저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Regulation of Astrocyte Functions by
a Parkinson
¢s Disease Gene, DJ-1
by
Dong-Joo Choi
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
Roles of Parkinson’s disease genes,
Regulation of Astrocyte Functions by
a Parkinson
¢s Disease Gene, DJ-1
by
Dong-Joo Choi
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Ph.D. of Biomedical Sciences
Supervised by
Euh-Hye Joe, Ph.D.
Major in Neuroscience
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Dong-Joo Choi is approved.
SUPERVISORY COMMITTEE
The Graduate School, Ajou University
July, 5th, 2016
i
Acknowledgment
약 6년 이란 시간 동안 남들 보다 부족한 저를 가르쳐 주시고, 기다려 주시고, 한 명의 연구자가 될 수 있도록 지도해 주신 조은혜 교수님께 진심으로 감사 인사를 드립니다. 신경과학에 대한 기초조차 없던 저를 수업과 많은 대화를 통해 지도해 주신 주일로 교수님, 박상면 교수님, 김병곤 교수님, 김은영 교수님, 이명애 교수님, 이상윤 교수님, 백은주 교수님, 강호철 교수님, 서혜영 교수님, 홍지만 교수님, 이진수 교수님 그리고 서울대에 계신 서영호 교수님께 감사 인사를 드립니다. 비록 지금 당장은 함께하지 못하지만 너무나 사랑하는 조글리아 식구들, 양명순 박사님, 경진누나, 범수형, 혜경누나, 종현이형, 인섭이, 준이, 지원이에게 감사 인사를 전하며, 현재 조글리아 식구들인, 양해걸 박사님, 신진영 박사님, 진화, 윌라에게도 감사 인사를 드립니다. 약리학교실에서 생활하는데 많은 도움을 주시는 주홍이형, 지훈누나, 수정누나, 현미, 선희, 유리, 서준, 지연, 우람, 재봉, 수진 에게도 감사 인사를 드립니다. 그 밖에도 함께 연구하고, 공부할 수 있었던, 신경과학전공, 뇌과학과 학우들에게 감사 인사를 드립니다. 마지막으로 31살이라는 늦은 나이에 다시 생물학을 하겠다는 결심을 응원해주시고, 지켜 봐 주신 부모님께 감사의 인사를 드립니다. 또한 부족한 형을 항상 응원해 준 동생에게도 감사를 전합니다. 끊임없이 배우고, 노력하고, 발전하는 신경과학을 연구하는 한 명의 연구자가 되도록 정진하겠습니다.ii - ABSTRACT -
Regulation of Astrocyte Functions by a Parkinsons’s Disease Gene,
DJ-1
Parkinson’s disease (PD) is a neurodegenerative motor disease, accompanied by
loss of dopaminergic neurons in the substantia nigra. PD genes have been studied to reveal
molecular mechanisms of onset and progression of PD. In this study, I investigated the effect
of a PD gene, DJ-1, on astrocyte function since astrocytes as the most abundant cells in the
brain play diverse roles for well-being and function of the brain. In the first part, I
investigated behavior of astrocytes in ATP-injured striatum. In response to injury, DJ-1 KO
astrocytes did not undergo proper gliosis: less increase in expression of GFAP, nestin, and
GDNF, and less hypertrophic. In addition, damage areas in DJ-1 KO brain were slowly
recovered with astrocytes and TH-positive neurites compared with WT brain. As an
underlying mechanism, I found that DJ-1 deficiency attenuateded activation of STAT3, a
transcription factor that regulates expression of GFAP and nestin. In the second part of the
study, I investigated anti-inflammatory roles of astrocytes regulated by DJ-1. DJ-1 deficient
iii
as iNOS and TNF-a. DJ-1 KO astrocytes compared with WT astrocytes less produced
anti-inflammatory prostaglandin, prostaglandin D2 (PGD2), through attenuated expression of
lipocalin-type PGD2 synthase (L-PGDS). DJ-1 regulates protein stability of Sox9, a
transcription factor that regulates L-PGDS expression. Thus, in the absence of DJ-1, Sox9
ubiquitination was increased and the protein was degraded. Taken together, these results
indicate that DJ-1 regulated numerous functions of astrocytes. Therefore, DJ-1 deficiency
may affect onset and/or progression of PD due to defects in astrocyte function in intact and
injured brain.
Keywords: Parkinson's disease; DJ-1; Astrocyte; Astrogliosis; Anti-inflammatory function;
iv
TABLE OF CONTENTS
ABSTRACT ... ii
TABLE OF CONTENTS ... iv
LIST OF FIGURES ... vii
LIST OF TABLE ... ix
ABBREVIATION ... xi.
INTRODUCTION ... 1
A. Parkinson's disease (PD) ... 1
B. Genes associated with PD ... 2
1. DJ-1 (PARK7) ... 2
2. Other genes ... 3
C. Function of astrocytes in the normal brain ... 6
D. Function of astrocytes in the injured brain ... 7
1. Reactive astrocytes : intermediate filaments (Ifs) and morphological features ... 7
2. Reactive astrocytes : function of astrogliosis in neural protection ... 8
3. Reactive astrocytes : function of astrogliosis for regeneration and repair ... 9
4. Reactive astorycytes : functin of astrogliosis for regulation of inflammation ... 11
E. Astrocyte dysfunction in neurodegenerative disease ... 12
F. Aim of this study ... 13
v
1. Ethics statement ………... 15
2. DJ-1 deficient mice ...………...………….……….. 15
3. Animal MRI ……….………... 15
4. Stereotaxic surgery and drug injection ... 16
5. Tissue preparation ... 16
6. Immunohistochemistry... 17
7. Histological quantification ... 18
8. Organotypic cortical slice cultures... 18
9. Cell culture ... 19
10. Western blot analysis... 19
11. Immunoprecipitation ... 21
12. Quantitative real-time PCR... 21
13. Reverse transcriptase PCR ... 22
14. DNA constructs... 23
15. Transfection... 23
16. Proximity ligation assay (PLA)... 23
17. Small interfering RNAs (siRNA)... 24
18. Prostaglandin D2 (PGD2) ELISA... 25
19. Inhibition of protein synthesis by cycloheximide... 25
20. Inhibition of Ubiquitination by MG132... 25
21. Measurement of nitric oxide... .25
vi
III. RESULTS ... 27
Part A. DJ-1, (PARK7), plays critical roles in astrogliosis by regulating of the pSTAT3 signaling pathway for tissue repair after brain injury... 27
1. Repair of brain injury was attenuated in DJ-1 KO mice……... 27
2. DJ-1 deficiency causes a defect of astrogliosis……… 30
3. Reduction of astrocyte derived GDNF expression and attenuated restoration of dopaminergic processes in the DJ-1 KO brain…... 41
4. DJ-1 rescued insufficient astrogliosis in DJ-1 KO brain slice cultures... .47
5. STAT3 activation (tyrosine phosphorylation of STAT3, pSTAT3) was attenuated in DJ- 1 KO slices...50
Part B. DJ-1 exerts the anti-inflammatory effects of astrocytes through stabilization of Sox9, a transcription factor that regulates lipocalin-type prostaglandin D2 synthase (L-PGDS) expression ……….………... 52
1. DJ-1 deficiency caused a defect of anti-inflammatory function of astrocytes ... 52
2. DJ-1 deficiency caused a defect of PGD2 expression by attenuation of L-PGDS in astrocytes……….……….. 57
3. DJ-1 deficiency caused a defect of Sox9 protein expression by regulation of ubiquitination in astrocytes ……….……….…………... 65
IV. DISCUSSION ... 73
V. SUMMARY AND CONCLUSION ... 85
REFERENCES ... 86
vii
LIST OF FIGURES
Figure 1. DJ-1 deficiency causes a defect of repair after brain damage ... 29
Figure 2. DJ-1 deficiency causes a defect of GFAP+ astrogliosis ... 31
Figure 3. DJ-1 deficiency causes a defect of progression of astrogliosis ... 33
Figure 4. DJ-1 deficiency causes a defect of morphology of GFAP+ reactive astrocytes .... 35
Figure 5. Normal astrocytes are not different between WT and DJ-1 KO mouse brain…… 36
Figure 6. Nestin locate in GFAP+ reactive astrocytes on penumbra from only damage near site after brain injury ………. 38
Figure 7. DJ-1 deficiency causes a defect of nestin+ astrogliosis ... 39
Figure 8. DJ-1 deficiency causes a defect of morphology of nestin+ reactive astrocytes .... 40
Figure 9. Several functional markers did not showed difference in WT and DJ-1 KO mouse after ATP injection ... 43
Figure 10. DJ-1 deficiency causes a defect of GDNF expression ... 44
Figure 11. Insufficient astrogliosis cause a defect of TH+ processes repair ... 46
Figure 12. Astrogliosis was induced by ATP in slice culture ... 48
Figure 13. DJ-1 regulates astrogliosis in slice cultured tissues ... 49
Figure 14. DJ-1 deficiency causes a defect of STAT3 phosphorylation in slice culture ... 51
Figure 15. DJ-1 deficiency causes a defect of anti-inflammatory function by ACM ... 54
Figure 16. DJ-1 deficiency causes a defect of ACM induced HO-1 expression in BV2 ... 56
viii
Figure 18. DJ-1 deficient astrocytes reduced L-PGDS expression ... 61
Figure 19. Expression levels of prostaglandin synthases, H-PGDS, mPGES, PGIS, were not different in WT and DJ-1 KO astrocytes ... 62
Figure 20. ACM from L-PGDS deficient astrocytes decreased anti-inflammatory effect ... 64
Figure 21. L-PGDS expression was regulated by Sox9 ... 66
Figure 22. DJ-1 regulated Sox9 expression in astrocytes ... 68
Figure 23. DJ-1 regulated Sox9 protein stability ... 70
ix
LIST OF TABLE
Table 1. Antibody...20 Table 2. Primer sequence...22 Table 3. siRNA sequence...24
x
ABBREVIATION
PD : Parkinson's disease
PINK1 : PTEN-induced putative kinase 1 LRRK2 : Leucine-rich repeat kinase 2 ATP : Adenosine triphosphate
GFAP : Glial fibrillary acidic protein GDNF : Glial derived neurotrophic factor
STAT: Signal-transducer and activator of transcription ACM : Astrocyte conditioned media
IFN-g : Interferon-gammar
iNOS : Inducible nitric oxide synthase HO-1 : Hemo oxygenas
PGD2 : Prostaglandin D2
L-PGDS: Lipocalin-type prostaglandin D2
TNF-a : Tumor necrosis factor-alpha PLA : in situ proximity ligation assay EthD-1 : Ethidium homodimer-1 NeuN : Neuronal Nuclei
1
I. INTRODUCTION
A. Parkinson's disease (PD)
Parkinson’s disease (PD) is a neurodegenerative motor disease with motor symptoms such as rigidity, resting tremor and postural instability (Olanow and Tatton, 1999). According to a recent report, PD also has non-motor symptoms such as cognitive impairment, depression and sleep disorder (Barbosa, 2013). Pathologically, PD is caused by the progressive loss of dopaminergic neurons in the substantia nigra (Baba, et al., 1998;Damier, et al., 1999). However, the factors affecting progression of PD are largely unknown because all cases of PD are of the sporadic form (90-95%). In investigating the progression of PD, many groups focused on the familial form (~10% of total PD) and reported the mutated genes related to familial forms of PD, such as alpha-synuclein, Parkin, PINK1, DJ-1 and LRRK2. These studies elucidated the molecular mechanism underlying neuronal death in PD, such as abnormal protein aggregation, production of reactive oxygen species (ROS), mitochondrial dysfunction, and excessive inflammation (Dawson and Dawson, 2003). However, animal models of PD based on mutation of these genes did not show PD phenotypes such as dopaminergic neuronal death and Lewy body formation (Chen, et al., 2005;Gispert, et al., 2009;Goldberg, et al., 2003;Kim, et al., 2005), although neuronal damage was enhanced in response to ischemic and/or mitochondrial toxin-induced injury. Therefore, it has been suggested that mutation of PD genes cooperate with certain environmental insults to cause PD.
2
B. Genes associated with PD 1. DJ-1 (PARK7)
DJ-1, PARK7, mutations are the cause of early onset autosomal-recessive PD. (Bonifati, et al., 2003a). DJ-1 has diverse roles in the brain although it has been identified as an oncogene that is linked to prostate cancer along with one member of the family of protein inhibitors of activated STAT (PIAS), PIASxa (Takahashi, et al., 2001;Tillman, et al., 2007). DJ-1 regulates intracellular ROS directly as an antioxidant (Mitsumoto, et al., 2001), and indirectly by inducing the expression of antioxidant enzymes through the stabilization of a transcriptional factor, Nrf2 (Clements, et al., 2006). DJ-1 inhibits cell death through interactions with apoptosis-associated protein such as Daxx, Bcl-XL and p53, or through
regulation of their stability and transcriptional activity (Fan, et al., 2008;Junn, et al., 2005;Ren, et al., 2011). It also promotes the degradation of protein aggregates through the inhibition of the fibrillation of a-synuclein (Shendelman, et al., 2004). In the brain, DJ-1 is expressed in astrocytes as well as in neurons (Bandopadhyay, et al., 2004;Mullett, et al., 2009). DJ-1 mediates the neuroprotective effect of astrocytes (Mullett and Hinkle, 2009), regulates endocytosis (Kim, et al., 2013b) and inflammation through regulating of LPS induced pro-inflammatory mediators, and interaction with SHP-1, a protein of the IFN-g signaling pathway (Kim, et al., 2013a;Waak, et al., 2009).
A 189 amino acid protein is encoded by the DJ-1 gene and is conserved among many species (Bader, et al., 2005;Bandopadhyay, et al., 2004;Kotaria, et al., 2005). Mutations of this protein were observed in population with early onset autosomal-recessive PD. A large deletion of DJ-1 and an L166P mutation of DJ-1 have been previously reported
3
(Bonifati, et al., 2003b). In particular, L166P was reported to lead to DJ-1 protein instability and rapid degradation due to impaired dimerization (Miller, et al., 2003;Moore, et al., 2003;Olzmann, et al., 2004). In addition, other mutants such as M26I, E64D and E163K have also been reported (Abou-Sleiman, et al., 2003;Hering, et al., 2004), but the effects of DJ-1 mutations are still largely unknown. In addition, animal models of PD based on mutation of DJ-1 did not demonstrate PD phenotypes such as dopaminergic neuronal death, and Lewy body formation (Chen, et al., 2005;Kim, et al., 2005;Kitada, et al., 2009). These studies suggest that examination of the functional links between mutated DJ-1 and the pathological condition are needed to better understand the development of PD
2. Other genes
In addition to DJ-1, the major PD related gene examined in this thesis, several genes, such as a-synuclein, PINK1, Parkin and LRRK2 have also been identified as important PD associated genes. First, a-synuclein (PARK1) is related to an early onset autosomal dominant for of PD (Chartier-Harlin, et al., 2004). a-synuclein is located mainly on the pre-synaptic terminals of the neuron and plays a role in their regulation thorough the maintenance of synaptic vesicles supplement (Maroteaux, et al., 1988). Mutation of a-synuclein leads to cellular toxicity as a result of a-synuclein aggregation (Ostrerova-Golts, et al., 2000;Pandey, et al., 2006). In addition, A50T mutation of a-synuclein in transgenic mice led to abnormal accumulation of a-synuclein in the neurons, causing neuronal degeneration (Lee, et al., 2002;Martin, et al., 2006).
4
dominant and late-onset familial PD. Interestingly, it has been reported that mutation of LRRK2 is also related with sporadic PD (Rajput, et al., 2006;Ross, et al., 2006;Zimprich, et al., 2004). LRRK2 has multiple domains, including ankyrin-like (ANK), leucin-rich repeat (LRR), Ras of complex (ROC), C-terminal of ROC (COR), kinase and WD40 domains. PD-related mutations have been identified in several domains, for example, R1441C/G.H in the ROC domain, G2019S in the kinase domain, and G2385R in the WD40 domain (Covy, et al., 2009;Paisan-Ruiz, et al., 2004;Wang, et al., 2010;Zheng, et al., 2011;Zimprich, et al., 2004). LRRK2 regulates filopodia protrusion and neurite outgrowth through interaction with actin regulatory protein, ERM and Rac1, and prevention of the actin polymerization process (Jaleel, et al., 2007;Meixner, et al., 2011;Parisiadou, et al., 2009). In addition, LRRK2 is related to calcium homeostasis (Cherra, et al., 2013;Gomez-Suaga and Hilfiker, 2012), inflammation (Gardet, et al., 2010;Kim, et al., 2012;Moehle, et al., 2012), mitochondria dynamics (Su and Qi, 2013), synaptic vesicle trafficking (Matta, et al., 2012;Piccoli, et al., 2011;Shin, et al., 2008), nuclear envelope integrity (Liu, et al., 2012), autophagy (Gomez-Suaga, et al., 2012;Manzoni, 2012), and microglia motility (Choi, et al., 2015).
Third, PTEN-induced kinase 1 (PINK1) is gene related to an autosomal recessive early-onset form of PD (Valente, et al., 2001). PINK1 has an N-terminal mitochondrial targeting sequence and a serine/threonine kinase domain. PINK1 is widely expressed throughout various brain regions (Blackinton, et al., 2007;Chiba, et al., 2009;Taymans, et al., 2006) and it is in expressed neurons, glial cells and neural stem cells (d'Amora, et al., 2011;Gandhi, et al., 2006). PINK1 has various roles such as the regulation of ATP generation, oxygen consumption (Beilina, et al., 2005;Liu, et al., 2009;Sim, et al., 2006), ROS
5
production (Gandhi, et al., 2009) and proliferation (Choi, et al., 2013). In addition, PINK1 deficient cells are more vulnerable to various insults compared with wild-type cells (Deng, et al., 2005;Haque, et al., 2008). Moreover, PINK1 regulates a mitochondrial clearance process called mitophagy by accumulating on the outer membrane of hyperpolarized mitochondria and recruiting another familial PD related protein, Parkin (Kondapalli, et al., 2012;Matsuda, et al., 2010;Narendra, et al., 2010;Vives-Bauza, et al., 2010).
Forth, Parkin is associated with form of autosomal recessive juvenile PD (Kitada, et al., 1998). Parkin has activity as an E3 ubiquitin ligase. Many studies have identified substrates of Parkin such as septins CDC-rel1, CDC-rel2 (Ageta-Ishihara, et al., 2013;Zhang, et al., 2000), synaptotagmin XI (Shimura, et al., 2000), synphilin-1 (Chung, et al., 2001), Paelr1 (Imai, et al., 2001), CHIP (Imai, et al., 2002), cyclinE (Staropoli, et al., 2003), and p38 in the tRNA synthase complex (Corti, et al., 2003).
6
C. Function of astrocytes in the normal brain
Astrocytes are the most abundant cells in the brain and play diverse roles in the well-being and function of the brain. Astrocytes maintain homeostasis of the brain’s microenvironment including neuronal metabolism, neurotransmitter synthesis, formation of the blood brain barrier (BBB), maintenance of the extracellular environment, and regulation of cerebral blood flow (Ransom and Ransom, 2012).
Typically, it has been reported that astrocytes provide nutrients and growth factors for neurons, (Banker, 1980;Walz and Mukerji, 1988). These cells also provide an energy source to adjacent neurons through lactate shuttling for metabolic regulation (Chih and Roberts Jr, 2003). Processes of astrocytes extended to the pre- and post- synaptic terminals, constructing a physical barrier that limit neurotransmitter diffusion (Araque, et al., 1999a;Araque, et al., 1999b). In addition, accumulated K+, a result of neuronal activity, is
rapidly removed and glutamate is converted to glutamine by the uptake of astrocytes to maintain the extracellular concentration of neurotransmitters (Verkhratsky and Kirchhoff, 2007). Astrocytes also have neurotransmitter receptors such as glutamatergic, GABAergic, adrenergic, and serotonergic receptors (Larsson, et al., 1980;Lee, et al., 2010;Perea, et al., 2009;Perez-Alvarez and Araque, 2013;Porter and McCarthy, 1997). In order to modulate homeostasis in brain regions, astrocytes have a cellular network where they couple with other astrocytes via gap junctions (Rouach, et al., 2000), where metabolic substrates and ion for cell-to-cell communication are exchanged (Gardner-Medwin, 1983). In addition, the gap junction between astrocytes plays a role in removing extracellular glutamate and potassium in order to regulate synaptic activity (Rouach, et al., 2008). Astrocytes also play a role in the
7
regulation of vessel permeability. Endfeet of astrocyte processes extend to capillaries and surround the endothelial cells of capillaries to form the BBB (Abbott, 2002;Mathiisen, et al., 2010). The BBB is an important regulatory factor between the brain’s bllod supply and the CNS. The movement of molecules, ions and cells between the brain and blood is tightly regulated by the BBB (Abbott and Friedman, 2012;Daneman, 2012;Luissint, et al., 2012;Wong, et al., 2013). Therefore, astrocytes are an important cell type in the maintenance of the brain’s environment and activation.
D. Function of astrocytes in the injured brain
Astrocytes are activated by various insults to the brain, such as trauma, ischemia and neurodegenerative disease, and respond via the process of astrogliosis. In the damaged brain, reactive astrocytes show different morphological features and expression of genes compared with normal astrocytes in order to protect and repair the injured brain (Eddleston and Mucke, 1993;Eng and Ghirnikar, 1994;Hernandez, et al., 2002;Pekny and Nilsson, 2005).
1. Reactive astrocytes: intermediate filaments (IFs) and morphological features
In the injured brain, astrocytes show high expression of intermediate filaments (IFs) such as glial fibrillary acidic protein (GFAP), nestin and vimentin. In particular, GFAP is the primary intermediate filament of astrocytes and has been used as a hallmark of reactive astrocytes in the injured brain (Correa-Cerro and Mandell, 2007;Eddleston and Mucke, 1993;Eng, et al., 2000). GFAP and the other IFs of reactive astrocytes are seen in a wide range of brain insults such as trauma, ischemic or hemorrhagic damage, epilepsy,
8
Alzheimer's disease, PD and multiple sclerosis (Burda and Sofroniew, 2014). Interestingly, a lack of GFAP and other IFs leads to the induction of neuronal damage by traumatic cerebrospinal injury, cerebral ischemia and traumatic or kainate excitotoxicity (Li, et al., 2008;Nawashiro, et al., 2000;Nawashiro, et al., 1998;Otani, et al., 2006).
Upregulated IFs cause morphological changes in reactive astrocytes such as hypertrophy of the cell body, and thickening and extension of the cellular processes (Wilhelmsson, et al., 2004). In a GFAP and vimentin dual KO mouse model, reactive astrocytes demonstrated less hypertrophy, process shortening and a reduction of glial scar formation when compared with WT.
2. Reactive astrocytes: function of astrogliosis in neural protection
Reactive astrocytes construct a physical barrier around damaged core regions with microglia/macrophages, extracellular matrix molecules, perivascular fibroblasts and pericytes, to isolate the site of injury (Burda and Sofroniew, 2014;Cregg, et al., 2014;Rolls, et al., 2009). After injury, the number of reactive astrocytes increases and surrounds the damaged core region (Fitch and Silver, 1997;Reier and Houle, 1988). Moreover, reactive astrocytes eliminate increased extracellular glutamate after injury (Rothstein, et al., 1996;Swanson, et al., 2004). Ablation of reactive astrocytes leads to a reduction in glutamate transporter expression and an induction of neuronal degeneration due to the excitotoxic effects of accumulating glutamate (Cui, et al., 2001). In addition, reactive astrocytes regulate oxidative stress after injury (Desagher, et al., 1996). For example, neuronal death due to nitric oxide (NO) or oxidative glutamate toxeicity was increased in a co-culture system with
9
glutathione deficient astrocytes (Chen, et al., 2001;Shih, et al., 2003). Reactive astrocytes also provide neuronal protection against ammonia toxicity. Ammonia, CNS dysfunction associated neurotoxin with hepatic encephalopathy, induced extensive degeneration in pure neuron culture. However, extensive degeneration was decreased in co-culture system with astrocytes (Rao, et al., 2005).
3. Reactive astrocytes: function of astrogliosis for regeneration and repair
In the past, various studies have suggested the potential negative effects of astrogliosis scar formation such as the elimination of neural regeneration. Numerous therapeutic studies have reported that the degradation of scar formation through techniques such as enzymes to eliminate scar formation (Moon, et al., 2001;Tester and Howland, 2008), and inhibition of astrocyte proliferation (Tian, et al., 2007), led to beneficial effects for regeneration. However, growing evidence has demonstrated that astrogliosis has important roles in regeneration and repair by regulating the supply of nutrients, angiogenesis, remyelination, neurotrophic factor s and neurogenesis (do Carmo Cunha, et al., 2007;Liberto, et al., 2004;Triolo, et al., 2006;White, et al., 2008). Reactive astrocytes showed increased glucose uptake and lactate release in hypoxic conditions (Marrif and Juurlink, 1999) and stored glycogen following stimulation by insulin-like growth factor-1 (IGF-1) (Dringen and Hamprecht, 1992) to provide energy support for neighboring neurons. In addition, astrocytes are associated with fibronectin, which outgrowth of dorsal root ganglion (DRG) neurites and axon regeneration in mature white matter (Tom, et al., 2004). Reactive astrocytes also correlated with neovascularization and angiogenesis, which provide oxygen and nutrients to
10
the injured brain area. Vascular endothelial growth factor (VEGF) is a key molecule in angiogenesis and is induced by platelet-activating factor (PAF) from reactive astrocytes after stab wounds, neural grafting and hypoxia (Krum and Rosenstein, 1998;Yoshida, et al., 2002). Reactive astrocytes also correlates with remyelination. Ciliary neurotrophic factor (CNTF) has been shown to regulate the induction of oligodendrocyte precursor proliferation through fibroblast growth factor-2 (FGF-2) for remyelination (Albrecht, et al., 2003). Interestingly, the levels of CNTF, which is present in normal astrocytes, were highly increased in reactive astrocytes after brain injury (Dallner, et al., 2002). In addition, CNTF induced FGF-2 expression in reactive astrocytes during remyelination in the spinal cord (Albrecht, et al., 2003). Moreover, it has been reported that reactive astrocytes express neurotrophic factor. For example, glial cell line-derived neurotrophic factor (GDNF) and its receptors are expressed during development and in the adult brain (Arenas, et al., 1995;Buj-Bello, et al., 1995;Oppenheim, et al., 1995). GDNF reported to effect neuronal survival (Kordower, et al., 2000;Perrelet, et al., 2002) and axonal regeneration (Bjorklund, et al., 1997;Iannotti, et al., 2003;Mills, et al., 2007). It has been reported that reactive astrocytes also express GDNF (Moretto, et al., 1996;Nakagawa and Schwartz, 2004). Recently, detection of reactive astrocyte derived neural stem and progenitor cells suggest that reactive astrocytes have stem cell-like properties after brain injury (Gotz, et al., 2015;Shimada, et al., 2012), since neural stem cell-like cells have been found in cortical tissues after various injuries such as stabbing injuries and stroke (Buffo, et al., 2008;Nakagomi, et al., 2009;Shimada, et al., 2010). Reactive astrocytes also express several stem cell associated protein such as GFAP, Nestin, RC2 and Sox2 (Buffo, et al., 2008;Pekny and Pekna, 2004;Shimada, et al., 2010).
11
Furthermore, glutamate aspartate transporter (GLAST)-positive reactive astrocytes showed de-differentiation and multipotent spheres formation potential (Buffo, et al., 2008).
4. Reactive astrocytes: function of astrogliosis for regulation of inflammation
Brain inflammation is a critical defense mechanism and process to regenerate the microenvironment after brain injury through the action of microglia and infiltrating marcrophages. Microglia and macrophages produce neurotrophic factors, such as transforming growth factor (TGF)-b1, neurotrophin (NT)-3 and brain-derived neurotrophic factor (BDNF). (Batchelor, et al., 1999;Elkabes, et al., 1996;Garg, et al., 2008;Glezer, et al., 2007;Jeong, et al., 2013a;Jeong, et al., 2013b;Lehrmann, et al., 1998;Schwartz, et al., 2006;Streit, 2005;Streit, 2002). However, uncontrolled brain inflammation could accelerate the progression of injury (Chao, et al., 1992;Choi, et al., 2003;Kitamura, et al., 1996). Many studies suggest that brain inflammation is a risk factor for neurodegenerative diseases such as Alzheimer's disease (AD), PD, and multiple sclerosis (MS) (Breitner, 1996;Chen, et al., 2003;Klegeris and McGeer, 2005;Raivich and Banati, 2004;Sheng, et al., 1998). Therefore, brain inflammation is tightly regulated to maintain its beneficial effects.
Following numerous studies, it has been demonstrated that astrocytes or reactive astrocyte have essential anti-inflammatory roles (Sofroniew, 2015). For example, astrocytes release various cytokines such as TGF-b, IL-6, IL-10, IL-11 IL-19, and IL-27 which activate anti-inflammatory signaling and immunosuppressive effects (Jensen, et al., 2013;John, et al., 2005;Meeuwsen, et al., 2003;Zamanian, et al., 2012). Furthermore astrocytes also produce prostaglandins E2 (PGE2) as an anti-inflammatory factor (Molina-Holgado, et al., 2000) and
12
astrocyte-conditioned media (ACM) suppresses nitrite, inducible nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-a expression in IFN-g treated BV2, primary microglia. ACM also induced endogenous NRF2 translocation and HO-1 expression. (Min KJ et al., 2006). In addition, several studies have suggested that the inflammatory response is increased in astrogliosis ablated transgenic mice after stroke (Li, et al., 2008;Liu, et al., 2014) and spinal cord injury (Herrmann, et al., 2008;Okada, et al., 2006).
E. Astrocyte dysfunction in neurodegenerative disease
Astrocyte dysfunction has been observed in various neurodegenerative diseases. Amyotrophic lateral sclerosis (ALS) is an adult motor neuron diseasecharacterized by the progressive degeneration of motor neurons in the cortex, the brainstem and spinal cord. Studies in human suggested that sporadic and familial ALS showed reduced levels of the astrocyte glutamate transporter, EAAT2 (Bristol and Rothstein, 1996). In a superoxide dismutase (SOD) mutated animal model, glutamate transport and GLT1 expression was decreased in astrocytes before neuronal degeneration (Bruijn, et al., 1997;Howland, et al., 2002). AD is the most common neurodegenerative disease and causes functional loss of cognitive abilities such as memory, language, and calculation (Selkoe, 2001). In human studies, neuron-derived amyloid material was accumulated in astrocytes and human amyloid-b (Aamyloid-b) activated astrocytes. In addition, Aamyloid-b affected the induction of calcium wave signaling in astrocytes (DeWitt, et al., 1998;Haughey and Mattson, 2003;Nagele, et al., 2004). In familial AD, calcium oscillations were founded in astrocytes with mutated presenilin 1 (PSEN1) leading to low concentrations of ATP and glutamate (Johnston, et al., 2006). In
13
animal models, expression of tau protein in astrocytes, which is on of the hallmarks of AD, caused a reduction in the expression and function of gluatamate transporters GLT1 and GLAST (Dabir, et al., 2006). PD is the second most common neurodegenerative disease, after AD. In human studies, GFAP immunoreactivity, a marker of reactive astrocytes, was found in the striatum and the substantia nigra pars compacta (SNpc) of postmortem PD cases (Dervan, et al., 2004;Mirza, et al., 2000). Interestingly, morphological changes such as enlarged cell bodies and distorted processed in the reactive astrocytes were different when compared with those in other neurodegenerative disease (Mirza, et al., 2000;Song, et al., 2009). In an animal model, it has been reported that astrocytes that expressed PD-related A53T mutant a-synuclein induced progressive paralysis. In addition, the mutated astrocytes were shown to have down-regulation of the astrocytic glutamate transporters (Gu, et al., 2010).
F. Aim of this study
Since it has been reported that PD is characterized by dopaminergic neuronal death in the SNpc, almost all studies investigating the progression of PD were focused on neuronal death. Unfortunately, little is known about the progression and development of PD. Recently, increasing evidence has suggested that dopaminergic neuronal death could be induced by functional defects in non-neuronal cells necessary for neuronal survival in either the intact brain or pathological conditions. Astrocytes are the most abundant glial cell and have numerous functions in the maintenance of the brain’s environment. In the intact healthy brain, astrocytes regulate brain homeostasis by regulating extracellular ion concentrations,
14
neurotransmitters, the BBB, and cerebral blood flow. In pathological conditions, reactive astrocytes perform a number of actins necessary to form a physical barrier to isolating the injury site through morphological change and proliferation, and provide neural protection by regulating of inflammation and oxidative stress. In addition, reactive astrocytes play a critical role in brain repair by providing energy support, releasing neurotrophic and growth factors, and regulating angiogenesis and remyelination. Therefore, dysfunction of astrocytes is intricately linked with the progression of neurodegeneration.
The specific aims of this study were
1. Whether DJ-1 deficiency attenuates endogenous repair and astrogliosis
2. Whether DJ-1 deficiency attenuates the anti-inflammatory function of astrocytes
As a result, Iexamined how the PD-related gene, DJ-1 affects the functions of astrocytes in pathological conditions.
15
II. Materials and methods
1. Ethics statement-
All experiments were performed in accordance with the approved animal protocols and guidelines established by the Ajou University School of Medicine Ethics Review Committee for animal experiments, and all animal work was approved by the Ethical Committee for Animal Research of Ajou University (2014-0029; AMC119).
2. DJ-1 deficient mice
The DJ-1 KO mice used in this study were a generous gift from Dr. UJ Kang (Chicago University, Chicago, IL, USA). DJ-1 KO mice were previously generated by deleting a 9.3- kb region of genomic DNA containing the first five exons and part of the promoter region of the DJ-1 gene (Chen, et al., 2005).
3. Animal MRI
c57/BL6 mouse were anesthetized by Isoflurane. Brain damage was measured by 9.4T MRI (BioSpec 94/30 US/R, BRUKER, USA) and Volume RF coil (Inner diameter 23 mm, BRUKER,USA) CNIR of Sungkyunkwan University. Using parameter was 2D T2 Turbo RARE sequence (TR/TE = 9000/33 ms; Resolution = 78 mM x 78 mM x 250 mM; Slice thickess = 250 mM; RARE factor = 8; Average = 2; Scan time = 9 m36 s).
16
4. Stereotaxic surgery and drug injection
Male c57/BL6 mouse were anesthetized by injection of Tribromethanol (250 mg/kg, i.p) and positioned in a stereotaxic apparatus (David Kopf instruments, USA). ATP (400 nmole) in 0.8 ml sterile phosphate-buffered saline (PBS), unilaterally administered in to the striatum (AP, +0.2 mm; ML,-2.5 mm; DV,-3 mm from bregma), according to the atlas of The mouse brain in sterotaxic coordinates (Paxinos and Franklin, second edition). ATP was purchased from sigma (USA). All animals were injected using a Hamilton syringe equipped with a 30-gauge blunt needle to minimize mechanical damage attached to a syringe pump (KD Scientific, New Hope, PA). Drugs were infused at a rate of 0.2 ml/min. After injection, the needle was held in plasce for an additional 4 min before removal. We collected data from at least 3 animals for each time point (1 day~28 day) after injection. The contralateral sides were used as a control.
5. Tissue preparation
Mouse were anesthetized and transcardially perfused with saline solution containing 0.5% sodium nitrate and heparin (10 u/ml), followed by 4% paraformaldehyde in a 0.1 M phosphate buffer, pH 7.2, for tissue fixation. Brains were obtained and post-fixed overnight at 4°C in 4% paraformaldehyde. After 2 day, fixed brains were stored at 4°C in a 30% sucrose solution until they sank. Six separate series of 40-mm coronal brain sections were obtained using a cryostat (Leica CM3050S, Germany) and stored in anti-freeze stock solution (phosphate buffer pH 7.2 containing 30% glycerol, 30% ethylene glycol) at 4°C before use.
17
For protein preparation, mouse was anesthetized and transcardially perfused with saline solution only for 2 min. Brains were removed and sliced with a Alto mouse brain slicer matrix (Stainless Steel Alto Coronal 1.0 mm Matrix (SA-2175), Roboz Surgical Instrument, USA) and a razor blade. A slice that included the needle injection spot was selected, and tissue blocks surrounding the needle tran were collected and stored at -70°C until use.
6. Immunohistochemistry
For 3,3’-diaminobenzidine (DAB) staining, every sixth serial section was selected, rinsed two times with PBS containing 0.2% Triton X-100 (PBST), treated with 3% H2O2 for 5 min and rinsed with PBST. Non-specific binding was blocked with 1% bovine serum albumen (BSA) in PBST. Tissue sections were incubated with antibodies (Table 1) during over night at room temperature. Sections were rinsed three times with PBST, incubated with biotinylated secondary antibodies (Vector Laboratories, USA), and visualized with avidin-biotin-peroxidase-DAB solution (0.05% DAB and 0.003% hydrogen peroxide in 0.1 M phosphate buffer) according to the manufacturer’s instructions. Sections were mounted on slides and examined under a bright field microscope (Olympus optical, BX51, Japan). Images were captured using PictureFrame Application 2.3 software. Photographs of the most damaged section were presented in the results.
For immunofluorescence staining, sections were washed in PBST, treated with 1% BSA, and incubated with combinations of antibodies summarized in Table 1. For visualization, Alexa Fluor 488- or Alexa Fluor 555-conjugated secondary antibodies (1:600 dilution; Invitrogen) were used. Counterstaining with 4', 6-diamidino-2-phenylindol (DAPI; Vector Laboratories)
18
was used to detect nuclei. Sections were analyzed under a confocal microscope (Carl Zeiss, Germany) with a water immersion objective. Images were captured using a confocal software (LSM Image Browser).
7. Histological quantification
Photographs of the most damaged section were taken. Sholl analysis and morphological change of GFAP and nestin positive cells were investigated using Neurolucida (MBF bioscience, Williston, VT, USA). Marker protein intensities were analyzed using Image J software.
8. Organotypic cortical slice cultures
Cortical slices were prepared as previously described (Stoppini et al, 1991) with a modification. c57/BL6 mice at postnatal day 7 were anesthetized, and the skull was removed. Cortical slices (400 mm thick) were prepared using a McIlwain tissue chopper (Mickle Laboratory Engineering, Goose Green, UK) in a slice culture medium (50% MEM containing, 25% Hanks’ balanced salt solution, 25% heat-inactivated horse serum, 6.5 mg/ml glucose, 1 mM L-glutamine, 10 U/ml penicillin-G, and 10 mg/ml streptomycin). Slices were cultured in culture inserts (Millipore, Bedford, MA, USA) in 6 well plates. Medium was changed every other day. At 7 day, ATP (50 mM) was added. Cell viability in slices was assayed using ethidium homodimer-1 (Etd-1, Molecular Probes, Eugene, OR, USA). To inhibit STAT3, slices were treated with 50 mM 5,15-diphenylporphyrin (DPP, Sigma).
19
9. Cell culture
Primary astrocytes cells were cultured from the fore brain of WT and DJ-1 KO c57/BL6 mouse. In briefly mouse skulls were rapidly removed and brain was placed in a culture dish with DMEM (Hyclone, Logan UT, USA) containing 10% FBS (Hyclone). Maninges was removed and brain wasmechanincally dissociated with gentle pipetting in the media. Dissociated brain cells were seeded on 75 cm2 T-flasks (0.5 hemisphere/flask), and
were incubated in 37°C, 5% CO2 incubator for 2 ~ 3 weeks. After 2 ~ 3 weeks Primary astrocytes were removed with 0.1% trypsin and were seeded culture dish in DMEM containing 10% FBS.
BV2 cells were cultured in DMEM containing 5% FBS. For activation of BV2, cells were incubated with 5 ng/ml recombinant murine IFN- g (PeproTech, Rocky Hill, NJ, USA). For antagonization of PGD2 receptor in BV2, cells were incubated with PGD2
receptors antagonist (CAY1047 and TM30089 for DP2; BWA868C for DP1, 10 mM each; Cayman Chemical Company). For inhibition of AKT phosphorylation, cells were incubated with PI3K inhibitors (LY294002, 2, 4 mM; Wortmanin, 50, 100 nM; Biomol, Plymouth Meeting, PA, USA). DJ-1 KO MEF cells were cultured in DMEM containing 10% FBS.
10. Western blot analysis
Protein was isolated from homogenated slices or brains tissues and prepared cells with modified RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM Na3VO4, and 1 mM NaF) containing protease inhibitor cocktail and phosphatase inhibitor cocktail (GenDEPOT, Barkor, TX, USA). Isolated proteins were
20
separated by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with specific antibodies (Table 1) overnight at 4°C. After washing with PBS, membranes were incubated with peroxidase-conjugated secondary antibodies, and visualized with enhanced chemiluminescence system (Daeil Lab Inc., Seoul, Korea). Band intensities were normalized with actin or GAPDH using Image J.
[Table 1] Antibody
Antibody Company / Country Dilution
GFAP SIGMA-ALDRICH, 1:5000 (WB) / 1:500 (IHC, ICC) TH Pel-Fressz BiologicalsRogers, 1:1000 (WB) / 1:1000(IHC) GDNF Santa Cruz Biotechnology 1:1000 (WB) / 1:200 (IHC, ICC)
NeuN Millipore 1:1000 (WB) / 1:200 (IHC, ICC) Sox9 Santa Cruz Biotechnology 1:1000 (WB)/ 1:200 (ICC, PLA) Ubiquitin Santa Cruz Biotechnology 1:1000 (WB) / 1:200 (IF, PLA)
Flag SIGMA-ALDRICH 1:1000 (WB) HA Santa Cruz Biotechnology 1:1000 (WB) DJ-1 Cell Signaling Technology 1:1000 (WB) Myc SIGMA-ALDRICH 1:1000 (WB) GAPDH Santa Cruz Biotechnology 1:2000 (WB) Actin Santa Cruz Biotechnology 1:2000 (WB) L-PGDS Cayman Chemical Company 1:1000 (WB) H-PGDS Santa Cruz Biotechnology 1:1000 (WB) mPGES Cayman Chemical Company 1:1000 (WB) PGIS Santa Cruz Biotechnology 1:1000 (WB) AQP4 Millipore, Bedford 1:1000 (WB) GLAST abcam, Cambridge 1:1000 (WB) GLT-1 abcam, Cambridge 1:1000 (WB) VEGF abcam, Cambridge 1:1000 (WB)
21
11. Immunoprecipitation
Prepared cells were washed twice with PBS and were isolated protein with immunoprecipitation lysis buffer (1% triton-X 100, 1 mM Na3VO4, 1 mM NaF, 150 mM NaCl, 1mM EDTA, 1mM EGTA, 50 mM HEPES, 0.5 mM DTT) containing protease inhibitor cocktail and phosphatase inhibitor cocktail (GenDEPOT). Isolated protein supernatants were incubated with specific antibody (Table 1) during 4 hour on 4°C spin rotor. After incubation, protein G agarose (Millipore, Bedford, MA, USA) was added with antibody incubated protein supernatants during 4 hour on 4°C spin rotor. Agarose beads were washed with PBS and proteins were isolated by boiling in 2x SDS-PAGE sample buffer. Final protein sample was analyzed by Western blotting.
12. Quantitative real-time PCR (qPCR)
Total RNA was isolated from primary astrocytes by easy-BLUE reagent (iNtRON Biotechnology, Seoul, Korea). cDNA was synthesized using 1mg of total RNA and the cDNA synthesis kit (iNtRON) at the following temperatures : Denaturation with oligo d(T)15
primers during 5 min 75°C, cDNA synthesis with AMV RT enzyme miture during 60 min 42 °C and Termination reaction during 5 min 70 °C. qPCR was done using 2x KAPA SYBR Fast Master Mix (Kapa Biosystem, Cape Town, South Africa) and was used by RG-6000 real-time amplification instrument (Corbett Research, Sydney, Australia). qPCR amplification using specific primer (Table 2). The cycle threshold (Ct) for the gene transcript was normalized to the average Ct for transcripts of the housekeeping gene, actin, amplified
22
in each reaction. Relative quantitation of normalized transcript abundance was determined using the comparative Ct method (DDCt), as described by the manufacturer.
13. Reverse transcriptase PCR (RT-PCR)
Total RNA was isolated from primary astrocytes by easy-BLUE reagent (iNtRON Biotechnology, Seoul, Korea). cDNA was synthesized using 1mg of total RNA and the cDNA synthesis kit (iNtRON) at the following temperatures : Denaturation with oligo d(T)15
primers during 5 min 75°C, cDNA synthesis with AMV RT enzyme miture during 60 min 42°C and Termination reaction during 5 min 70°C. RT-PCR was performed using specific primer (Table 2). The amplified products were separated by electrophoresis on a 1.5% agarose gel and detected under UV light. Band intensities were analyzed using Image J.
[Table 2] Primer sequence
Primer Sense Anti-sense
TNF-a 5’-GTAGCCCACGTCGTAGCAAA 5’-CCCTTCTCCAGCTGGGAGAC HO-1 5’-TGCAGGTGATGCTGACAGAGG 5’-GGGATGAGCTAGTGCTGATCTGG L-PGDS 5’-GACACAGTGCAGCCCAACTTTC 5’-GGGCTACCACTGTCTTGCACATA Sox9 5’-GGACAACACATGCCTCTGCAA 5’-TCTCCAGCCACAGCAGTGAGTAA H-PGDS 5’-GCCAGTGCCACACACAGCTAA 5’-CGTCTTGCCCATGTCACCA
mPGES 5’-ACAGTGGTTTCAGCAGGGTGTC 5’-GTCCAGATTTGCAGCCAGGAG PGIS 5’-TGCGTACACGGCTGGACTTC 5’-CCTGCAGGTCTCTGTGCATCA Actin 5’-GCTCTGGCTCCTAGCACCAT 5’-GCCACCGATCCACACAGAGT
23
14. DNA constructs
Plasmid DNA for p3x FLAG-hDJ-1, hL166P, hE64D and hC106A plasmid was gifts from Prof. Park (Ajou University, Woncheon-dong Youngtong-gu Suwon, Kyunggi-do, Korea). HA-Ubi was gifts from Prof. Jou (Ajou University). pWPXL was a gift from Didier Trono (Addgene plasmid # 12257) and pWPXL-Sox9 was a gift from Bob Weinberg (Addgene plasmid # 36979).
15. Transfection
Cells were transiently transfected with plasmid DNA using jetPEI DNA transfection reagent (Polyplus Transfection, Boulevard Sébastien Brant, 67401 Illkirch Graffenstaden, FR), as explained by the manufacturer. Briefly, cells were incubated to DNA plasmid and jetPEI mixture for 4 hours. After 4 hour, media were replaced with fresh media. 3 days later, transfected cells were used for experiments.
Cortical slices plasmid DNA transfection was performed using a modified Zou &Crews method (Zou and Crews, 2010). Cortical slice culture tissues were transiently transfected with plasmid DNA using jetPEI DNA transfection reagent as explained by the manufacturer. The transfection mixture was added to slice culture media and incubated in a total volume of 1.5 ml (0.5 ml on top of slices and 1 ml at bottom of the cultures) during 4 hours. After 4 hour, media were replaced with fresh media. 3 days later, transfected slice culture tissues were used for experiments.
24
Primary astrocytes were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and treated with 1% bovine serum albumen. Primary astrocytes were incubated with Sox9 and ubiquitin antibodies during over night at RT. After overnight, the sample was washed with PBS containing 0.1% Triton X-100(PBST) and was incubated with DNA probe-conjugated secondary antibodies (Olink Bioscience, Uppsala, Sweden) for 1 h at 37°C. The sample was washed and DNA probes were ligated for 30 min at 37°C, amplified for 2 h at 37°C, and examined under an Axiovert 200M microscope (Carl Zeiss).
17. Small interfering RNAs (siRNA)
The expression of target proteins, Sox9 and L-PGDS were knocked down by transiently transfection of mouse specific small interfering RNAs (siRNA; Genolution Pharmaceuticals, Seoul, Korea) as in Table 3. For transfections, the medium was replaced with Opti-MEM (Invitrogen) and astrocytes were treated with 10 nM siRNA and RNAiMAX transfection reagent, according to the manufacturer’s instructions (Invitrogen) for 5 days. Knockdown of targets was confirmed by qPCR, RT-PCR and Western blot.
[Table 3] siRNA sequence
Primer Sense
Sox9 siRNA #1 5’- UUG UUA UAG UAA CAU AAA UAA UAU U-3’ Sox9 siRNA #2 5’- GGG GAA UAA ACA GAU AAC AUA GAU U-3’ L-PGDS siRNA #1 5’ GGGAGAAGAAAGCUGUAUUGUAUUU 3’ L-PGDS siRNA #2 5’ GGAGAAGAAAGCUGUAUUGUAUAUU 3’
25
18. Prostaglandin D2 (PGD2) ELISA
PGD2 expression level was measured by a commercial ELISA kits, according to the
manufacturer’s instructions (Prostaglandind D2-MOX EIA kit, Cayman Chemical Company).
19. Inhibition of protein synthesis by cycloheximide
Cells were treated with 10 mg/ml cycloheximide (SIGMA-ALDRICH) for 1, 2 and 3 hours. After appropriate time intervals, cells were harvested and total proteins were prepared.
20. Inhibition of proteasomal degradation by MG132
Cells were treated with 10 mg/ml MG132 (SIGMA-ALDRICH) for 4 hours. After 4 hours, cells were harvested with lysis buffer containing 20 mM N-Ethylmaleimide (NEM; SIGMA-ALDRICH) and total proteins were prepared.
21. Measurement of nitric oxide
The amount of nitrite formed from nitric oxide (NO) was measured by culture mediaum (50 mL) with an equal volume of Griess reagent (0.1% naphthylenthylene diamine, 1% sulfanilamide, 2.5% H3PO4), and then measuring optical density at 540 nm. (Ding, et al.,
26
22. Statistical analysis
The statistical significance of differences between two groups was determined using unpaired two-tailed Student’s t tests.
27
III. RESULTS
PART A.
DJ-1, (PARK7), plays critical roles in astrogliosis by regulating of thepSTAT3 signaling pathway for tissue repair after brain injury
1.
Repair of brain injury was attenuated in DJ-1 KO miceTo examine whether PD-related genes may be involved in the regeneration of the injured brain, I examined time-dependent changes following brain injury in wild type and DJ-1 KO mice. Brain injury was produced by stereotaxic injection of ATP into the striatum since ATP, a component of damage associated molecule patterns (DAMPs), induces brain damage (Amadio, et al., 2002;Cavaliere, et al., 2001a;Cavaliere, et al., 2001b;Jeong, et al., 2010;Melani, et al., 2005). The damaged sites were chased using a 9.4T MRI scanner (Fig. 1A), reconstructed using Neurolucida 3D-modeling (Fig. 1B), and calculated the volume of damaged tissue (Fig. 1C). At 1 day after injection, the volume of the damaged tissue did not differ between WT and DJ-1 KO mice brain: 4.5+0.14 mm3 and 4.4±0.27 mm3 in WT and
DJ-1 KO, respectively (Fig. 1C, D). Thereafter, the volume of the damaged sites rapidly decreased within 1 week in both WT and DJ-1 KO mice brain and then slowly for up to 29 days (Fig. 1C, D). Interestingly, however, the repair of damage was retarded in DJ-1 KO mice brain. The volume of damaged tissue in WT group reduced to 1.6±0.1 mm3 (65±3.28%
of damaged volume at 1d after ATP injection) at 8 d, 1.0±0.02 mm3 (78±1.18%) at 15 d,
28
volumes reduced to 2.6±0.2 mm3 (42±6.38%) at 8 d, 2.1±0.13 mm3 (53±4.57%) at 15 d,
and 0.5±0.01 mm3 (90±0.81%) at 29 d (Fig. 1C, D). These results showed that DJ-1
29
Figure 1. DJ-1 deficiency causes a defect of repair after brain damage. Brain damage
was induced by ATP (400 nmloe) injection in striatum in WT and DJ-1 KO mouse (n = 3 mice). (A) Damage areas were chased by 9.4T MRI at indicated time points after ATP injection. Yellow arrows showed damage areas. (B) ATP induced damage (red) were reconstructed by 3D-modeling of Neurolucida. (C) Damage volume was calculated (D) Repair ratio was quantified. Values are means ± SEMs of 3 mice (*P < 0.05).
30
2. DJ-1 deficiency causes a defect of astrogliosis
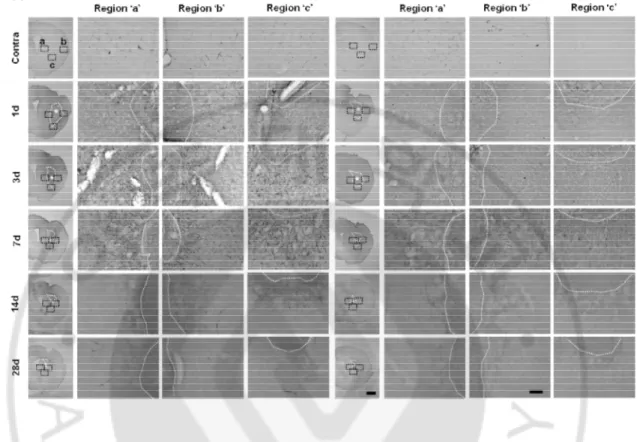
In response to injury, astrocytes are activated and increase the expression of several proteins required for the maintenance of brain homeostasis and reconstruction of damaged brain (Eddleston and Mucke, 1993;Eng and Ghirnikar, 1994;Pekny and Nilsson, 2005;Sofroniew, 2009) Therefore, I examined whether the retarded repair of damage in the DJ-1 KO group may have been related to the responses of astrocytes. For this, brain sections were obtained from WT and DJ-1 KO mice from 1 d to 29 d after ATP injection, and the response of astrocytes was analyzed with GFAP specific antibodies (Fig. 2). Interestingly, astrogliosis was delayed in the DJ-1 KO brain in all penumbral regions, right, left, and below the damaged core region (Fig. 2A). I detected that reactive astrocytes showed progression of typical of astrogliosis in the WT brain, however progression was slowed and showed insufficient morphological change in the DJ-1 KO brain from 3 d to 7 d after ATP injection.
31
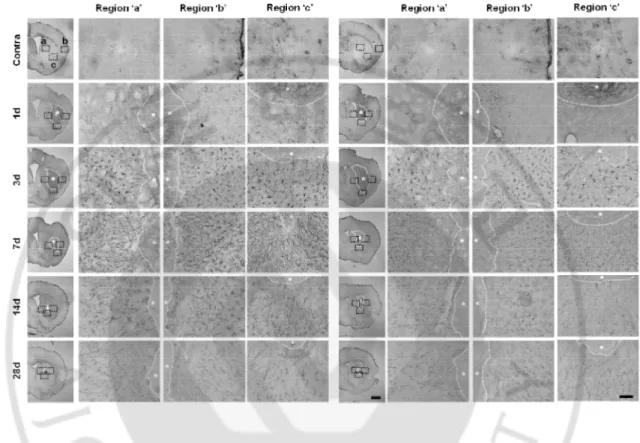
Figure 2. DJ-1 deficiency causes a defect of GFAP+ astrogliosis. (A) Brain damage was
induced by ATP (400 nmloe) injection in striatum in WT and DJ-1 KO mouse (n = 4-10 mice). Brain sections were prepared at the indicated times after ATP injection and stained with GFAP antibody. Photographs of the most damaged sections were obtained and were analyzed with serial sections (*, damage core region). Penumbra regions were divided left (a), right (b) and bottom (c). Scale bars, 1 mm, 50 mm
32
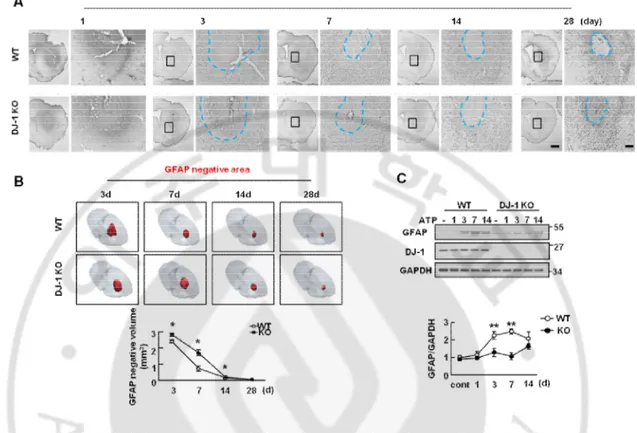
In response to the ATP injection, astrogliosis was detectable in the penumbral region at 3 d after injection of ATP (Fig. 3A). Since astrogliosis was not yet detectable at 1 d (Fig. 2A, 3A), I measured the astrocyte response from 3 d. According to the MRI results, GFAP-negative areas (blue dotted lines) reduced between 3 d to 29 d in both WT and KO brain (Fig. 3A). The volume of damaged tissue at each time point was quantified by Neurolucida 3D-modeling based on the analysis of at least eight sections from each animal stained with GFAP antibodies (Fig. 3B). As with the MRI data, the damaged volumes of tissue reduced more rapidly in the WT than in KO brain (Fig. 3B, C). GFAP negative volumes at 7 d were about 30% of that at 3 d in the WT brain, but about 60% in DJ-1 KO brain. At 14 d, however, GFAP negative volumes were reduced to about 90% in both WT and DJ-1 KO brain (Fig. 3B, C). In Western blot analysis using brain lysates, GFAP expression in the WT brain increased 2-2.5 folds at 3 d, 7 d and 14 d after the ATP injection, while GFAP expression in the DJ-1 KO brain only slightly (less than 1.8-folds) increased at 3 d, 7 d, and 14 d after injury (Fig. 3D). In the intact WT and KO brain, GFAP expression was similar (Fig. 3D). The smaller increase in GFAP expression in Western blot compared with that in immunostaining may be due to the inclusion of damaged tissue in the brain lysates.
33
Figure 3. DJ-1 deficiency causes a defect of progression of astrogliosis. (A) Brain damage
was induced by ATP (400 nmloe) injection in striatum in WT and DJ-1 KO mouse (n = 4-10 mice). Brain sections were prepared at the indicated times after ATP injection and stained with GFAP antibody. Photographs of the most damaged sections were obtained and were analyzed with serial sections. Blue line showed endpoints of astrogliosis (B) Negative area of astrogliosis (red) were reconstructed by 3D-modeling of Neurolucida. Negative area was quantified (lower panel). (C) Protein samples were obtained to striatum at indicated time points after ATP injection and GFAP expression level was measured by Western blot with GFAP specific antibody (n = 3 mice). GFAP intensities were quantified (right panel). GAPDH was used as a loading control. Values are means ± SEMs of 4-10 mice (A , B) and 3 mice (C) (*P < 0.05; ** P < 0.005). Scale bars, 1 mm, 200 mm (A)
34
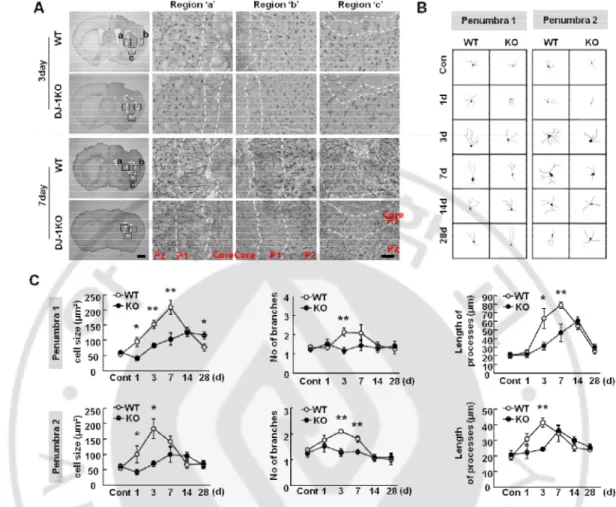
Next, I further examined the morphology of astrocytes in the WT and KO brain (Fig. 4). After the ATP injection, reactive astrocytes showed heterogeneous morphological features (Ding, 2014;Shimada, et al., 2012), important for the progression and function of astrogliosis (Namekawa, et al., 2002;Nawashiro, et al., 2000;Nawashiro, et al., 1998;Otani, et al., 2006). In addition, insufficient morphological change of reactive astrocytes was also observed in PD patients (Mirza, et al., 2000;Song, et al., 2009). Interestingly, the patterns of astrogliosis were different in between the WT and DJ-1 KO brain in all penumbral regions, right, left, and below the damaged core, particularly, at 3 d and 7 d after injury (Fig. 4A). Astrocytes in the penumbral region next to the damaged core (Penumbra 1, P1) had a polarized morphology with long processes extended toward the damage core, but the cells in the penumbra region next to P1 (Penumbra 2, P2) had a hypertrophic but non polarized morphology (Fig. 4A, B). Interestingly, astrocytes from the DJ-1 KO brain in both P1 and P2 showed less activated morphology compared with WT astrocytes: shorter processes, fewer hypertrophic cell bodies, and a smaller increase in GFAP intensity (Fig. 4A, B, C), although astrocyte morphology and GFAP expression were similar in the intact brain of both groups (Fig. 5). Sholl analysis also showed that all parameters, cell volume, and number and length of processe, were reduced in both P1 and P2 in the KO astrocytes, particularly at 3 d and 7 d (Fig. 4A, B, C).
35
Figure 4. DJ-1 deficiency causes a defect of morphology of GFAP+ reactive astrocytes. (A) Brain sections were prepared at the indicated times after ATP injection and stained with
GFAP antibody. Photographs of the most damaged sections were obtained and were analyzed with serial sections (n = 4-10 mice). Core region were showed damage area and penumbra 1 (P1) and penumbra 2 (P2) were separated by morphology of GFAP+ reactive astrocytes. (B) Morphology of GFAP+ reactive astrocytes between WT and DJ-1 KO was measured by Neurolucida on each penumbra region. (C) Morphological features of GFAP+ reactive astrocytes were quantified by Sholl analysis. Values are means ± SEMs of 40-50 cells (*P < 0.05; **P<0.005). Scale bar, 1 mm, 50 mm (A).
36
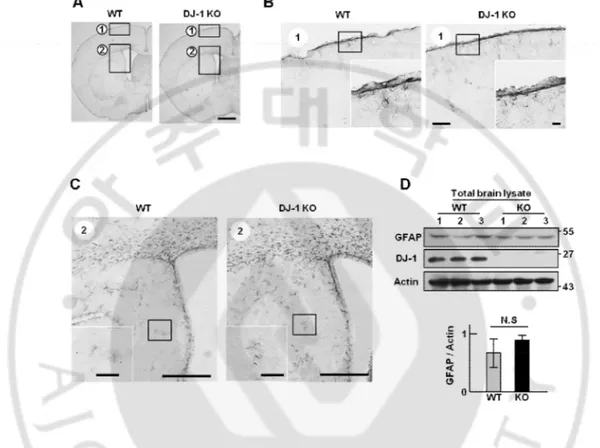
Figure 5. Normal astrocytes are not different between WT and DJ-1 KO mouse brain. (A) Brain sections were prepared and stained with GFAP antibody (n = 5 mice). (B) Normal
cortical astrocytes were investigated by GFAP staining. (C) Normal striatal astrocytes were investigated by GFAP staining. (D) Protein samples were obtained from total brain and GFAP expression was analyzed by Western blot with GFAP specific antibody. GFAP intensities were quantified (lower panel). Actin was used as a loading control. Values are means ± SEMs of 3 mice (*P < 0.05; **P<0.005). Scale bars, 1 mm (A), 200 mm (B), 20 mm (B, inset), 500 mm (C), 20 mm (C, inset).
37
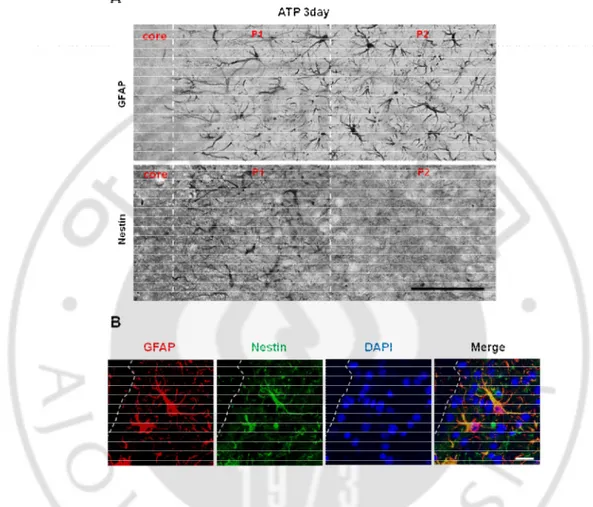
Next, I compared nestin expression in the WT and DJ-1 KO brain since nestin, a marker of reactive astrocytes, has been known to regulate the repair of injury in the brain (Duan, et al., 2015;Lin, et al., 1995;Sirko, et al., 2013;Wiese, et al., 2004), and repair-related processes of proliferation, migration, invasion of cells (Akiyama, et al., 2013;Narita, et al., 2014;Zhao, et al., 2014), and angiogenesis (Matsuda, et al., 2013). After nestin staining, I found that nestin expression dramatically increased in P1 but not in P2 at 3 d and 7 d after ATP injection (Fig 6A, 7A) and nestin expressing cells were GFAP positive reactive astrocytes after ATP injection (Fig 6B). As expected, nestin expression was delayed and reduced in the striatum of the DJ-1 KO brain (Fig 7A, Fig 8A, B) although nestin was barely expressed in both the intact striatum of either group (Fig 7A). Sholl analysis of nestin-positive cells confirmed that morphological parameters such as cell volume, number of process branches, and length of processes, were reduced in the DJ-1 KO brain (Fig 8C, D). At 3 d and 7 d, the morphological features of reactive astrocytes in the DJ-1 KO striatum showed a 30-50% reduction in all parameters. Taken together, these data showed that DJ-1 deficiency causes abnormal responses of in the astrocytes of the injured brain, which led me to examine the expression of functional markers of astrocytes.
38
Figure 6. Nestin locate in GFAP+ reactive astrocytes on penumbra from only damage near site after brain injury. (A) Brain sections were prepared and stained with nestin
antibody. Nestin+ reactive astrecytes were located on penumbra ‘1’ region of GFAP+ astrocytes at 3 d after ATP injection (B) Nestin+ reactive astrocytes were localized with GFAP+ reactive astrocytes. Scale bars, 100 mm (A), 20 mm (B).
39
Figure 7. DJ-1 deficiency causes a defect of nestin+ astrogliosis. (A) Brain damage was
induced by ATP (400 nmloe) injection in striatum in WT and DJ-1 KO mouse. Brain sections were prepared at the indicated times after ATP injection and stained with nestin antibody. Photographs of the most damaged sections were obtained (*, damage core region). Penumbra regions were divided left (a), right (b) and bottom (c). Scale bars, 1 mm, 50 mm
40
Figure 8. DJ-1 deficiency causes a defect of morphology of nestin+ reactive astrocytes. (A) Brain sections were prepared at the indicated times after ATP injection and stained with
nestin antibody. Photographs of the most damaged sections were obtained. Core region was damage area. P1 and P2 region were separated by features of reactive astrocytes (B) Sections obtained at 3 d were double-labeled with nestin and GFAP antibodies (C) Morphology of nestin+ reactive astrocytes between WT and DJ-1 KO was measured by Neurolucida on penumbra region at indicated time points. (D) Features of reactive astrocytes were quantified by Sholl analysis of Neurolucida. Values are means ± SEMs of 40–50 cells (*P < 0.05; **P <0.005). Scale bars, 1 mm, 50 mm (A), 10 mm (B).
41
3. Reduction of astrocyte derived GDNF expression and attenuated restoration
of dopaminergic processes in the DJ-1 KO brain
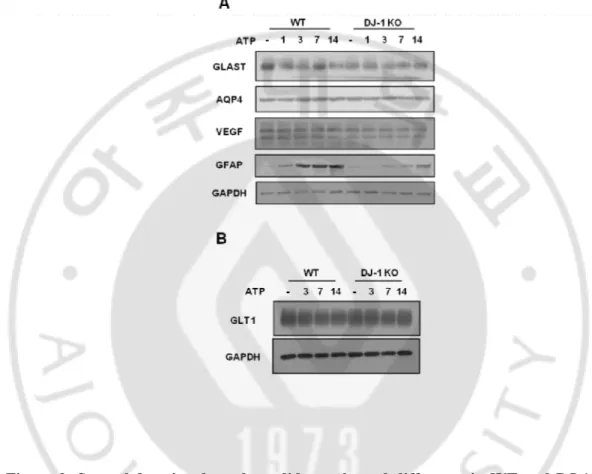
Recently, growing evidences has demonstrated that astrogliosis has an important roles in regeneration and repair through the regulation of the supply of nutrients, angiongenesis, remyelination, neurotrophic factors and neurogenesis (do Carmo Cunha, et al., 2007;Liberto, et al., 2004;Triolo, et al., 2006;White, et al., 2008). Therefore, I examined whether astrocyte function was affected by the insufficient astrogliosis in DJ-1 KO injured mouse. Using Western blot with several functional markers, I found that GLAST, GLT1, AQP4 and VEGF were not affected by DJ-1 deficiency (Fig 9A, B).
However, I determined that DJ-1 KO induced insufficient astrogliosis led to a deficiency of neurotrophic factor. GDNF is neurotrophic factor that is well known to act upon dopaminergic neurons (Lin, et al., 1993) and regulate axonal regeneration of dopaminergic and sensory neurons after brain and spinal cord injury (Bjorklund, et al., 1997;Iannotti, et al., 2003;Mills, et al., 2007). Since GDNF expression is increased in reactive astrocytes in the injured brain (Nakagawa, et al., 2005), I examined GDNF expression in WT and KO brain. Using GDNF staining, GDNF expression was detectable in astrocytes found in P1 and P2 in both WT and DJ-1 KO brain from 3 d to 7 d post-ATP injection, but less weakly in the DJ-1 KO brain (Fig 10A). I also found that GDNF expression in GFAP-positive reactive astrocytes was reduced in P1 and P2 in the striatum of DJ-1 KO mice after brain injury (Fig 10B). After ATP injection, I prepared brain lysates from the ATP-injected WT and DJ-1 KO striatum at 3 d and 7 d when dramatic changes in astrogliosis had been observed. Using Western blot, I found that GDNF expression level was
42
significantly decreased (by approx. 50%) in the DJ-1 KO striatum compared with WT striatum at 3 d and 7 d (Fig 10C). Taken together, these results indicate that astrocyte responses in the injured brain, astrogliosis, and GDNF expression are insufficient in the DJ-1 KO brain.
43
Figure 9. Several functional markers did not showed difference in WT and DJ-1 KO mouse after ATP injection. (A) Protein samples were obtained to striatum at indicated time
points after ATP injection and GFAP and several functional markers expression level were measured by Western blot with specific antibodies. GAPDH was used as a loading control (A, B).
44
Figure 10. DJ-1 deficiency causes a defect of GDNF expression. (A) Brain sections were
prepared at the indicated times after ATP injection and stained with GDNF antibody. Photographs of the most damaged sections were obtained. GDNF expression was detected in reactive astrocytes (yellow arrow). (B) Sections obrained at 3 d were double-labeled with nestin and GFAP antibodies. (C) Protein samples were obtained to striatum at indicated time points after ATP injection and GDNF was measured by Western blot with specific antibodies. GAPDH was used as a loading control. Data shown are representative of at least three independent experiments. Values are means ± SEMs of 3 mice (*P < 0.05; **P <0.005). Scale bars, 50 mm, 20 mm (A), 50 mm (B).