이학
이학
이학
이학 석사학위
석사학위
석사학위 논문
석사학위
논문
논문
논문
Protective effects of Human bone
marrow-derived Mesenchymal stem cells on Apoptosis
Induced Human Neuroblastoma SH-SY5Y cells
아
아
아
아
주
주
주
주
대
대
대
대
학
학
학
학
교
교
교
교
대
대
대
대
학
학
학
학
원
원
원
원
의
의
의
의
학
학
학
학
과
과
과
과
신
신
신
신
진
진
진
진
영
영
영
영
Protective effects of Human bone
marrow-derived Mesenchymal stem cells on Apoptosis
Induced Human Neuroblastoma SH-SY5Y cells
by
Jin Young Shin
A Dissertation Submitted to the Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
MASTER OF SCIENCES
Supervised by
Young Hwan Ahn, M.D., Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
신진영의
신진영의
신진영의
신진영의
이학
이학
이학
이학
석사학위
석사학위
석사학위
석사학위
논문을
논문을
논문을
논문을
인준함
인준함
인준함
인준함
.
.
.
.
심사위원장
심사위원장
심사위원장
심사위원장
안
안
안
안
영
영
영
영
환
환
환
환
인
인
인
인
심사위원
심사위원
심사위원
심사위원
이
이
이
이
필
필
필
필
휴
휴
휴
휴
인
인
인
인
심사위원
심사위원
심사위원
심사위원
이
이
이
이
광
광
광
광
인
인
인
인
아
아
아
아 주
주
주
주 대
대
대
대 학
학
학
학 교
교 대
교
교
대
대
대 학
학
학
학 원
원
원
원
2006
2006
2006
2006
년
년
년
년 12
12
12
12
월 22
월
월
월
22
22
22
일
일
일
일
i
ACKNOWLEDGEMENT
먼저 석사 과정 2년 동안 부족했던 저를 이끌어 주시고 가르침을 주신
안영환 선생님께 감사를 드립니다. 더불어 이 논문을 심사해 주시고 격려
와 충고를 해 주셨던 이필휴 선생님과 이광 선생님을 비롯한 방오영 선생
님, 김세혁 선생님, 남효석 선생님, 백만정 선생님 박창석선생님께도 감사
의 마음을 전합니다.
2년 동안 힘들거나 어려운 일이 있을 때 항상 옆에서 격려해주고 항상
도움을 주셨던 민선언니, 지친 생활의 비타민 같던 착한 동생이자 친구
수영이 덕분에 많은 것을 배웠고 힘이 되어줘서 너무 고맙다는 말을 하고
싶습니다. 실험실 생활의 도움과 가르침을 준 창미 언니, 재호 오빠, 소윤
언니, 근우 오빠와 소아과 정화씨 감사 드립니다. 그리고 고락을 같이했던
우리 실험실 식구들-문옥 언니, 화정 언니, 우영 오빠, 윤정이, 경아, 선
철이, 유정이-기쁜 일도 함께하고 힘든 시간도 함께한 친구들이었습니다.
누구보다 늘 옆에서 어려울 때마다 도와주고 충고를 아끼지 않은 현정 언
니와 언제나 힘이 되어주고 따뜻한 위로가 되어준 성우에게 진심으로 감
사의 마음 전하고 싶고 바쁘다는 핑계로 소홀히 한 친구 모두에게 이 논
문을 빌어 고맙다는 말을 전하고 싶습니다.
마지막으로 힘들하고 투정하는 저를 묵묵히 도와주시고, 따뜻하게 감싸
주신 부모님과 오빠한테도 고맙고 사랑한다고 말하고 싶습니다.
ii
-ABSTRACT-
Protective effects of Human Bone Marrow-Derived Mesenchymal Stem Cells on
Apoptosis Induced Human Neuroblastoma SH-SY5Y Cells
Apoptotic cell death is important and anti-apoptotic strategies are crucial in treatment of neurological disorders including Alzheimer’s disease, Parkinson’s disease and stroke. Cell therapy using human bone marrow-derived mesenchymal stem cells (hMSCs) is an attractive tool to alleviate neurological disease We investigate whether culture media of hMSCs and coculted hMSC using by transwell have effects against apoptosis induced SY5Ys. SH-ST5Ys were treated with 0.25M Stausrosporine(STS) for 24hrs to induce apoptosis. The media of hMSCs was collected after 2days of hMSC culture and added into STS-treated SH-SY5Ys. hMSCs plated in transwell were cocultured with STS-treated SH-SH-SY5Ys. Cell viability was assessed by trypan blue dye exclusion method and MTS assay for 5days. Morphological, biochemical and Bcl-2 family western blot analysis were also performed. Number of viable cells was decreased progressively in STS-treated SH-SY5Ys in time, concentration-dependent manner and was not stopped with addition of new media including 10% FBS or media of hMSCs. Two days after application of transwell coculture system of hBM-MSCs, number of cells begin to increased significantly. Caspase-3 activity was maintained high and peaked at day 3 of addition of transwell hMSCs. Expression of bax was
iii
increased in STS-treated SH-SY5Y cocultured without hSMCs, but reversed progressively after hMSCs addition. Expression of Bcl-2 and surviving protein were increased in cocultured with hMSCs. These finding indicate that coculture with hMSCs protect apoptosis, but not only with addition of hMSCs culture media. Our results suggested that the hBM-MSCs might have an effect to induce intrinsic ‘self-repair’ and regenerative capacity. These results might further support the potential therapeutic use of hBM-MSCs in neurological disease
iv
TABLE OF CONTENTS
ABSTRACT. ... ⅰ TABLE OF CONTENTS... ⅱ LIST OF FIGURES ... ⅳ Ⅰ. INTRODUCTION ... 1Ⅱ. MATRIALS AND METHODS ... 5
1. Matrials ... 5
2. Isolation of hBM-MSCs and Culture maintenance ... 6
3.Culture of human SH-SY5Y neuroblastoma cell culture and induction of apoptotic cell death with staurosporine treatment ... 6
4. Experimental design to effect of hBM-MSCs against apoptosis ... 7
4.1. Co-culture of STS treated SH-SY5Ys with media of hBM-MSCs ... 7
4.2 Co-culture of STS treated SH-SY5Ys with transwell hMSCs inserts ... 7
5. Cell viability assay ... 7
6. Morphological analysis... 8
7. Flow cytometric measurement of cell death using annexin-V/PI ... 8
8. Caspase-3 activity assay ... 9
9.Immunobloting ... 10
v
Ⅲ.RESULTS ... 11
1 Programmed cell death induction by staurosporine... 11
2. Effects of medium of hBM-MSCs on apoptosis induced SH-SY5Ys ... 11
3. Neuroprotective Effect of hMSC on apoptosis induced SH-SY5Ys ... 12
4. Flow cytometric measurement of cell death using annexin-v/PI... 12
5. Western blot analysis ... 13
6. Caspase-3 activity assay ... 13
Ⅳ. DISCUSSION... 23
vi
LIST OF FIGURES
Fig. 1 Experimental design... 15
Fig.2 Induction of cell death by using STS ... 16
Fig.3 Effects of medium of hMSCs... 17
Fig.4 Effects of hMSCs on apoptosis induced SH-SY5Ys... 18
Fig.5 Flow cytometry analysis of apoptosis induced SH-SY5Ys cocultured with hMSCs or not... 19
Fig.6 Pro-apoptotic protein Bax expression ... 20
Fig.7 Anti-apoptotic protein Bcl-2 expression... 21
- 1 -
. INTRODUCTION
Ⅰ
Ⅰ
Ⅰ
Ⅰ
Degeneration and death of neurons is the fundamental process responsible for the clinical manifestations of many different neurological disorders of aging, including
Alzheimer’s disease(AD)(Mattson, Pedersen et al. 1999), Parkinson’s
disease(PD)(Jenner and Olanow 1998; Mattson, Pedersen et al. 1999), and stroke(Sacco, Wolf et al. 1999). AD results from degeneration and death of neurons in brain regions(such as the hippocampus and cerebral cortex) that are involved in learning and memory processes. PD exhibit profound motor dysfunction that results from degeneration of dopaminergic neurons in the substantia nigra. Stroke manifests a range of neurological deficits that are related to death of neurons supplied by the affected blood vessels. While different populations of neurons in the brain die in each disorder, many aspects of the biochemical cascades that result in neuronal death are shared.(Mattson, Duan et al. 2001)
Neuronal apoptosis in age-related neurodegenerative disorders may be triggered by oxidative damage to proteins, lipids and DNA, metabolic compromise resulting from impaired glucose metabolism and mitochondrial dysfunction, and over activation of glutamate receptors resulting in disruption of neuronal calcium homeostasis.(Mattson, Duan et al. 2001) Apoptosis can be triggered by various stimuli. including withdrawal of growth factors, application of chemotherapeutic drugs, and cross-linking of death signal transmitting receptors.(Murphy, Ranganathan et al. 2000) Three major apoptotic
- 2 -
pathways originating from three separate subcellular compartments have been identified as the death receptor-mediated pathway, the mitochondrial apoptotic pathway, and the endoplasmic reticulum pathway. Although each pathway is initially mediated b different mechanisms, they share a common final phase of apoptosis, consisting of the activation of the executioner caspases and dismantling of substrates critical for cell survival. The mechanisms involved induction of apoptosis by chemotherapeutic agents such as alkylating agents, topoisomerase inhibitors, and antimitotic agents are believed to be largely mediated by the mitochondrial apoptotic pathway. This involves release of mitochondrial apoptotic proteins such as cytochrome c, apoptosis inducting factor(AIF), second mitochondrial-directed activator of caspase/ direct inhibitor of apoptosis(IAP) protein binding protein with low pI (Smac/DIABLO), endonuclease G and Oni1/HtrA2.[3] Also it involves that mitochondria integrate numerous different pro-apoptotic signal transducing pathways. Prominent proteins which translocate to mitochondria upon apoptosis induction include the pro-apoptotic members of the Bcl-2 family. Thus, Bax, a member of the Bcl-2 family proteins, which can promote the apoptosis translocates from the cytosol to mitochondria in response to multiple different apoptosis inducers. Bax translocation is linked to a conformational change, insertion into the mitochondrial membranes, and oligomerization. One mechanism triggering Bax translocation is cytosolic alkalinization (which can be triggered by growth factor withdrawal).(Sacco, Wolf et al. 1999; Debatin, Poncet et al. 2002) Anti-apoptotic proteins from the Bcl-2 family (Bcl-2, Bcl-XL), certain exzymes involved in
- 3 -
mitochondrial membranes), certain kinases (e.g. protein kinase A, which phosphorylates and inactivates Bad), anti-oxidant enzymes, as well as to energy-rich metabolites (glucose, ATP, ADP, NADH+), implying a major cross-talk between signal transduction, redox regulation and intermediate metabolism at the mitochondrial level. [4] Bcl-2 and Bcl-XL are two prominent native inhibitor of apoptosis and Bax function. Early
investigations focused on the ability of Bcl-2 to regulate inner mitochondrial permeability transition, cellular redox state, and oxidative damage-induced cell death. The ability of Bcl-2 and Bcl-XL to strengthen antioxidant defenses, to inhibit
mitochondrial permeability transition, and pore-independent cytochrome c release makes these proteins attractive multitarget neuroprotective candidates.(Polster and Fiskum 2004)
Since the capacity of the adult brain to regenerate after acute or chronic lesions is limited, novel concepts use the regenerative potential of stem cells in order to protect or replace damaged neurons, or to achieve functional recovery through enhanced neuronal plasticity.(Isele, Lee et al. 2006) Mesenchymal stem cells(MSCs) are introduced in clinic for treatment of several degenerative diseases. MSC therapy should provide an exogenous supply of cells capable of neurogenesis or modulatory effects, or both, on the environment to enhance plasticity and the survival and differentiation of host cells. A common source of MSCs is BM, which contains both MSCs and HSCs. These stem cell characteristics are based on their ability to differentiate into multiple cell types including osteoblases, chondrocytes, endothelial cells and even neuronal-like cells. MSCs administraton has been shown to promote neuronal survival and limit the severity
- 4 -
of neurological impairment in animal models of induced stroke and traumatic brain injury. The neuroprotective effects of hMSCs are thought to result, in part, from their ability to replace diseased or damaged neurons via cellular differentiation.(Raff 2003; Kassem, Kristiansen et al. 2004; Bang, Lee et al. 2005)
There are no studies reporting the interaction between hBM-MSCs and apoptosis. We investigated whether hBM-MSCs could trigger endogenous survival in apoptotic neuronal cells. Neuronal cells were cocultured with hBM-MSC by using transwell prior to an apoptotic insult to test whether such treatment could achieve neuroprotection against apoptotic stress and mediate such protective effects through anti-apoptosis function of Bcl-2.
- 5 -
Ⅱ
Ⅱ
Ⅱ
Ⅱ. MATRIALS AND METHODS
1. Materials
Human bone marrow-derived mesenchymal stem cells (hBM-MSCs; FCB-Pharmicell co.Ltd, Sungnam, korea) were culture-expanded from the bone marrow aspirate from the persons who agree to use his cells for research purpose. We obtained written informed consent from all persons who provide their BM aspirates according to the guideline of institutional Review board(AJIRB-04015) and Korea Food and Drug administration (KFDA-CEPT Approval No.37). And human neuroblastoma cell line(#22266, Korea cell line Bank, Seoul) were used.
For this experiments, High glucose and Low glucose Dulbecco's modified Eagle's medium (DMEM;Gibco-BLR,Grand Island, NY), trypsin-EDTA (Gibco BLR, NY), fetal bovine serum (FBS; Gibco BLR, NY), penicillin/streptomycin(Gibco BLR, NY), Cell culture dish (Corning), and Staurosporine (STS; Sigma, St Louis, MO) were used. The cell titer 96 [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-ulfophenyl)-2H-tetrazolium, inner salt (MTS), Fluorescein isothiocyanate (FITC)-labeled annexin V (BD Bioscience), Protein assay (Bio-rad), rotease inhibitor cocktail(Sigma), and nitrocellulose membrane(bio-rad) were also used for evaluation procedures,
- 6 -
Stressgen) were used. HRP-goat anti-mouse IgG(H+L) conjugate (zymed) antibody (1:2000; Amersham) were also used.
2. Isolation of hBM-MSCs and Culture maintenance
We obtained written informed consent from all the persons who agree to use his cells for research purpose. Their BM aspirates were prepared and mononuclear cells were isolated by Ficoll density centrifugation. Mononuclear cells were placed in a 10 cm dishes and were cultivated in low-glucose DMEM containing 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37℃ under 5% CO2. Medium containing non-adherent cells were then replaced every 4days of culture. When dish was reached 70-80% confluence, the cells were trypsinized and subcultured. Culture-expanded hBM-MSCs were provided for these trials. Passage 6 hBM-MSCs were used in all experiments [14, 15].
3. Culture of human SH-SY5Y neuroblastoma cell culture and induction of apoptotic cell death with staurosporine treatment
SH-SY5Y cells were cultured in 6-well plate and maintained in high glucose DMEM
containing 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37℃
under 5% CO2 [11]. SH-SY5Ys were plated in a number of 3x10
4
cells in 24-well plate for these trials. After three days of culture, several different concentration of STS ranging from 0.25 to 2µM were treated for different incubation period of 3, 6, 12 and 24 hours to induce apoptotic cell death. For further trials, SH-SY5Y cells were
- 7 -
preconditioned with 0.25 µM STS for 24 hours.
4. Experimental design to effect of hBM-MSCs against apoptosis
4.1 part 1. Co-culture of STS treated SH-SY5Ys with media of hBM-MSCs
For these trials, SH-SY5Ys were plated to density of 3x104 cells. After 3days, SH-SY5Ys were treated with 0.25µM STS for 24hrs to induce apoptotic cell death. Subsequently, medium of STS-treated SH-SY5Ys was replaced with 1) fresh DMEM containing 10% FBS, 2) 100% of hBM-MSCs media, and 3) media composed of fresh media and hBM-MSCs culture media (1:1 fresh media hBM-MSCs media ratio). These culture was maintained for 5 days and was repeated in triplicate.
4.2 part 2. Co-culture of STS treated SH-SY5Ys with transwell hMSCs inserts After three day culture of SH-SY5Ys in 24-well plate, those were treated with 0.25µM
STS for 24hrs to induce cell death. Cultured hBM-MSCs, and SH-SY5Ys were maintained in each transwell insert for 24 hrs. Each transwell insert with Cultured hBM-MSCs, or SH-SY5Ys, subsequently, was dipped in the basal plate of STS-treated SH-SY5Ys, to make share the same culture environment without direct contacts between hBM-MSCs and STS-treated SH-SY5Ys.
5. Cell viability assay
After addition of culture media of MSCs and trans-well application of hBM-MSCs, the viability of co-cultured SH-SY5Ys was assessed at day 1, 2, 3, and 5 over
- 8 -
time. The viability of SH-SY5Ys was assessed by two different methods including trypan blue dye exclusion method and measurement of metabolic activity of the SY5Y cells using MTS assay. The metabolic activity was measured by cell titer 96[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxylmethoxyphenyl)-2-(4-ulfophenyl)-2H-tetrazolium, innersalt(MTS)]. The harvested cells at the different time points were incubated in serum-free medium containing MTS solution (20 v/v) at 37℃ for 1hrs. Following incubation, MTS were quantified at 490nm by ELISA plate reader (Bio-Tek instruments. Winooski, VT). The viability of the harvested cells, determined as the ratio between dead (blue) and viable (unstained) cells was also determined by the trypan blue dye exclusion method. Viable and nonviable (blue cells) cells were counted on a hematocytometer.
6. Morphological analysis
To observe morphological changes overtime, cultured SH-SY5Ys at day 1, 2, 3, and 5 were fixed in 4% paraformaldehyde in 0.2M phosphate buffer for 10 min at room temperature. After fixation of cells, they were washed with PBS three times. SH-SY5Ys morphology was visualized by phase contrast microscopic optics (Nicon, Tokyo, Japan) fitted with a model D70s (Nicon, Tokyo, Japan).
7. Flow cytometric measurement of cell death using Annexin-V/PI
Cultured and STS treated SH-SY5Ys with or without hBM-MSCs were harvested by trypsinization and pelleted by centrifugation at 1500rpm for 5min. Cell pellets were
- 9 -
washed once in ice cold PBS, followed by gentle re-suspension in 100µl of annexin-V binding buffer containing 5µl of FITC-labeled annexin V and 5µl propidium iodide (PI; 100g/ml stock in PBS) solution for 15min. And then add 400µl 1x binding buffer. Samples were immediately kept on ice and analyzed on a FACScan at 530nm(FL-1) for FITC, and red PI at above 600nm (FL-2). FL-1and FL-2 were collected on log-scale with voltages of 450 and 458, respectively. Signal overlap was compensated for electronically. Data was acquired and analyzed using Winmdi software. Acquisition gates were wet using the forward and side light scatter of the cells and a minimum of 1,000 events were collected for each sample (Martin, Reutelingsperger et al. 1995; Azuhata, Scott et al. 2006; Zafar, Inayat-Hussain et al. 2006)
8. Caspase -3 activity assay
The caspase-3 activity was measured by caspase-3 activity assay kit(Chemicon) Caspase-3 activity was determined by monitoring proteolysis of corresponding colorimetric substrates. Cells were collected and washed in ice-cold PBS pH 7.0. Cells were subsequently lysed in 1x lysis buffer for 10min in ice and the lysates were clarified by centrifugation at 13000rpm. After centrifugation for 10mins, cytosolic extracts of SH-SY5Ys were transferred to a fresh tube and putted on ice. Then 30µg of the caspase-3-specific colorimetric substrate acetyl-Asp-Glu-Val-Asp-7-p-nitroaniline (Ac-DEVD-pNA) was added in cytosolic extracts. They were incubated for 1hrs at 37℃. The release of DEVD-pNA were quantified at 405nm by ELISA plate reader.(Lopez and Ferrer 2000)
- 10 - 9. Immunobloting
SH-SY5Ys were lysed in phosphate-buffered saline containing 1%, triton X-100, 1% DOC, 0.1% SDS and 1% protease inhibitor cocktail. The lysed cells were centrifuged at 13000rpm to remove cellular debris. Protein concentration of the extracts was determined by the Bradford assay. Equal amounts of protein were separated by SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and blocked with 5% skim milk in TBST for 2hrs. The membranes were incubated with Bax(Stressgen, 1:1000), Bcl-2(Stressgen, 1:1000) which were diluted with 5% skim milk in TBST at 4℃ with gentle shaking, overnight. After washing, the membrane was incubated with a HRP-goat anti-mouse IgG (H+L) conjugate antibody(zymed) for 1hr. After several washes, the blots were developed. (Lombet, Zujovic et al. 2001; Azuhata, Scott et al. 2006)
10. Statistical analysis
Statistical analyses were performed using a t-test on SigmaStat software (SigmaStat version 2.03 for Windows). A p value<0.05 was considered statistically significant.
- 11 -
Ⅲ
Ⅲ
Ⅲ
Ⅲ. RESULTS
1. Programmed cell death induction by staurosporine
To determine the optimal condition of concentration and duration of STS treatment that induce cell death, SH-SY5Ys were cultured with various concentrations (0.25~2µM) of STS for 24hrs. Cell viability declined in a concentration-dependant and a
time-dependent manner (Fig. 2). When SH-SY5Ys were treated with 0.25M STS, viability
was 80% at 24 hours, but treated with 2µM in concentration, viability decreased to 55% (data not shown). With phase contrast microscope, we observed cells showing morphological characteristics of apoptosis as was reported before(Lopez and Ferrer 2000)
2. Effects of medium of hBM-MSCs on apoptosis induced SH-SY5Ys
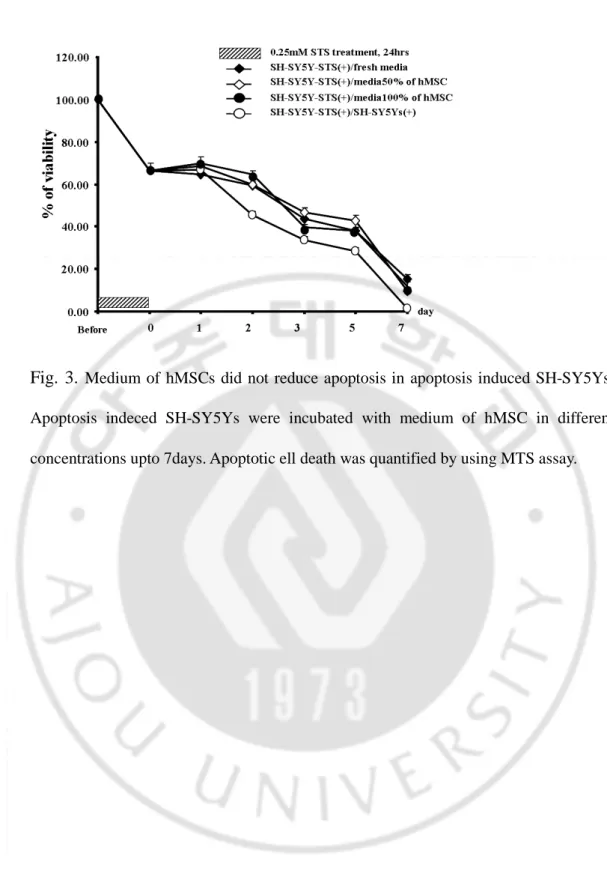
To determined whether addition of medium of hBM-MSCs has neuroprotective effects against apoptotic cell death, the media of SH-SY5Ys were replaced by the media of hBM-MSCs. The media of hBM-MSCs did not show any protective effect against apoptosis. To eliminate the possibility of the depletion of nutritional supports due to 24 hours culture of hBM-MSCs, hBM-MSCs were diluted with fresh DMEM with 10% FBS in 1:1 ratio. As shown in Fig. 3, apoptotic cell death induced SH-SY5Ys were not protected with medium of hBM-MSCs. These results demonstrated that hBM-MSCs medium alone is not enough to protect cells from apoptotic cell death.
- 12 -
3. Neuroprotective effect of hBM-MSCs on apoptosis induced SH-SY5Ys
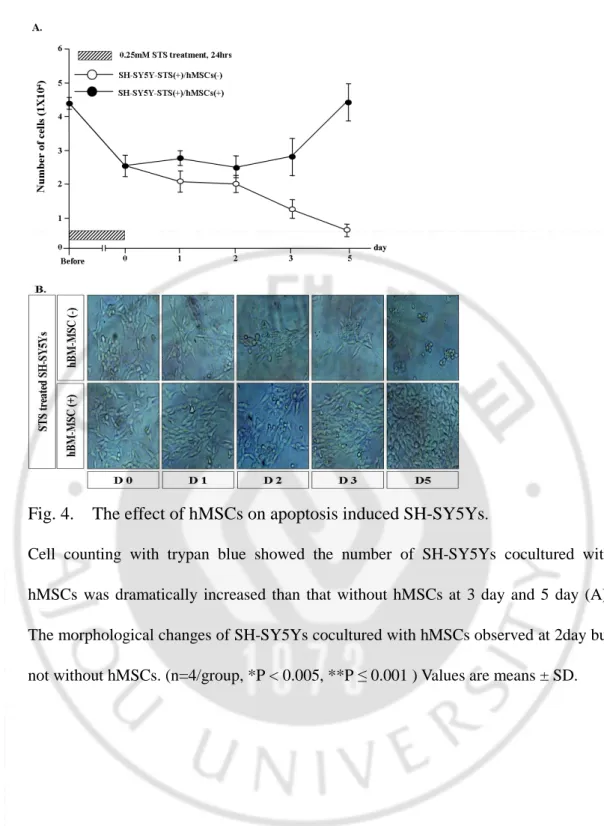
To determine the effect of hBM-MSCs on apoptosis induced SH-SY5Ys, apoptosis induced SH-SY5Ys were cocultured with hMSCs using transwell inserts for 5days. Number of viable cells was counted and compared with control that was cocultured with SH-SY5Ys using same transwell inserts from before coculture until 5 days after. For initial two to three days of coculture, number of Trypan blue excluded viable cells did not changed (Fig 4). However, number of SH-SY5Ys increased significantly at day 3 (p<0.05) and day 5 (p<0.01). When apoptosis induced SH-SY5Ys were cocultured with hBM-MSCs using transwell inserts for 5days, number of cells did not changed for 3 days (Fig. 4). Under phase contrast microscope, cells exhibited morphological characteristics of apoptosis, including extreme nuclear shrinkage and chromatin concentration, as well as apoptotic bodies were observed before coculture with hBM-MSCs and were decreased in number progressively.
4. Flow cytometric measurement of cell death using annexin-v/PI
To observe the influence of hBM-MSCs on the apoptosis induced SH-SY5Ys overtime, repeated flow cytometric assays was performed. Cultured and STS treated SH-SY5Ys with or without hBM-MSCs were analyzed. It was demonstrated that amount of Annexin V positive cells was same in pattern with data from trypan blue exclusion cell counting. These results indicate that hBM-MSCs protect SH-SY5Ys from apoptotic cell death. As shown in Fig. 5, cell proportion of live cells stained negative for both annexin-V and PI (PS-/PI-), in the lower left quadrant of each diagram was higher in hBM-MSCs
- 13 -
co-cultured group(38.1and 33.3%) than cells without hBM-MSCs (69.3 and 84.6%) at 3 days and 5 days. Annexin-V positive and PI negative (PS+/PI-) cell population in early stages of apoptosis but not in phase of necrosis (lower right quadrant) were increased significantly in Cultured SH-SY5Ys without hBM-MSCs. Cell population according to the response to annexin-V and PI were quantified and compared between cells with hBM-MSCs treated or not (Fig 5-B).
5. Western blot analysis
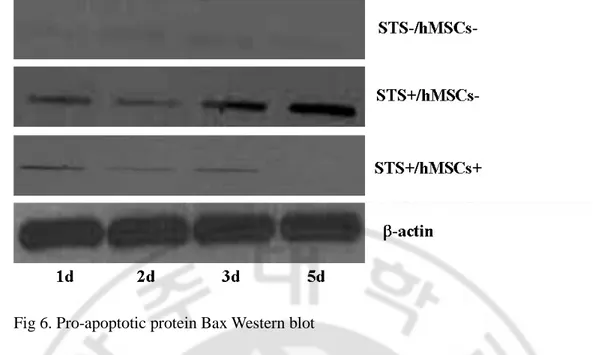
To investigate role of Bcl-2 family in recovery of apoptosis induced cell death, western blot analysis of bcl-family including bcl-2 (apoptosis suppressor) and bax (apoptosis promoter) were performed in cultured and STS treated SH-SY5Ys with or without hBM-MSCs. According to our data, expression of bax marked at day 1, increased progressively and peaked at day 5 in SH-SY5Ys without hBM-MSCs but, decreased progressively and significantly cells with hBM-MSCs (Fig 6). However, expression of bcl-2 increased progressively and reached normal level at five days after addition of hBM-MSCs (Fig 7).
6. Caspase-3 activity assay
To determine further apoptosis related cell death, activation of cysteine proteases in cultured and STS treated SH-SY5Ys with or without hBM-MSCs was analyzed. We examined the activation of caspase-3 proteases directly in cell lysates by analyzing the hydrolysis of specific colorimetric substrates. There was significant decrease of
caspase-- 14 -
3 activity in STS treated SH-SY5Ys with hBM-MSCs than cells without hBM-MSCs overtime (Fig 8). Significant decrease in caspase-3 observed at 2,3 and 5day following coculture with hMSC.
- 15 - Fig. 1. Experimental design
- 16 -
Fig.2. The ability of STS to act as an effective inducer of apoptosis upon the SH-SY5Y cells was initially characterized by analyzing its effect in a range from 0.25mM to 2mM. The cell death phenomenon appeared to be concentration, time-dependent in SH-SY5Y cells. Loss of cell viability was monitored by MTS assy
- 17 -
Fig. 3.
Medium of hMSCs did not reduce apoptosis in apoptosis induced SH-SY5Ys. Apoptosis indeced SH-SY5Ys were incubated with medium of hMSC in different concentrations upto 7days. Apoptotic ell death was quantified by using MTS assay.- 18 -
Fig. 4. The effect of hMSCs on apoptosis induced SH-SY5Ys
.Cell counting with trypan blue showed the number of SH-SY5Ys cocultured with hMSCs was dramatically increased than that without hMSCs at 3 day and 5 day (A). The morphological changes of SH-SY5Ys cocultured with hMSCs observed at 2day but not without hMSCs. (n=4/group, *P < 0.005, **P ≤ 0.001 ) Values are means ± SD.
- 19 -
Fig. 5. Flow cytometry analysis of apoptosis induced SH-SY5Ys cocultured with hMSCs or not. Folw cytogram showing cell death as assessed by flow cytometric analysis using annexin V/PI (A). Histogram showing cell death as calculated flow cytometric analysis (B). Annexin V+ cells were reduced in SH-SY5Ys cocultured with hMSCs at 2days. (n=4/group, *P < 0.005, **P ≤ 0.001 ) Values are means ± SD.
- 20 -
Fig 6. Pro-apoptotic protein Bax Western blot
Bax translocation was induced in SH-SY5Y. However hMSC inhibit translocation of Bax. Western blot analysis was performed to determine the expression levels of Bax(21kDa). Actin-b was used as the loading control for cytosolic proteins. Sighificant difference is between the apoptotic SH-SY5Y cocultured with hMSC and without hMSC.
- 21 -
Fig. 7 Anti-apoptotic protein Bcl-2 Western blot
Westrn blot analysis of untreated SH-SY5Ys, apoptotic SH-SY5Ys cocultured with hMSCs and without hMSCs for 1-5days. The proteins were stained using specific antibodies against Bcl-2(30kd). β-actin was used as the loading control
- 22 -
Fig.8. Caspase-3 activities in SH-SY5Y cocultured with or without hMSCs treated with staurosporine. Activity was quantified by monitoring the hydrolysis of the corresponding caspase-3 specific colorimetric substrate. No change of caspase-3 activity is found in normal SH-SY5Y. A significant decrease in caspase-3 occurs at 2day, 3day and 5day. Activity of caspase-3 decrease at day in SH-SY5Y cocultured with hMSCs, whereas caspase-3 activity peaks at 3day in SH-SY5Y without hMSCs (n=3/group, *P < 0.005, **P ≤ 0.001 ). Values are means ± SD.
- 23 -
Ⅳ
Ⅳ
Ⅳ
Ⅳ. Discussion
Apoptosis is a mechanism of programmed cell death involved in the homeostasis of nervous tissues and it’s dysregulation has been associated with the pathology of neurodegenerative diseases, stroke, and neurotrauma. Therefore, therapeutic strategies to prevent neuronal cell apoptosis is critical in Neurologic diseases (Siren, Fratelli et al. 2001; Pregi, Vittori et al. 2006) Recent experimental studies raised the possibility of using hMSCs in neurological disease therapy. There is increasing evidence that hMSC promote fuctional recovery in animal models. In this regard, hMSCs are considered to have neuroprotective role.(Bang, Lee et al. 2005) This study investigates that hMSCs have protective effects against apoptosic stress by expression of anti-apoptotic protein Bcl-2.
Staurosporine is able to induce a prominent neuritogenesis in human neuroblastoma cells. Because of this neurotrophin-like effect, staurosporine has been proposed as a potential prototype for future neurothrphic drugs, that could stimulate the process of regeneration and neurite outgrowth in damaged neurons.(Rasouly and Lazarovici 1994) However staurosproine can also be toxic for cells of neural origine at the concentrations and neurite outgrowth occurs early and cell death phenomenon takes place at later times.(Boix, Llecha et al. 1997) SH-SY5Ys have been used extensively in the study of neuronal cell death. Staurosporine-induced cell death in SH-SY5Ys is accompanied by endogenous Bax translocation and concomitant translocation of cytochome c from the
- 24 -
mitochondrial to the cytosolic fractions.(McGinnis, Gnegy et al. 1999; Lopez and Ferrer 2000) Our results indicate that staurosporine not only triggers the apoptotic phenomenon upon the SH-SY5Ys but also induce cell death in concentration-, and time-dependent manner. We decided that 0.25uM of staurosporine treatment for 24 hours was optimal to observe the effect of hBM-MSCs against the STS-induced apoptotic cell death. Also I obtained the results that treatment with both fresh culture media including 10% FBS and culture media of the hMSCs failed to rescue the apoptotic stress of SH-SY5Ys. Isele N et al reported that the protective effects of hMSCs conditioned medium was further accelerated when hMSCs were incubated with Neurobasal medium of neurons previously exposed to apoptotic stress indication a potential cross talk between stressed neurons and hMSCs. But heating of the conditioned medium abolish the protective effect, suggesting that heat labile factors, i.e. protein, released by hMSCs mediated the observed anti-apoptotic effect in the neurons(Isele, Lee et al. 2006). And we found that the presence of hMSCs reduced cell death, which suggested that hMSCs exert protective roles on cell death. This indicates the release of protective factors which exert their effects across species barriers, which is typical for growth factors and cytokines. In addition, these results offer the perspective to use hMSCs in forcal cerebral ischemia. Li et al. reported that hMSCs treatment of stroke in rats increased astrocytic proliferation and activation in the subventricular zone, and an increased expression of an axonal marker(GAP-43) was observed among reactive astrocytes in the scar boundary zone and SVZ. (Li, Chen et al. 2005) This indicates that the beneficial beneficial functional recovery that hMSCs provide after stroke may be derived from the
- 25 -
protection of astrocytes from cell death post-ischemia. The increased survival of astorcytes in the injured brain might subsequently increase neuronal cell survival, enhance neuroregeneration, and promote plasticity.(Gao, Li et al. 2005)
Another important aspect is that the neuroprotective effect of hMSCs required coculture with SH-SY5Ys induced cell death. When cultured media of hMSCs was supplied simultaneously with the apoptotic challenge, the neuroprotective effect was not detectable, whereas coculture of apoptosis induced SH-SY5Ys with hMSCs significantly attenuated neuronal apoptosis. This supports the idea that hMSCs mediated neuroprotection depends on expression of survival factors.
To further understand the mechanism underlying hMSCs effects on apoptosis, we examined Bcl-2 family protein. Bax translocates from the cytosol to the mitochondria in neuronal cells undergoing apoptosis. Bax has been previously found in various cell types.(Jurgensmeier, Xie et al. 1998) Bax translocation is accompanied by release of cytochrome c from the mitochondria in both CFNs and SH-SY5Ys. Cytochrom c released from the mitochondria is a cofactor for caspase-3 activity.(McGinnis, Gnegy et al. 1999) Apoptotic signals induced Bax translocation in SH-SY5Ys suggest that Bax translocation is a universal component of apoptosis in neuronal cells. Bax expression is known to be critical for neuronal apoptosis. Anti-apoptotic protein such as Bcl-2 inhibit the mitochondrial permeability transition and cytochrom c release, although effects downstream of cychrome c release have been documented.(Kluck, Bossy-Wetzel et al. 1997; Rosse, Olivier et al. 1998) Cytochrome c plays an active role in the apoptotic cascade by facilitating activation of downstream caspases through processing of
- 26 -
procaspase-9.(Li, Nijhawan et al. 1997) While it has been suggested that the prima(Susin, Zamzami et al. 1996; Kluck, Bossy-Wetzel et al. 1997)ry site for Bcl-2 regulation of apoptosis is by preventing cytochrome release.(Susin, Zamzami et al. 1996; Kluck, Bossy-Wetzel et al. 1997) (Murphy, Ranganathan et al. 2000)In monitoring Bax expression during coculturing period, Bax expression was increased in time dependent manner. It was peaked at 5days. While Bcl-2 expression was gradually increased. These our results suggest that the mechanism by which Bcl-2 blocks drug-induced apoptosis is by preventing Bax overexpression. These finding suggests that the hMSCs neuroprotective effects on apoptosis induced cells might partially be derived from upregulation of anti-apoptotic protein and downregulation of pro-apoptotic protein.
In summary, out findings strongly suggest that hMSCs secrete protective factors that prevent neuronal apoptosis that prevent neuronal apoptosis through stimulation of expression of endogenous anti-apoptotic protein. These findings suggest that the therapeutic benefit hMSCs provide to injured brain after ischemic infarct may be inpart due to hMSCs stimulation of neuroprotection.
- 27 -
Ⅴ
Ⅴ
Ⅴ
Ⅴ. Reference
Azuhata, T., D. Scott, et al. (2006). "Survivin inhibits apoptosis induced by TRAIL, and the ratio between survivin and TRAIL receptors is predictive of recurrent disease in neuroblastoma." J Pediatr Surg 41(8): 1431-40.
Bang, O. Y., J. S. Lee, et al. (2005). "Autologous mesenchymal stem cell transplantation in stroke patients." Ann Neurol 57(6): 874-82.
Boix, J., N. Llecha, et al. (1997). "Characterization of the cell death process induced by staurosporine in human neuroblastoma cell lines." Neuropharmacology 36(6): 811-21.
Debatin, K. M., D. Poncet, et al. (2002). "Chemotherapy: targeting the mitochondrial cell death pathway." Oncogene 21(57): 8786-803.
Gao, Q., Y. Li, et al. (2005). "Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult." Neuroscience 136(1): 123-34.
Isele, N. B., H. S. Lee, et al. (2006). "Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons." Neurochem Int.
Jenner, P. and C. W. Olanow (1998). "Understanding cell death in Parkinson's disease." Ann Neurol 44(3 Suppl 1): S72-84.
Jurgensmeier, J. M., Z. Xie, et al. (1998). "Bax directly induces release of cytochrome c from isolated mitochondria." Proc Natl Acad Sci U S A 95(9): 4997-5002.
- 28 -
Kassem, M., M. Kristiansen, et al. (2004). "Mesenchymal stem cells: cell biology and potential use in therapy." Basic Clin Pharmacol Toxicol 95(5): 209-14.
Kluck, R. M., E. Bossy-Wetzel, et al. (1997). "The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis." Science
275(5303): 1132-6.
Li, P., D. Nijhawan, et al. (1997). "Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade." Cell 91(4): 479-89.
Li, Y., J. Chen, et al. (2005). "Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells." Glia 49(3): 407-17.
Lombet, A., V. Zujovic, et al. (2001). "Resistance to induced apoptosis in the human neuroblastoma cell line SK-N-SH in relation to neuronal differentiation. Role of Bcl-2 protein family." Eur J Biochem 268(5): 1352-62.
Lopez, E. and I. Ferrer (2000). "Staurosporine- and H-7-induced cell death in SH-SY5Y neuroblastoma cells is associated with caspase-2 and caspase-3 activation, but not with activation of the FAS/FAS-L-caspase-8 signaling pathway." Brain Res Mol Brain Res 85(1-2): 61-7.
Martin, S. J., C. P. Reutelingsperger, et al. (1995). "Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl." J Exp Med
182(5): 1545-56.
Mattson, M. P., W. Duan, et al. (2001). "Neurodegenerative disorders and ischemic brain diseases." Apoptosis 6(1-2): 69-81.
- 29 -
Mattson, M. P., W. A. Pedersen, et al. (1999). "Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases." Ann N Y Acad Sci 893: 154-75.
McGinnis, K. M., M. E. Gnegy, et al. (1999). "Endogenous bax translocation in SH-SY5Y human neuroblastoma cells and cerebellar granule neurons undergoing apoptosis." J Neurochem 72(5): 1899-906.
Murphy, K. M., V. Ranganathan, et al. (2000). "Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells." Cell Death Differ 7(1): 102-11.
Polster, B. M. and G. Fiskum (2004). "Mitochondrial mechanisms of neural cell apoptosis." J Neurochem 90(6): 1281-9.
Pregi, N., D. Vittori, et al. (2006). "Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells." Biochim Biophys Acta 1763(2): 238-46.
Raff, M. (2003). "Adult stem cell plasticity: fact or artifact?" Annu Rev Cell Dev Biol
19: 1-22.
Rasouly, D. and P. Lazarovici (1994). "Staurosporine induces tyrosine phosphorylation of a 145 kDa protein but does not activate gp140trk in PC12 cells." Eur J Pharmacol 269(2): 255-64.
Rosse, T., R. Olivier, et al. (1998). "Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c." Nature 391(6666): 496-9.
Sacco, R. L., P. A. Wolf, et al. (1999). "Risk factors and their management for stroke prevention: outlook for 1999 and beyond." Neurology 53(7 Suppl 4): S15-24.
- 30 -
Siren, A. L., M. Fratelli, et al. (2001). "Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress." Proc Natl Acad Sci U S A 98(7): 4044-9.
Susin, S. A., N. Zamzami, et al. (1996). "Bcl-2 inhibits the mitochondrial release of an apoptogenic protease." J Exp Med 184(4): 1331-41.
Zafar, K. S., S. H. Inayat-Hussain, et al. (2006). "Overexpression of NQO1 protects human SK-N-MC neuroblastoma cells against dopamine-induced cell death." Toxicol Lett 166(3): 261-7.