이학
이학
이학
이학 석사학위
석사학위
석사학위
석사학위 논문
논문
논문
논문
Neuroprotective Effect of Human
Mesenchymal Stem Cell on Dopaminergic
Neurons by Anti-inflammatory Action
아
아
아
아 주
주
주 대
주
대
대
대 학
학
학 교
학
교 대
교
교
대
대
대 학
학
학 원
학
원
원
원
의
의
의
의 학
학
학 과
학
과
과
과
김
김
김
김 유
유
유 정
유
정
정
정
Neuroprotective Effect of Human
Mesenchymal Stem Cell on Dopaminergic
Neurons by Anti-inflammatory Action
by
You Joung Kim
A Dissertation Submitted to the Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
MASTER OF SCIENCES
Supervised by
Phil Hyu Lee, M.D., Ph.D
Department of Medical Sciences
The Graduate School, Ajou University
김유정의
김유정의
김유정의
김유정의 이학
이학
이학
이학 석사학위
석사학위
석사학위
석사학위 논문을
논문을 인준함
논문을
논문을
인준함
인준함
인준함
.
.
.
.
심사위원장
심사위원장
심사위원장
심사위원장
이
이 필
이
이
필 휴
필
필
휴
휴 인
휴
인
인
인
심 사 위 원
심 사 위 원
심 사 위 원
심 사 위 원
안
안 영
안
안
영 환
영
영
환
환 인
환
인
인
인
심 사 위 원
심 사 위 원
심 사 위 원
심 사 위 원
이
이 광
이
이
광
광 인
광
인
인
인
아
아
아
아 주
주
주 대
주
대
대
대 학
학
학 교
학
교 대
교
교
대
대
대 학
학
학 원
학
원
원
원
2007
2007
2007
2007
년
년 12
년
년
12
12
12
월 21
월
월
월
21
21
21
일
일
일
일
-ABSTRACT-
Neuroprotective Effect of Human Mesenchymal Stem Cell on
Dopaminergic Neurons by Anti-inflammatory Action
Parkinson's disease (PD) is a common progressive neurodegenerative disorder caused by the
loss of dopaminergic neurons in the substantia nigra (SN). Numerous studies have provided
evidence suggesting that neuroinflammation plays a critical role in the pathogenesis of PD. The
present study was to evaluate effect of human bone marrow-derived mesenchymal stem cell
(hMSCs) on modulation of neuroinflammation. We used lipopolysaccharide (LPS)-induced in
vitro and in vivo inflammation models to investigate whether hMSCs have a protective effect on
the dopaminergic system through anti-inflammatory mechanism. hMSCs treatment significantly
decreased LPS-induced microglial activation and production of pro-inflammatory cytokines
compared to LPS-only treatment group. In co-culture cells of microglia and mesencephalic
dopaminergic neurons, hMSCs treatment resulted in a significant reduction of dopaminergic
neuronal loss induced by LPS stimulation. In animal study, hMSCs treatment significantly
increased survival of TH-ip cells at 7 and 14 days following hMSCS injection, approximately
two times more than the LPS-only treatment, which was clearly accompanied by a decrease in
activation of microglia. Additionally, cytokine assay in the SN showed that hMSCs treatment
significantly down regulated the LPS-induced increase in the expression of TNF-α and iNOS
mRNA following LPS stimulation. TNF-α and iNOS release were also significantly decreased
following hMSCS injection.
The present study demonstrated that MSCs have a neuroprotective effect on dopaminergic
neurons via an anti-inflammatory mechanism mediated by the modulation of microglial
activation. Along with various trophic effect and transdifferentiational potency,
anti-inflammatory mechanism of MSCs could have major therapeutic implication in the treatment of
PD.
Key words: Human mesenchymal stem cell, anti-inflammation, dopaminergic neurons,
TABLE OF CONTENTS
ABSTRACT ···ⅰ
TABLE OF CONTENTS ···ⅱ
LIST OF FIGURES ···ⅳ
Ⅰ. INTRODUCTION ···1
Ⅱ. MATERIALS AND METHODS ···3
A. MATERIALS ···3
B. METHODS ···4
1. Isolation of hMSCs and culture maintenance ···4
2. Co-culture of LPS treated enriched-microglia or neuron-glia culture with transwell hMSCs insert ···4
3. Microglia enriched cultures ···5
4. Co-cultures of microglia and mesencephalic neuron cultures ···5
5. Determination of NO release ·······6
6. Animals study ···6
7. Reverse transcription PCR (RT-PCR) ···7
8. Quantification of TNF-α release ···8
9. Tissue preparation ···8
10. Immunocytochemistry and immunohistochemistry ···9
11. Stereological cell counts ···9
Ⅲ. RESULTS ···11
1. hMSCs decreased LPS-induced microglial activation ···11
2. hMSCs treatment significantly decreased LPS-induced expression of inflammatory cytokine ···11
3. hMSCs treatment significantly reduced dopaminergic neuronal death induced by LPS stimulation in mesencephalic tissue and microglia co-cultured system ···12
4. hMSCs treatment significantly decreased dopaminergic neuronal loss and microglia activation induced by LPS stimulation in the SN ···12
5. hMSCs reduced the protein expression of TNF-α release induced by LPS stimulation ···13
Ⅳ. DISCUSSION ···23
REFERENCES ···27
LIST OF FIGURES
Fig. 1. The change of microglia morphology in vitro by LPS and hMSCs ······14
Fig. 2. Effect of hMSCs co-culture on LPS induced production of pro-inflammatory factors
and their gene expression in microglial ···15
Fig. 3. Neuroprotective effects of hMSCs on LPS-induced neurotoxicity on mesencephalic
neuron cultures ···17
Fig. 4. Morphological evidence of the protective effect of hMSCs against LPS induce damage
to dopaminergic neurons in the SN ···19
Fig. 5. Analysis of TNF-α and iNOS gene expression was conducted via RT-PCR ······21
. INTRODUCTION
Ⅰ
Ⅰ
Ⅰ
Ⅰ
Parkinson’s disease (PD) is a chronic neurodegenerative disease characterized by selective
loss of dopaminergic neurons and the presence of Lewy bodies, proteinaceous inclusions that
contain α-synuclein, synphilin-1, components of the ubiquitin proteasomal pathway, and parkin in the substantia nigra (SN) (Moore et al. 2005). Recent advances have revealed that the
pathogenesis of neuronal degeneration in PD may involve several molecular and cellular events
including oxidative stress, accumulation of toxic proteins resulting from dysfunction of the
protein degradation system, proapoptotic mechanisms, and mitochondrial dysfunction (Moore et
al. 2005; von Bohlen und Halbach et al. 2004). However, the relationship between these
biochemical changes and their temporal context remain unresolved issues.
According to recent human and animal studies, a glial reaction and inflammatory processes
may participate in the cascade of neuronal degeneration in PD. A post-mortem study described
extensive proliferation of reactive amoeboid microglia the SN of PD patients (McGeer et al.
1988), indicating that activated microglia may lead to dopaminergic neurodegeneration.
Additional pathological study demonstrated the conspicuous presence of activated microglia in
the SN of PD patients who were exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
(MPTP) (Langston et al. 1999), suggesting an ongoing stimulus could lead to disease
progression long after the initial toxic insult.
A positron emission tomography study using radiotracer for activated microglia revealed that
microglial activation occurs in patients with early PD and is closely linked to the degree of
as tumor necrosis factor (TNF)-α, interleukin-1β, and interferon-γ have been demonstrated in the SN of PD patients (Boka et al. 1994; Hunot et al. 1999; Nagatsu et al. 2000). The evidence
of inflammation in the dopaminergic neuronal death have also been documented in animal
models of PD created by numerous neurotoxins such as MPTP, 6-hydroxydopamine, or
rotenone (Cicchetti et al. 2002; Gao et al. 2002; Liberatore et al. 1999).
Human bone marrow-derived Mesenchymal stem cells (hMSCs) are present in adult bone
marrow and represent <0.01% of all nucleated bone marrow cells. hMSCs are themselves
capable of multipotency, with differentiation under appropriate conditions into chondrocytes,
skeletal myocytes, and neurons (Makino et al. 1999; Pittenger et al. 1999; Woodbury et al.
2000). Recent reports showed that the neuroprotective effect of hMSCs may be mediated not
only by their differentiation into neuron-like cells, but also by their ability to produce various
trophic factors that may contribute to functional recovery, neuronal cell survival, and
stimulation of endogenous regeneration (Barry and Murphy 2004). Of these, anti-inflammation
role of hMSCs transplantation as a protective mechanism has been demonstrated in vitro and in
animal model of ischemic heart disease and experimental autoimmune encephalomyelitis
(Gerdoni et al. 2007; Guo et al. 2007; Zappia et al. 2005).
Although few studies reported the effect of hMSCs transplantation in animal model of PD,
the application of hMSCs in neurodegenerative diseases with the viewpoint of neuroprotective
effect through anti-inflammatory action has not been studied. In the present study, we used
lipopolysaccharide (LPS)-induced in vitro and in vivo inflammation models to investigate
whether hMSCs have a protective effect on the dopaminergic system through anti-inflammatory
. MATERIALS AND METHODS
Ⅱ
Ⅱ
Ⅱ
Ⅱ
A. MATERIALS
Human bone marrow-derived mesenchymal stem cells (hBM-MSCs; FCB-Pgarmicell co.Ltd,
Sungnam, Korea) were culture-expanded from the bone marrow aspirate from the persons who
agree to use his cells for research purpose. We obtained written informed consent from all
persons who provide their BM aspirates according to the guideline of institutional Review board
(AJIRB-04015) and Korea Food and Drug administration (KFDA-CEPT Approval No.37).
For this experiments, Low glucose Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BLR, Grand Island, NY), minimum essential medium (MEM; Sigma, St Louis, MO, USA),
trypsine-EDTA (Gibco BLR, NY), fetal bovine serum (FBS; Gibco BLR, NY),
penicillin/streptomycin (Gibco BLR, NY), Cell culture dish (Corning), and LPS(Sigma).
Antibodies and dilutions used in this include: mouse anti-TH (1:2000 dilution for brain tissue;
1:7500 dilution for cell culture, Pel-freez, USA), OX-42 (1:200, Seratec, Raleight, NC, USA)
B. METHODS
1. Isolation of hMSCs and culture maintenance
We obtained written informed consent from all the persons who agree to use his cells for
research purpose. Their bone marrow aspirates were prepared and mononuclear cells were
isolated by Ficoll density centrifugation. Mononuclear cells were placed in a 10cm dishes and
were cultivated in low-glucose DMEM containing 10% FBS and 1% penicillin/streptomycin in
a humidified incubator at 37 °C under 5% CO2. Medium containing non-adherent cells were
then replaced every 4 days of culture. When dish was reached 70-80% confluence, the cells
were trypsinized and subcultured. Culture expanded hMSCs were provided for these trials. At
passage 6 of hMSCs, cells were injected by tail vain into the rat (1x106 cells/ 1ml PBS) and
co-culturing (3x105 cells/well, using transwell).
2. Co-culture of LPS treated enriched-microglia or neuron-glia culture with transwell
hMSCs insert
Cultured hMSCs (3x105 cells/well) and DMEM containing 10% FBS were maintained in the
bottom side of an 0.4 µm pore size Costar transwell (Cirnong, NY 14831) insert were incubated in a humidified incubator at 37 °C and 5% CO2 for 24 hr. Both enriched-microglia and
neuron-glia culture, after one day seeding of microneuron-glia in plate, those were treated with LPS for 4 hr to
induced inflammation. And then each transwell with cultured hMSCs (3x105 cells/well) or
DMEM, was dipped in the basal plate of LPS treated culture system. The cells and supernatants
3. Microglia enriched cultures
Microglia was cultured from the cerebral cortices of a day old Sprague–Dawley (SD) rats
(Chung et al. 2001; Ryu et al. 2000). The cortices were rinsed twice in minimum essential
medium (MEM; Sigma, St Louis, MO, USA) containing 10% fetal bovine serum and
mechanically triturated. The dissociated cells were plated with 75-cm2 T-flasks. After 13 to 15
days, the microglia were detached from the flasks, applied to a nylon mesh to remove astrocytes,
and seeded onto 24-well plates (1×105 cells/well) or 6-well plates (1×106 cells/well). After 30
min to 1 hr, the culture medium was replaced with MEM containing 5% fetal bovine serum and
24 hr later, treated with LPS (100 ng/ml; Sigma) for 4 hr (Shytle et al. 2004) to induce
inflammation. Each transwell insert with cultured hMSCs (3x105 cells/well) or DMEM, was
dipped in the basal plate of LPS treated culture system. After 6, 24, 48, 72 hr, the microglia was
then used in immunocytochemistry, polymerase chain reaction (PCR), nitric oxide (NO) assay,
and enzyme-linked immunosorbent assay (ELISA).
4. Co-cultures of microglia and mesencephalic neuron cultures
Dopaminergic neurons were cultured from the SN of 14-day embryonic SD rats (Chung et al.
2001; Han et al. 2003). The tissue was incubated in Ca2+-, Mg2+- free Hanks’ balanced salt
solution (CMF–HBSS) for 10 min and a 0.01% trypsin solution in CMF–HBSS for 9 min at
37 °C. The cultures were rinsed twice in RF (Dulbecco’s modified Eagle’s medium
supplemented with 10% fetal bovine serum, 6 mg/ml glucose, 204 µg/ml L-glutamine, and 100 U/ml penicillin/streptomycin (P/S)) for trypsin inhibition and then dissociated into single cells
culture slides pre-coated with 0.1 mg/ml poly-D-lysin and 4 µg/ml laminin, and seeded in 24-well culture plates at a density of 1×105 cells/coverslip or slide. The cells were incubated in a
humidified incubator at 37 °C and 5% CO2 for 24 hr. In two-day-old in vitro cultures (DIV), the
culture medium was replaced with chemically defined serum-free medium composed of Ham’s
nutrient mixture (F12–DMEM) and with 1% ITS (insulin, transferring, selenium), glucose,
L-glutamine, and P/S. In DIV 3, the culture was co-cultured with 1×105 cortical microglia/well
(Kim et al. 2000; Lee et al. 2005; Liu et al. 2000), and in DIV 4, and 24 hr later, treated with
LPS (100 ng/ml; Sigma) for 4 hr to induce inflammation. Each transwell insert with cultured
hMSCs (3x105 cells/well) or DMEM, was dipped in the basal plate of LPS treated culture
system. The cells were then used in immunocytochemistry at 6, 24, 48 and 72 hr later.
5. Determination of NO release
The amount of NO in a conditioned microglia medium was determined at 24, 48 and 72 hr
following hMSCs or vehicle co-culturing. The amount of nitrite converted from NO (Chung et
al. 2001; Lee et al. 2005; Yang et al. 2002) was measured by mixing the culture medium (50 µl)
with an equal volume of Griess reagent (0.1% naphthlethylene diamine, 1% sulfanilamide, and
2.5% H3PO4). The optical density was measured at 540 nm.
6. Animals study
Male Sprague-Dawley rats (weighing 220-240g) were allowed to acclimate for used 3 days
before the experiments. All of the experiments were conducted using male rats (weighing
apparatus. For injection of LPS (5 µg/µl; 3 µl) into the left SN, the following coordinates were used: 5.3 mm posterior, 2.3 mm lateral, 7.7 mm ventral from the bregma, and injected at a rate
of 3 µl/5min using a 26-gauge Hamolton syringe attached to an automated microinjector (Paxinos & Watson, 1998). The needle was then left in place for an additional 10 min before
slow retraction. The control group was injected 1M PBS into same position and method with
LPS injection group. And at 8 hr following LPS stimulation, hMSCs (1x106 cells/ 1ml PBS) or
vehicle was injected by tail vain into the rat. At 4 hr, 3 day, 7 day and 14 day following hMSCs
injection, the rats were sacrificed. For ELISA and RNA extraction, the SN area was rapidly
removed from the brains and frozen at –70 °C.
7. Reverse transcription PCR (RT-PCR)
Total RNA was extracted from dissected tissue using TRIzol (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer’s instructions and quantified spectrophotometrically.
Reverse transcription (RT) was performed using Superscript reverse transcription (Invitrogen),
with total RNA (2 µg) as the template for the reaction. The PCR reaction was performed using 10 pmol each of 5’ and 3’ set of primers for TNF-α (sense 5’-GTA GCC CAC GTC GTA GCA AA-3’ antisense 5’-CCC TTCTCC AGC TGG GAG AC-3’), iNOS (sense 5’-GCA GAA TGT
GAC CAT CAT GG-3’ antisense 5’-ACA ACC TTG GTG TTG AAG GC-3’), GAPDH (sense
5’-TCC CTC AAG ATT GTC AGC AA-3’ antisense 5’-AGA TCC ACA ACG GAT ACA TT-3’).
After an initial denaturation at 94 °C for 5 min, 30 cycles of PCR were performed consisting of
denaturation (30 sec, 95 °C), annealing (1 min; 56 °C (TNF-α, GAPDH), 52 °C (iNOS)), extension (1 min, 72 °C); and final extension (10 min, 72 °C). The PCR product was separated
by electrophoresis on a 2% agarose gel, stained with ethidium bromide. Gels were detected
under UV light.
8. Quantification of TNF-α release
Brain tissues from the ipsilateral SN were dissected and homogenized in MEM containing 1%
P/S with sea sand. The tissue homogenates was centrifuged for 20 min at 15,000 rpm (4 °C) and
the supernatant was stored in fresh tubes. And collected cell culture supernatants were prepared.
The release of TNF-α into the culture supernatant and the supernatant of tissue homogenates were determined by ELISA (Pyo et al. 1998). The supernatants (50 µl) were placed on an ELISA kit (Biosource, Camarillo, CA, USA) and a sandwich ELISA was performed according
to the manufacturer’s instructions. The optical density was measured at 450 nm.
9. Tissue preparation
For immunohistochemistry, the animals, anesthetized with 10% chloral hydrate, were
perfused with a saline solution containing 0.5% sodium nitrate and heparin (10 U/ml) and fixed
with 4% paraformaldehyde dissolved in 0.1M PB (both at almost 200 ml/rat) at 4 hr, 3 day, 7
day and 14 day following hMSCs injection. The brain was removed from the skull, post-fixed
for 3 days in buffered 4% paraformaldehyde at 4 °C and stored in a 30% sucrose solution for 1
to 2 days at 4 °C until they sank. They were then sectioned on a sliding microtome to obtain a
50-µm-thick coronal section. All sections were stored in tissue stock solution (30% glycerol, 30% etholene glycol, 30% 3rd D.W., 10% 0.2M PB (pH 7.2) at 4 °C until required.
10. Immunocytochemistry and immunohistochemistry
The brain sections and co-cultured cells were rinsed twice in PBS and incubated in 0.2%
Triton X-100 for 30 min at room temperature. They were rinsed thrice with 0.5% bovine serum
albumin (BSA) in 1x PBS for blocking. After blocking, they were incubated overnight at 4 ◦C with primary antibodies; the primary antibodies for OX-42 and tyrosine hydroxylase (TH) were
OX-42 (1:200 for immunohistochemistry, 1:500 for immunocytochemistry; Serotec, Raleigh,
NC, USA), TH (1:2000 for immunohistochemistry, 1:7500 for immunocytochemistry; Pel-freez,
St. Rogers, AR, USA). After 16 hr, the cultures and brain sections were rinsed thrice in 0.5%
BSA in 1x PBS (10 min each rinse) and incubated with appropriate biotinylated secondary
antibody and avidin–biotin complex (Elite Kit; Vector Laboratories, Burlingame, CA, USA) for
1 hr at RT. The bound antiserum was visualized by incubating with 0.05% diaminobenzidine–
HCL (DAB) and 0.003% hydrogen peroxide in 0.1 M PB. The cultures and brain sections were
rinsed with 0.1 M PB for DAB inhibition. The immunostained cells were analyzed under
bright-field microscopy.
11. Stereological cell counts
The unbiased stereological estimation of the total number of the TH-immunopositive (TH-ip)
and activated microglial cells in the SN was made using the optical fractionator, as previously
described in detail with some modifications (Kirik et al. 1998). This sampling technique is not
affected by tissue volume changes and does not require reference volume determinations (West
et al. 1991). The sections used for counting covered the entire SN, from the rostral tip of the
in a series. Sampling was performed using the Olympus C.A.S.T.-Grid system (Olympus
Denmark A/S, Denmark), using an Olympus BX51 microscope, connected to the stage and
feeding the computer with the distance information in the Z-axis. SN was delineated at 1.25×
objective. A counting frame (60%, 35,650µm2) was placed randomly on the first counting area and systemically moved though all counting areas until the entire delineated area was sampled.
Actual counting was performed using a 40× oil objective. Guard volume (4µm from the top 4-6µm from the bottom of the section) were excluded from both surfaces to avoid the problem of lost cap, and only the profiles that came into focus within the counting volume (with depth of
10µm) were counted. The total of TH-ip cells was calculated according to the optical fractionator formula for more details, see (West et al. 1991).
12. Statistical analysis
The mean of the groups were compared using the Mann–Whitney U-test for pairs and the
Kruskal–Wallis analysis for multiple comparisons. The statistical significance was indicated
when p < 0.05. Statistical analyses were performed using commerciallyavailable software
. RESULTS
Ⅲ
Ⅲ
Ⅲ
Ⅲ
1. hMSCs decreased LPS-induced microglial activation
We analyzed the morphological change of microglia using OX-42 in order to examine the
effect of hMSCs on microglial activation. With the introduction of LPS, the morphology of
microglia drastically changed from spherical shape to process-bearing cells at 6 and 24 hr after
co-culture with vehicle. However, co-culture with hMSCs (3 × 105/well, using transwell) after
LPS treatment significantly decreased the number of process-bearing, activated microglia at 6
and 24 hr after co-culture with hMSCs (Fig. 1 A and B).
2. hMSCs treatment significantly decreased LPS-induced expression of inflammatory
cytokine
To investigate the effect of hMSCs co-culture on LPS-induced production of inflammatory
cytokines and their gene expression in microglial, microglia enriched cultures were treated with
LPS for 4 hr and then co-cultured with vehicle or hMSCs using transwell. After 6, 24, 48 and 72
hr, culture supernatants and cells were collected for RT-PCR, nitrite assay and TNF-α ELISA. LPS treatment significantly induced the mRNA expression of TNF-α and iNOS, accumulation of nitrite compared with control group, whereas co-culture with hMSCs showed significant
reduction of TNF-α and iNOS mRNA expression and amount of nitrite when compared to those treated only with LPS at 24, 48, and 72 hr (Fig. 2 A - C). And co-culture of hMSCs responsively
inhibited the LPS induced increase in TNF-α protein levels, the levels of TNF-α were measured by ELISA. As shown in Fig. 3 D, at 24 hr after injection hMSCs, the production of TNF-α was
dramatically increased by LPS alone injection and the level gap was maintained until 72 hr. The
stimulated levels of TNF-α were significantly reduced in injection of hMSCs group.
3. hMSCs treatment significantly reduced dopaminergic neuronal death induced by LPS
stimulation in mesencephalic tissue and microglia co-cultured system
Co-cultures of microglia and mesencephalic neurons were treated with LPS for 4 hr and then
co-cultured with vehicle or hMSCs to determine the effect of hMSCs-induced anti-microglial
activation on dopaminergic neurons. After 6 or 24 hr, cultures were immunostained with
anti-TH antibody. As shown in Fig. 3, the LPS treatment resulted in a significant loss of anti-TH-ip cells,
while there was significantly decreased the loss of TH-ip cells in cultures that were treated with
hMSCs (Fig. 3 A). A cell counting analysis showed that co-culture with hMSCs resulted in an
incremental survival of TH-ip cells at 6 and 24 hr following hMSCs treatment (Fig. 3 B).
4. hMSCs treatment significantly decreased dopaminergic neuronal loss and microglial
activation induced by LPS stimulation in the SN
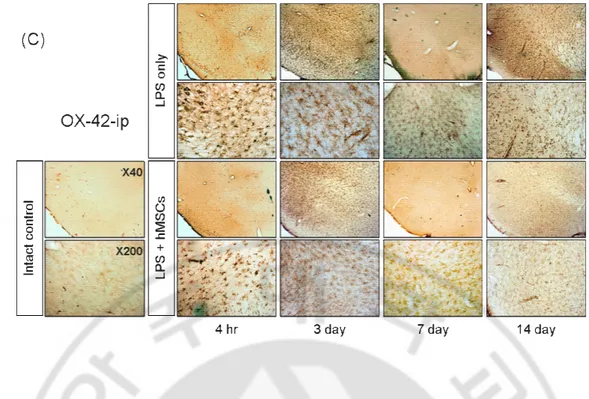
In animal study, hMSCs was infused via tail vein 8 hr after the injection of LPS to examine
the effect of hMSCs on LPS-induced microglial activation and dopaminergic neuronal death in
rat SN. LPS stimulation in the SN resulted in a considerable loss of TH-ip cells with a
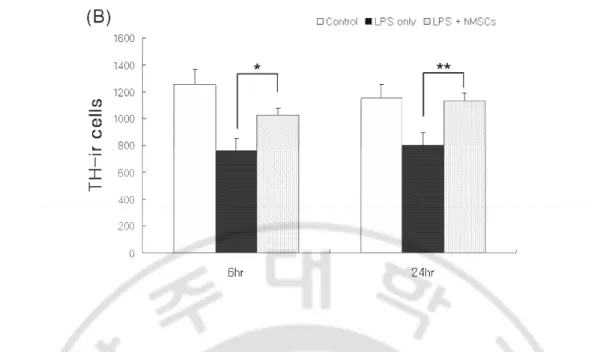
concomitant activation of microglia (Fig. 4 A and C). hMSCs treatment showed that the loss of
TH-ip cells induced by LPS stimulation in the SN was considerably reduced (Fig. 4 A). As well,
hMSCs treatment was clearly accompanied by attenuation of microglial activation, which was
increased survival of TH-ip cells at 7 and 14 days following hMSCs injection, approximately
two times more than the LPS-only treatment (Fig. 4 B). Additionally, cytokine assay in the SN
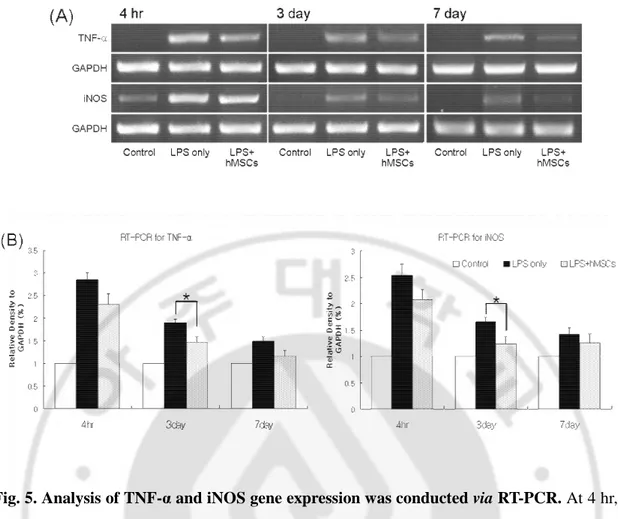
showed that hMSCs treatment significantly down regulated the LPS-induced increase in the
expression of TNF-α and iNOS mRNA at 3 day following LPS stimulation (Fig. 5 A). TNF-α and iNOS release were significantly decreased in the hMSCs group when compared to the
LPS-only treatment group at 4 hr and 3 day following hMSCs injection (Fig. 5 B).
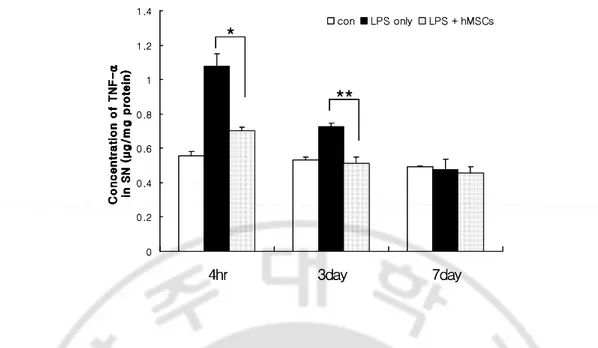
5. hMSCs reduced the protein expression of TNF-α release induced by LPS stimulation
Injection of hMSCs responsively inhibited the LPS induced increase in TNF-α protein levels, the levels of TNF-α were measured by ELISA. As shown in Fig. 6, at 4 hr after injection hMSCs, the production of TNF-α was dramatically increased by LPS alone injection. The stimulated levels of TNF-α were significantly reduced in injection of hMSCs group. In 7 day after injection of vehicle or hMSCs, the amount of TNF-α present in the SN was reduced to levels comparable to the level of the control.
Fig. 1. hMSCs inhibited LPS-induced microglial activation. Rat microglia-enriched cultures
1 day after plating were treated with the hMSCs for 6 and 24 hr with 100 ng/ml LPS. The
microglia were immunostained with OX-42 antibody. The morphology of microglia
dramatically changed from spherical shape to process-bearing cells at 6 and 24 hr after
co-culture with vehicle. However, co-co-culture with hMSCs after LPS treatment significantly
decreased the number of process-bearing, activated microglia at 6 and 24 hr after co-culture
with hMSCs (A and B). The data are displayed as the mean (column) ± SEM (bar) values. The
results are representative of three to five in each group. **P < 0.01. ***P < 0.001. Scale bar, 100
0 10 20 30 40 N it ri te ( μ M ) 48hr 24hr 72hr ** * ** (C) 0 0.2 0.4 0.6 0.8 1 1.2 1.4 6hr 24hr 48hr 72hr C o n c e n tr a ti o n o f T N F -α (μ g /m g p ro te in ) Control LPS only LPS + hMSCs * * * (D) 0 10 20 30 40 N it ri te ( μ M ) 48hr 24hr 72hr ** * ** (C) 0 10 20 30 40 N it ri te ( μ M ) 48hr 24hr 72hr ** * ** 0 10 20 30 40 N it ri te ( μ M ) 48hr 24hr 72hr ** ** ** ** ** (C) 0 0.2 0.4 0.6 0.8 1 1.2 1.4 6hr 24hr 48hr 72hr C o n c e n tr a ti o n o f T N F -α (μ g /m g p ro te in ) Control LPS only LPS + hMSCs * * * (D) 0 0.2 0.4 0.6 0.8 1 1.2 1.4 6hr 24hr 48hr 72hr C o n c e n tr a ti o n o f T N F -α (μ g /m g p ro te in ) Control LPS only LPS + hMSCs * * * 0 0.2 0.4 0.6 0.8 1 1.2 1.4 6hr 24hr 48hr 72hr C o n c e n tr a ti o n o f T N F -α (μ g /m g p ro te in ) Control LPS only LPS + hMSCs ** ** ** (D)
Fig. 2. Effect of hMSCs co-culture on LPS induced production of proinflammatory factors
and their gene expression in microglial. Microglia en-riched cultures treated with LPS for 4 hr
and then co-cultured with vehicle or hMSCs (3x105 cells) using transwell. After 6, 24, 48 and 72
hr, culture supernatants and cells were collected for RT-PCR, nitrite assay and TNF-α ELISA. LPS treatment induced the mRNA expression of TNF-α and iNOS and accumulation of nitrite compared with control group, whereas hMSCs co-culture group was significantly reduced the
LPS-induced increase in the expression of TNF-α and iNOS mRNA levels (A and B) and amount of nitrite (C). And the stimulated levels of TNF-α were significantly reduced in hMSCs co-cultured group. The data are displayed as the mean (column) ± SEM (bar) values. The results
Fig. 3. Neuroprotective effects of hMSCs on LPS-induced neurotoxicity on mesencephalic
neuron-glia cultures. In 4-day-old in vitro cultures, the medium was replaced with a treatment
medium and treated with the vehicle or hMSCs 4 hr later LPS treatment. After 6 or 24 hr,
cultures were immunostained with anti-TH antibody. LPS treatment led to a loss of TH-
immunopositive (TH-ip) neurons and the TH-ip neurons were unhealthy, with dramatically
shortened and damaged neuritis. The hMSCs treatment blocked LPS-induced morphological
changes of mesencephalic dopaminergic neurons. The data are displayed as the mean (column)
± SEM (bar) values. The results are representative of three to five in each group. *P < 0.05. **P
Fig. 4. Morphological evidence of the protective effect of hMSCs against LPS induced
damage to dopaminergic neurons in the SN. LPS injection (5 µg/3 µl) in the SN and 8hr later,
vehicle or hMSCs (1x106 cells) were injected to tail vain. On 4hr, 3day, 7day and 14 day, rats
were transcardially perfused by 4% paraformaldehyde. Frozen sections (50 µm in thickness) were cut and TH was detected by immunohistochemical staining to show dopaminergic neurons
(A) and OX-42 was detected to show microglial activation (C) in the SN. The stereological
estimation of the total number of TH-ip cells in the SN using the optical fractionator
demonstrated that hMSCs injection significantly decreased LPS-induced dopaminergic neuron
death (B) and decreased activation of microglia compared with the LPS only treatment group
(C). The data are displayed as the mean (column) ± SEM (bar) values. The results are
Fig. 5. Analysis of TNF-α and iNOS gene expression was conducted via RT-PCR. At 4 hr, 3
day and 7 day after vehicle or hMSCs injection, rats were decapitated and SN was dissected and
total RNA (2 µg) was extracted from dissected tissue using TRIzol. LPS elicited an increase in the mRNA expression of TNF-α and iNOS, whereas hMSCs injection group was significantly down regulated the LPS-induced increase in the expression of TNF-α and iNOS mRNA levels. The data are displayed as the mean (column) ± SEM (bar) values. The results are representative
0 0.2 0.4 0.6 0.8 1 1.2 1.4 4hr 3day 7day C o n c e n tr a ti o n o f T N F -α C o n c e n tr a ti o n o f T N F -α C o n c e n tr a ti o n o f T N F -α C o n c e n tr a ti o n o f T N F -α in S N ( μ g / m g p ro te in ) in S N ( μ g / m g p ro te in ) in S N ( μ g / m g p ro te in ) in S N ( μ g / m g p ro te in ) con LPS only LPS + hMSCs * ** 0 0.2 0.4 0.6 0.8 1 1.2 1.4 4hr 3day 7day C o n c e n tr a ti o n o f T N F -α C o n c e n tr a ti o n o f T N F -α C o n c e n tr a ti o n o f T N F -α C o n c e n tr a ti o n o f T N F -α in S N ( μ g / m g p ro te in ) in S N ( μ g / m g p ro te in ) in S N ( μ g / m g p ro te in ) in S N ( μ g / m g p ro te in ) con LPS only LPS + hMSCs * * ** **
Fig. 6. The effect of hMSCs treatment on the TNF-α release induced by LPS stimulation.
At 4 hr, 3 day and 7 day after vehicle or hMSCs injection, rats were decapitated and SN was
dissected. The concentration of TNF-α was detected by commercial ELISA kit. A significant decrease in TNF-α occurs at 4 hr and 3 day. The data are displayed as the mean (column) ± SEM (bar) values. The results are representative of three to five rats in each group. *P < 0.05,
.
Ⅳ
Ⅳ
Ⅳ
Ⅳ DISCUSSION
The present study demonstrated that hMSCs has a protective effect on dopaminergic neurons
through anti-inflammatory actions. First, we demonstrated that in microglia and mesencephalic
neuron co-cultures, hMSCs treatment prevented dopaminergic neuronal death by reducing
LPS-induced release of the pro-inflammatory cytokines. Second, we confirmed that in rats, hMSCs
injection significantly reduced LPS- induced dopaminergic neuronal loss in the SN.
Besides regenerative capacity of hMSCs, it has been known that hMSCs also possess
immunoregulatory properties. Although the exact mechanism responsible for hMSC-mediated
immunoregulation is not fully understood, in vitro studies suggested that hMSCs can not only
inhibit nearly all cells participating in the immune response cell-cell contact-dependant
mechanism, but also release a variety of soluble factors, which may be implicated in the
immunosuppressive activity of hMSCs (Karussis et al. 2007; Krampera et al. 2006; Nauta and
Fibbe 2007). Recent animal studies in a model of experimental autoimmune encephalomyelitis
reported that hMSCs treatment showed a significantly milder disease and fewer relapses
compared with control mice, with decreased number of inflammatory infiltrates, reduced
demyelination, and axonal loss (Gerdoni et al. 2007; Zappia et al. 2005). Additionally, Guo et al.
(Gerdoni et al. 2007) reported that MSC transplantation decreased protein production and gene
expression of inflammation cytokines as well as increased functional recovery from myocardial
infarct. These studies suggest that anti-inflammation action of hMSCs might be one of
underlying mechanisms for the tissue protective effect.
dopaminergic neuronal loss induced by LPS stimulation. In co-cultures of microglia and
mesencephalic neurons, these anti-inflammatory actions of hMSCs actually led to a significant
decrease (up to ~40%) in dopaminergic neuronal death induced by LPS stimulation.
Furthermore, hMSCs administration dramatically decreased the dopaminergic neuronal loss in
the SN induced by LPS stimulation, which was clearly accompanied by attenuation of
microglial activation and a reduction in the formation of TNF-α and iNOS. In addition to a variety of pleiotrophic mechanisms of hMSCs as a trophic mediator (Caplan and Dennis 2006),
our present data suggest that the neuroprotective property of hMSCs through its
anti-inflammatory behavior also works in animal model of PD.
A large body of experimental evidence indicates that inhibition of the inflammatory response
can prevent degeneration of nigrostriatal dopaminergic neurons. For example, sodium salicylate,
COX-2 inhibitor, or minocycline have been shown to significantly reduce striatal dopaminergic
depletion and dopaminergic neuronal loss induced by MPTP or LPS-models (Aubin et al. 1998;
Du et al. 2001; He et al. 2001). A large cohort study of patients has shown that the risk of
developing PD in regular nonsteroidal anti-inflammatory drugs (NSAID) users was decreased
by up to 45% compared with those who take NSAIDs on a non-regular basis and higher
exposure to NSAID demonstrates a trend toward a greater benefit (Chen et al. 2003), supporting
the neuroprotective effects of NSAID in the development or progression of PD. A very recent
epidemiological study also supports that NSAIDs are protective against PD, with a particularly
strong protective effect evident among regular nonaspirin NSAID users (Wahner et al. 2007).
Therefore, the evidence demonstrating the neuroprotective effect of anti-inflammatory agents on
revitalized interest in identifying inhibition of inflammation as a possible strategy in the
treatment of PD.
Recent studies indicate that human hMSCs can be induced to differentiate into neuron-like
cells (Mareschi et al. 2006; Pittenger et al. 1999; Woodbury et al. 2000). Additionally, hMSCs
express an expression of several specific neuronal markers and transcriptional factors, of which
a large proportion of the genes was participating in the neuro-dopaminergic system, suggesting
that expression of neural gene as well as gene associated with the dopaminergic system is a
widespread phenomenon of hMSCs (Blondheim et al. 2006). There have been a few reports
about application of hMSCs in animal model of PD. Li et al. (Li et al. 2001) and Blondheim et
al. (Blondheim et al. 2006) reported that using MPTP and 6-hydroxydopamine-treated PD
models, respectively, hMSCs injected intrastriatally exhibited the phenotype of dopaminergic
neurons. Along with possible transdifferention potency of hMSCs into dopaminergic phenotype,
neuroprotective property of hMSCs on dopaminergic neurons through anti-inflammatory actions
may raise the possibility of clinical application of hMSCs as a possible strategy in the treatment
of PD. In addition to the molecular and cellular benefits of hMSCs, cell therapy with hMSCs
has an advantage in clinical applications. hMSCs can be easily harvested from self bone marrow,
cultured in vitro, and administered to patients via various roots including intravenous,
intraarterial, intrathecal, or intralesional infusion. In contrast with embryonic stem cell therapy,
there is no immunological rejection, and cell therapy with hMSCs is free from ethical issues.
Importantly, regarding the safety of hMSCs application, our group recently documented that cell
therapy with hMSCs in patients with multiple system atrophy and ischemic stroke is feasible
In summary, the present study demonstrated that hMSCs have a neuroprotective effect on
dopaminergic neurons via an anti-inflammatory mechanism mediated by the modulation of
microglial activation. Along with various trophic effect and transdifferentiational potency,
anti-inflammatory mechanism of hMSCs could have major therapeutic implication in the treatment
REFERENCE
1. Aubin N, Curet O, Deffois A, and Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. Journal of neurochemistry 71: 1635-1642, 1998
2. Bang OY, Lee JS, Lee PH, and Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of neurology 57: 874-882, 2005
3. Barry FP, and Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. The international journal of biochemistry & cell biology 36: 568-584, 2004
4. Blondheim NR, Levy YS, Ben-Zur T, Burshtein A, Cherlow T, Kan I, Barzilai R, Bahat-Stromza M, Barhum Y, Bulvik S, Melamed E, and Offen D. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem cells and development 15: 141-164, 2006
5. Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, and Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neuroscience
letters 172: 151-154, 1994
6. Caplan AI, and Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular
biochemistry 98: 1076-1084, 2006
7. Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, and Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease.
Archives of neurology 60: 1059-1064, 2003
8. Chung ES, Joe EH, Ryu JK, Kim J, Lee YB, Cho KG, Oh YJ, Maeng SH, Baik HH, Kim SU, and Jin BK. GT1b ganglioside induces death of dopaminergic neurons in rat mesencephalic cultures. Neuroreport 12: 611-614, 2001
9. Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, and Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. The European journal of
neuroscience 15: 991-998, 2002
10. Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, and Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease.
Proceedings of the National Academy of Sciences of the United States of America 98:
14669-14674, 2001
11. Gao HM, Hong JS, Zhang W, and Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22: 782-790, 2002
12. Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, and Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Annals of
neurology 61: 219-227, 2007
13. Guo J, Lin GS, Bao CY, Hu ZM, and Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation 30: 97-104, 2007
14. Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, and Oh YJ. Caspasedependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci 23: 5069-5078, 2003
15. He Y, Appel S, and Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain research 909: 187-193, 2001
16. Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debre P, Agid Y, Dugas B, and Hirsch EC. FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci 19: 3440-3447, 1999
17. Karussis D, Kassis I, Basan GS, and Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): A proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 2007
18. Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, and Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia.
J Neurosci 20: 6309-6316, 2000
19. Kirik D, Rosenblad C, and Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Experimental neurology 152: 259-277, 1998
20. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, and Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Current opinion in
pharmacology 6: 435-441, 2006
21. Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, and Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Annals of neurology 46: 598-605, 1999
22. Lee DY, Oh YJ, and Jin BK. Thrombin-activated microglia contribute to death of dopaminergic neurons in rat mesencephalic cultures: dual roles of mitogen-activated protein kinase signaling pathways. Glia 51: 98-110, 2005
23. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, and Huh K. Autologous Mesenchymal Stem Cell Therapy Delays the Progression of Neurological Deficits in Patients With Multiple System Atrophy. Clin Pharmacol Ther 2007
24. Li Y, Chen J, Wang L, Zhang L, Lu M, and Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience letters 316: 67-70, 2001
25. Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, and Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nature
medicine 5: 1403-1409, 1999
26. Liu B, Jiang JW, Wilson BC, Du L, Yang SN, Wang JY, Wu GC, Cao XD, and Hong JS. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. The Journal of
pharmacology and experimental therapeutics 295: 125-132, 2000
27. Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, and Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. The Journal of clinical investigation 103: 697-705, 1999
28. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, Medico E, Madon E, Vercelli A, and Fagioli F. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Experimental
hematology 34: 1563-1572, 2006
29. McGeer PL, Itagaki S, Boyes BE, and McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38: 1285-1291, 1988
30. Moore DJ, West AB, Dawson VL, and Dawson TM. Molecular pathophysiology of Parkinson's disease. Annual review of neuroscience 28: 57-87, 2005
31. Nagatsu T, Mogi M, Ichinose H, and Togari A. Cytokines in Parkinson's disease. Journal of
32. Nauta AJ, and Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells.
Blood 110: 3499-3506, 2007
33. Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, and Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Annals of
neurology 57: 168-175, 2005
34. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, and Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science (New York, NY 284: 143-147, 1999
35. Pyo H, Jou I, Jung S, Hong S, and Joe EH. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. Neuroreport 9: 871-874, 1998
36. Ryu J, Pyo H, Jou I, and Joe E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-kappa B. The Journal of
biological chemistry 275: 29955-29959, 2000
37. Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, and Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. Journal of neurochemistry 89: 337-343, 2004
38. von Bohlen und Halbach O, Schober A, and Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson's disease. Progress in neurobiology 73: 151-177, 2004
39. Wahner AD, Bronstein JM, Bordelon YM, and Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology 69: 1836-1842, 2007
40. West MJ, Slomianka L, and Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator.
41. Woodbury D, Schwarz EJ, Prockop DJ, and Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of neuroscience research 61: 364-370, 2000
42. Yang MS, Park EJ, Sohn S, Kwon HJ, Shin WH, Pyo HK, Jin B, Choi KS, Jou I, and Joe EH. Interleukin-13 and -4 induce death of activated microglia. Glia 38: 273-280, 2002
43. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, and Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755-1761, 2005
-국문요약
국문요약
국문요약
국문요약-
성체
성체
성체
성체 중간엽
중간엽
중간엽
중간엽 줄기세포의
줄기세포의
줄기세포의 항염증
줄기세포의
항염증
항염증
항염증 작용을
작용을
작용을
작용을 통한
통한
통한
통한
도파민
도파민
도파민
도파민 신경세포의
신경세포의
신경세포의
신경세포의 보호
보호
보호
보호 효과
효과
효과
효과
김 유 정 아주대학교 대학원 의학과 (지도교수: 이필휴) 파킨슨 씨 병은 흑색질 안에 도파민 신경세포의 감소가 원인이 되는 퇴행성 뇌질환이다. 이미 여러 연구에서 신경염증이 파킨슨 씨 병의 발병에 중요한 역할을 함이 증명되었다. 현 우리 실험에서는 성체 중간엽 세포가 신경염증을 완화하는 효과를 확인하였다. 우리는 lipopolysaccharide (LPS)로 실헌 동물 모델과 세포 모델을 만들어 염증을 유도한 후 성체 중간엽 줄기세포가 항염증 메커니즘을 통하여 도파민 신경세포를 보호하는 효과를 갖는지 확인하였다. 성체 중간엽 줄기세포를 처리하였을 때 LPS만 처리한 개체군과 비교하여 LPS에 의해 유도된 전구염증 사이토카인의 증가와 미세아교세포의 활성이 유의미하게 감소함을 알 수 있었다. 또한 미세아교세포와 중뇌 도파민 신경세포의 co-culture system에서 성체 중간엽 줄기세포를 처리한 경우 LPS로 인한 도파민 신경세포의 감소가 현저히 줄어듦을확인하였다. 그리고 동물 실험을 통하여 성체 중간엽 줄기세포를 주입하였을 때 성체 중간엽 줄기세포를 주입한지 7, 14일이 지난 후 TH-immunopositive (TH-ip)를 표현하는 세포가 증가함을 확인하였으며 이는 LPS 개체군에 비하여 그 양이 대략 두 배 이상이었고 흑색질에서 미세아교세포의 활성의 감소도 수반하였다. 그리고 흑색질 안의 사이토카인 정량 실험 결과, 성체 중간엽 줄기세포를 주입한 개체군은 LPS에 의해 증가된 TNF-α와 iNOS mRNA의 발현을 조절억제 되었고, 성체 중간엽 줄기세포가 주입된지 4시간과 3일 후의 개체군에서 TNF-α와 iNOS의 발현 역시 LPS만 투여된 개체군에 비교하여 유의미하게 감소됨을 관찰하였다. 본 연구는 성체 중간엽 줄기세포가 항염증 메커니즘을 통하여 미세아교세포의 활성을 조절함으로 도파민 신경세포를 보호하는 것을 확인하였고, 이는 다양한
trophic effect와 transdifferentiation 가능성과 함께 성체 중간엽 줄기세포의 항염증
메커니즘이 파킨슨 씨 병의 치료에 주요하게 사용될 수 있음을 시사한다. 핵심어 핵심어 핵심어 핵심어: 성체 중간엽 줄기세포, 항염증, 도파민 신경세포, 미세아교세포, 파킨슨씨병