proteins, are responsible for endocytosis of 3D8 scFv. HSPG (SDC2 and GPC3) and CSPG (CD44 and BCAN)
overexpressing HeLa cells were incubated with 500 mIU/ml heparinase III or 500 mIU/ml chondroitinase ABC for 2 h at 37 °C, prior to treatment with 3D8 scFv for 6 h at 37 °C. Lysates were immunoprecipitated with GFP, GPC3, CD44, or α-HA antibodies, followed by western blot.
J. Production of IL-8 and TNF-α by internalizing IgG is not mediated by TRIM21 signaling pathway.
The TRIM21 intracellular Fc receptor is important in the ADIN process of pathogen recognition involving cytosolic antibody. As an autoantigen, TRIM21 is highly expressed in SLE patients (Ben-Chetrit et al., 1988). We hypothesized that anti-DNA antibodies with high titer in SLE are associated with TRIM21. To investigate whether the cytokine signaling by freely internalized 3D8 IgG is mediated by TRIM21, we purified the 3D8 IgG N434D mutant, which cannot interact with TRIM21 due to a single amino acid change in the CH3 domain. The
internalization of the TRIM21 binding mutant was confirmed by confocal microscopy (Fig. 10A). Physical interaction of IgGs (wild-type and N434D) and TRIM21 was confirmed by immunoprecipitation (Fig. 10B). The lysates of THP-1 cells treated with IgGs were precipitated with protein A/G agarose beads. TRIM21 was detected only in the pull-downed lysates from the 3D8 IgG wild type, indicating the interaction between 3D8 IgG wild-type and endogenous TRIM21. When THP-1 cells were treated with 5 µM of IgG proteins for 6 h, the secretion of IL-8 and TNF-α were increased by approximately 40% in the cells treated with 3D8 IgG N434D mutant compared to the cells treated with 3D8 IgG wild-type (Fig. 10C). In addition, IL-8 and TNF-α production by the 3D8 IgG wild-type was increased by 20% in TRIM21-knockdown THP-1 cells (Fig. 10D, E). The results indicate that TRIM21 is not responsible for the production of cytokines by 3D8 IgG, but seems to play a role as a negative regulator, and also suggests the possibility of an unknown intracellular Fc receptor instead of TRIM21.