저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
의학 석사학위 논문
Impaired cerebral & peripheral endothelial function
in patients with restless legs syndrome
아 주 대 학 교 대 학 원
의 학 과
김 민 승
Impaired cerebral & peripheral
endothelial function in patients with
restless legs syndrome
지도교수 홍 지 만
이 논문을 의학 석사학위 논문으로 제출함.
2019 년 2 월
아 주 대 학 교 대 학 원
의 학 과
김 민 승
김민승의 의학 석사학위 논문을 인준함.
심사위원장 홍 지 만 인
심 사 위 원 문 소 영 인
심 사 위 원 이 진 수 인
아 주 대 학 교 대 학 원
2018년 12월 24일
i
- Abstract -
Impaired cerebral & peripheral endothelial function in
patients with restless legs syndrome
Instroduction: Restless legs syndrome (RLS) is a sleep related movement disorders,
characterized by an uncomfortable sensation usually in the legs, particularly at night. Although it is widely accepted that dysfunction of dopaminergic system and iron system plays an important role in RLS, its pathophysiology has not been fully elucidated as yet. Several studies reported association between RLS and cardiovascular disease. Endothelial dysfunction is known to be an early stage in the development of atherosclerosis and associated with cardiovascular events. We investigated the flow-mediated dilatation (FMD) and vasomotor reactivity (VMR) to evaluate the endothelial dysfunction on RLS patients.
Method: Patients with RLS were included who visited the Parkinson’s Disease and
Movement Disorders Clinic in the Department of Neurology between March 2014 and April 2017. After 2 days of withdrawal of medication, cerebral vasomotor reactivity (VMR) and flow-mediated dilation (FMD) were measured. To evaluate the association between responsiveness to dopaminergic treatment and the degree of endothelial dysfunction, we asked to 34 of RLS patients about improvement of their RLS symptoms after administration of dopamine agonist.
Result: The values of VMR in both MCA (Lt MCA 51.3±9.5%, vs 58.9±9.2%,
p=0.003, Rt MCA 50.8±10.4%, vs 57.7±7.4%, p=0.006) and BA (51.1±9.6%, vs 57.1±11.3%, p=0.030) were significantly lower in RLS group than control group. The values of FMD (7.1±1.5% vs 8.5±1.8%, p=0.006) also was significantly lower in RLS patients. Baseline cerebral VMR (right MCA: complete responsive vs partial responsive vs nonresponsive 44.9±6.2% vs 49.9±11.3 vs 59.1±8.5%, p=0.004) showed significant difference between these three groups.
ii
Conclusion: This study demonstrated that RLS patients have poorer cerebral and
systemic endothelial function than normal healthy subjects. This findings provide further evidence of a possible association between RLS and cardiovascular disease, including ischemic stroke, and also the role of vascular pathologies in the pathogenesis of the RLS.
iii
Table of Contents
Abstracts ···ⅰ Table of Contents ···ⅲ List of Figures ···ⅳ List of Tables ···v Ⅰ. Introduction ···1 Ⅱ. Method ···2 Ⅲ. Results ···6 Ⅳ. Discussion ···13 Ⅴ. Conclusion ···17 References ···18 국문요약 ···22iv
List of Figures
v
List of Tables
Table 1.··· 7
Table 2.··· 9
1
Ⅰ. Introduction
Restless legs syndrome (RLS) is a sleep related movement disorders, characterized by an uncomfortable sensation usually in the legs, particularly at night1. Although it is widely accepted that dysfunction of dopaminergic system and iron system plays an important role in RLS, its pathophysiology has not been fully elucidated as yet.2 The association between RLS and cardiovascular disease was first reported in 20013, and several following epidemiological studies also demonstrate RLS patients to have a higher risk of developing vascular disease such as hypertension, myocardial infarction (MI) and ischemic stroke4-8. Small vessel disease (SVD) also recently reported to be significantly correlated with RLS9. Endothelial dysfunction is known to be an early stage in the development of atherosclerosis and associated with cardiovascular events10,11. Flow-mediated dilatation (FMD) is a widely used method to assess systemic endothelial function.12 We previously suggested that patients with RLS had a poor peripheral endothelial function, as lower values of flow-mediated diameter (FMD)13. Albeit, cerebral
endothelial function was not evaluated by FMD, thus insufficient to explain the high incidence of ischemic stroke in patients with RLS. The vasomotor reactivity (VMR) evaluate the compensatory potential of the flow-regulating vessels of brain, reflects the function of cerebral arteries.
The aim of this study is to assess the association between RLS and cerebral & systemic endothelial dysfunction using FMD and VMR, to support the association between RLS and cerebrovascular diseases and to support the pathophysiology of RLS associated with microvasculature alteration.
2
Ⅱ. Methods
Subjects
Patients with RLS were included who visited the Parkinson’s Disease and Movement Disorders Clinic in the Department of Neurology between March 2014 and April 2017. Final enrollment consisted of 34 RLS patients and 36 age- and sex- matched normal controls. RLS was diagnosed according to the 2012 updated diagnostic criteria of the International Restless Legs Syndrome Study Group (IRLSSG)14. IRLSSG scale (IRLS) was used to measure the severity of RLS15. The
36 healthy subjects with no active neurologic disorders were included in the control group. A experienced neurologist performed clinical and neurological examinations on all participants. After 2 days of withdrawal of medication, cerebral vasomotor reactivity (VMR) and flow-mediated dilation (FMD) were measured. To exclude a secondary RLS, several laboratory studies of serum ferritin, iron, iron-binding capacity, folic acid, vitamin B12, fasting glucose, BUN, and creatinine levels were investigated. A standard nerve conduction study was also performed to exclude subjects with active neuropathy.
To evaluate the association between responsiveness to dopaminergic treatment and the degree of endothelial dysfunction, we asked to 34 of RLS patients about improvement of their RLS symptoms after administration of dopamine agonist. The pramipexole or ropinirole were administered for more than two months and then responsiveness was evaluated. Subjects were classified as good response, partial response or no response. Responsiveness was evaluated by patients’ IRLSS after usage of dopamine agonist. Good response was defined as decreased IRLSS more than 50% compared with previous score, and partial response definded as decreased IRLSS between 10% to 50%. Patients with less than 10% decreased or increased IRLSS were sorted to the group of no response.
3
The inclusion criteria for patients were as follows: (i) aged 18–70 years and (ii) no prior treatment for RLS. We excluded a patients who might have a secondary RLS, who had comorbidities such as pregnancy, chronic kidney disease, iron deficiency or peripheral neuropathy. Several medical conditions that could mimic RLS symptoms (such as neuropathic pain syndromes, myalgia, nocturnal leg cramps, venous stasis, leg edema, arthritis, positional discomfort, habitual foot tapping) also excluded. Finally, patients with uncontrolled hypertension, diabetes mellitus, and dyslipidemia also excluded.
All protocols were approved by the institutional review board prior to initiation of the study.
FMD protocol
Endothelial function was evaluated at the brachial artery in response to hyperemia16. Participants were instructed to stop smoking, alcohol, and caffeine within 24 hour prior to the examination. All FMD studies were performed during the early morning, around 8:00 am. The probe were fixed by fixing device on the right arm of the subject to ensure that the diameters were measured at the same location. All procedure was performed by experienced sonographer. We used a 10- to 12-MHz broadband linear-array transducer for brachial artery ultrasound imaging. A Logiq S6 ultrasonographic system (GE Healthcare, Waukesha, WI, USA) was used. To induce the occlusion of brachial artery, a blood pressure cuff was placed on the right forearm and inflating the cuff, at least 50 mmHg above the subject's systolic pressure for 5 min. The pressure was then rapidly released to induce hand and forearm hyperemia and subsequent reactive vasodilation of brachial artery. The procedure was performed twice at 30 minutes intervals, and the brachial artery examined in cross-sectional area. In cross-section imaging, we recorded M-mode tracings from 10 seconds before cuff inflation to 90 seconds after cuff deflation. The minimum
4
(systolic) and maximum (diastolic) diameters of the brachial artery were obtained by obtained sonographic images from the M-mode and B-mode studies. Images were measured at 10 seconds before inflation of the pressure cuff (baseline diameter) and every 10 seconds until 90 seconds after deflation. FMD was defined as the maximum percentage change in brachial artery diameter relative to baseline, after reactive hyperemia procedure.
VMR protocol
The VMR values were measured as percentage increase of the mean flow velocity on transcranial Doppler (TCD) in response to hypercapnia induced by the rebreathing method. TCD was performed using PMD 100M (Spencer Technologies). This system also calculates a power M-mode Doppler (PMD) image with a single-gate spectrogram. VMR was evaluated at the both middle cerebral artery (MCA) and basilar artery (BA) in each patient. Subjects sat in a comfortable and quiet room. Then, subjects were asked to relax and breathe as usual. The subjects’ cardiac rhythm and HR were continuously monitored. Baseline heart rate (HR) and blood pressure (BP) were checked after 5 minutes of rest. During the examination, BP was recorded at intervals of 30 seconds. The stop value, which means HR that the test would be terminated was defined as a 1.1-fold increase in baseline value. As for the re-breathing, the examiner instructed the subjects to fit the mask on their face and breathe 15 times per minute through a 3-liter, limited volume vinyl re-breather bag. The re-breathing procedure was terminated if (i) the mean flow velocity no longer increased, (ii) the HR reached the calculated “stop value,” (iii) or the subject no longer be able to withstand the re-breathing. During re-breathing, the velocities and spectra of the MCAs and BA were simultaneously monitored in response to re-breathing hypercapnia. Baseline mean-flow velocity (Vbase) was measured after a 1 minute of rest. The maximal mean velocity (Vmax) was defined as the mean flow
5
velocity at which no further velocity increase occurred. The VMR was calculated as (Vmax − Vbase) × 100/Vbase17.
Statistics
The Student's t-test and Fisher’s exact test were used to compare the categorical and continuous variables between groups. The correlation between continuous variables, including FMD, VMR and IRLSS was assessed by Spearman's correlation analysis. All statistical analyses were performed using SPSS, ver. 18.0 and a p-value 0.05 was set to indicate statistical significance.
6
Ⅲ. Results
Baseline characteristics
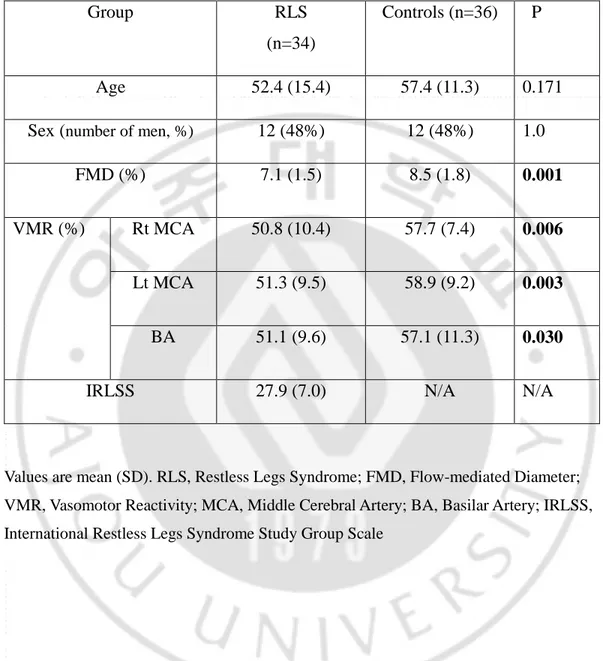
The demographic characteristics of the subjects are shown in Table 1. The average ages of the RLS patients and normal controls were 52.4 (±15.4) and 57.4 (±11.3), respectively. The mean IRLSS in RLS patients was 27.9 (±7.0).
7
Table 1. Basic characteristics and clinical features
Group RLS
(n=34)
Controls (n=36) P
Age 52.4 (15.4) 57.4 (11.3) 0.171
Sex (number of men, %) 12 (48%) 12 (48%) 1.0
FMD (%) 7.1 (1.5) 8.5 (1.8) 0.001
VMR (%) Rt MCA 50.8 (10.4) 57.7 (7.4) 0.006
Lt MCA 51.3 (9.5) 58.9 (9.2) 0.003
BA 51.1 (9.6) 57.1 (11.3) 0.030
IRLSS 27.9 (7.0) N/A N/A
Values are mean (SD). RLS, Restless Legs Syndrome; FMD, Flow-mediated Diameter; VMR, Vasomotor Reactivity; MCA, Middle Cerebral Artery; BA, Basilar Artery; IRLSS, International Restless Legs Syndrome Study Group Scale
8
FMD & VMR responses
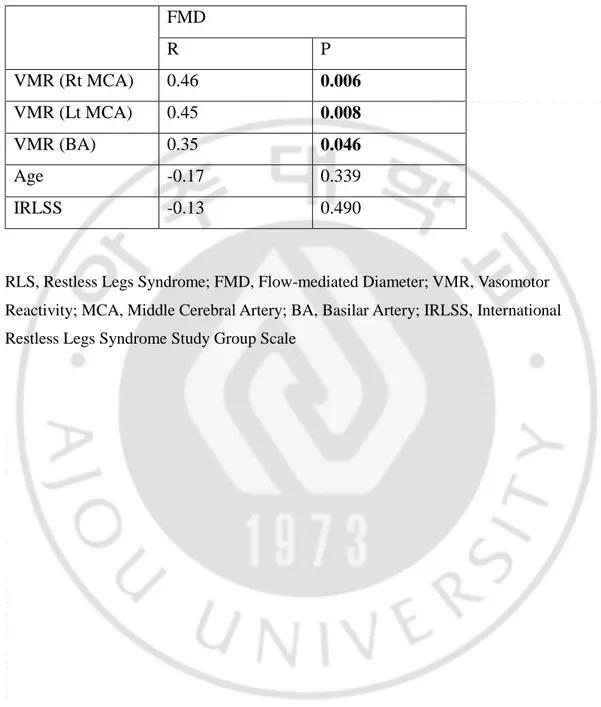
The values of VMR in both MCA (Lt MCA 51.3±9.5%, vs 58.9±9.2%, p=0.003, Rt MCA 50.8±10.4%, vs 57.7±7.4%, p=0.006) and BA (51.1±9.6%, vs 57.1±11.3%, p=0.030) were significantly lower in RLS group than control group. The values of FMD (7.1±1.5% vs 8.5±1.8%, p=0.006) also was significantly lower in RLS patients. The significant correlation between FMD and VMR of every arteries was noted (p=0.006 in Rt MCA, p=0.008 in Lt MCA, p=0.046 in BA). Age and severity of RLS (IRLSS) was not correlated with neither VMR nor FMD (Table 2).
9
Table 2. Correlation between FMD and other values in RLS group
FMD R P VMR (Rt MCA) 0.46 0.006 VMR (Lt MCA) 0.45 0.008 VMR (BA) 0.35 0.046 Age -0.17 0.339 IRLSS -0.13 0.490
RLS, Restless Legs Syndrome; FMD, Flow-mediated Diameter; VMR, Vasomotor Reactivity; MCA, Middle Cerebral Artery; BA, Basilar Artery; IRLSS, International Restless Legs Syndrome Study Group Scale
10
Relationship between responsiveness to dopaminergic treatment and cerebral VMR & FMD
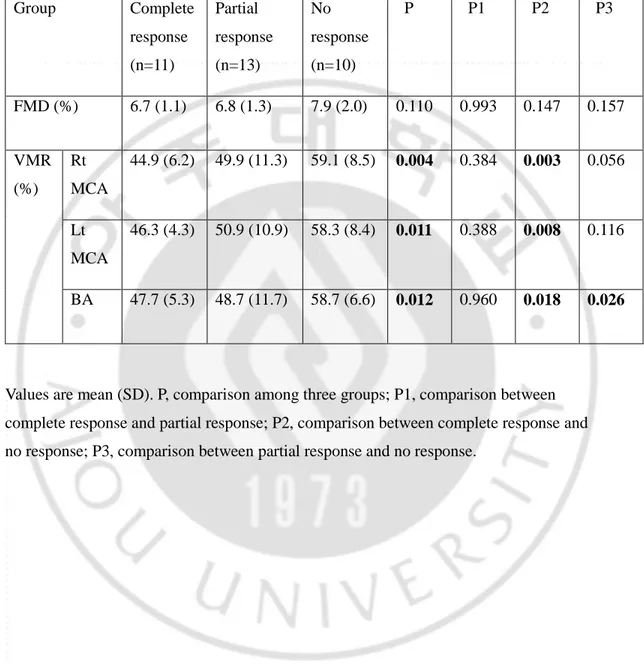
Of the 34 RLS patients, 11 (32.4%) patients reported completely responsive, 13 (38.2%) patients were partially responsive and 10 (29.4%) patients were nonresponsive to dopaminergic treatment. Baseline cerebral VMR (right MCA: complete responsive vs partial responsive vs nonresponsive 44.9±6.2% vs 49.9±11.3 vs 59.1±8.5%, p=0.004) showed significant difference between these three groups. As compared each groups, patients with complete response to dopamine had significantly lower values of VMR in all of tested vessels (both MCA and BA). (Table 3). Patients with partial response to dopamine showed no significant difference with other groups, except values of VMR in BA compared with no response group. But, although no statistical significance, VMR values of partial response group were lower than values of no response group and higher than complete response group (Figure 1). FMD showed no significant difference with other groups (6.7±1.1% vs 6.8±1.3 vs 7.9±2.0%, p=0.11).
11
Table 3. Relationship between responsiveness to dopaminergic treatment and cerebral VMR & FMD Group Complete response (n=11) Partial response (n=13) No response (n=10) P P1 P2 P3 FMD (%) 6.7 (1.1) 6.8 (1.3) 7.9 (2.0) 0.110 0.993 0.147 0.157 VMR (%) Rt MCA 44.9 (6.2) 49.9 (11.3) 59.1 (8.5) 0.004 0.384 0.003 0.056 Lt MCA 46.3 (4.3) 50.9 (10.9) 58.3 (8.4) 0.011 0.388 0.008 0.116 BA 47.7 (5.3) 48.7 (11.7) 58.7 (6.6) 0.012 0.960 0.018 0.026
Values are mean (SD). P, comparison among three groups; P1, comparison between complete response and partial response; P2, comparison between complete response and no response; P3, comparison between partial response and no response.
12
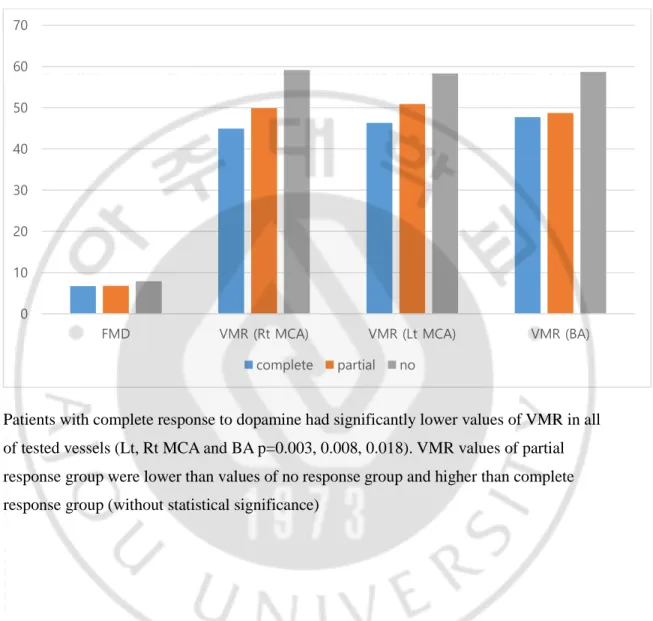
Figure 1. Relationship between responsiveness to dopaminergic treatment and cerebral VMR & FMD
Patients with complete response to dopamine had significantly lower values of VMR in all of tested vessels (Lt, Rt MCA and BA p=0.003, 0.008, 0.018). VMR values of partial response group were lower than values of no response group and higher than complete response group (without statistical significance)
0 10 20 30 40 50 60 70
FMD VMR (Rt MCA) VMR (Lt MCA) VMR (BA)
13
Ⅵ. Discussion
This study shows that VMR & FMD were significantly impaired in RLS patients than healthy controls, and those values of FMD and VMR had a correlation. And, relationship between dopamine response and VMR & FMD is also identified. These findings imply the impaired endothelial function in RLS, that support the correlation between cerebrovascular disease, especially stroke, and RLS. To the best of our knowledge, this study is the first to evaluate both cerebral and systemic peripheral endothelial function in patients with RLS.
The pathophysiology of RLS is poorly understood. The dysfunction of dopaminergic system and iron dysregulation, but definite mechanisms of disease is unclear. Other many hypothesis are suggested, such as opioid system, glutamatergic system, genetics, anatomical network vulnerability, alterations in vascular structure or hypoxia2,18. Of that, the hypothesis that vascular pathologies in the central nervous system or the periphery lead to RLS or PLMS also proposed7. In a number of studies supporting this, RLS patients have shown changes in the peripheral microvasculature or cardiovascular autonomic system, including altered leg blood flow, capillary tortuosity, skin temperatures, heart rate variability and peripheral hypoxia19-23.
Although recent studies have observed the association between RLS and cardiovascular disease, the specific mechanisms underlying this relationship are not yet clear. The periodic limb movement disorder (PLMD) present in the majority of patients with RLS, and these limb movements may induce transient increases in heart rate and blood pressure. These sympathetic hyperactivity was suggested for one of risk factors of cardiovascular disaease7,24. However, Bauer et al reported that, during
polysomnography, blood pressure elevation event was occurred but more than half of these events were recorded during non-PLMD period25. Sleep disturbance
possibly caused by sensory symptoms in RLS also suggested to be a cause of vascular events in RLS4.
14
Our findings could be one of theories to support these vascular pathologies in RLS. Endothelial function is associated with cardiovascular risk factor and is considered to be an early process of atherosclerosis26,27. Shimbo et al reported that endothelial dysfunction assessed by impaired FMD predicted cardiovascular events (MI, stroke and vascular death)11. Leukoaraiosis, migraine and obstructive sleep apnea, which were known to be associated with vascular pathogenesis, demonstrate the impaired FMD and VMR, suggest the relationship between cerebral & systemic endothelial dysfunction and vascular pathologies28-31. In RLS, several research about relationship between vascular disease and RLS was reported, but this study is the first to approach to the vascular event with endothelial dysfunction.
The mechanisms of endothelial dysfunction in RLS could be explained by NO signal pathway. A few studies report that RLS patients have decreased NO levels, increased NOS expression23 and variation in the NOS gene22. As is well known, NO plays an important role in the endothelial function of blood vessel32, thus decrement of NO production can lead to a decreased local blood flow and localized hypoxia. These changes may trigger main clinical symptoms in RLS, as the lower oxygen partial pressure could trigger the firing of peripheral nociceptive C fibers in the legs, and this firing may trigger pain and sensory symptoms in lower limbs33. In addition,
hypoxia inducible factor pathway activation occurs in substantia nigra and brain microvasculature in patients with RLS, and this pathway also associated with NO signal. This hypoxia inducible pathway can result from, or contribute to cellular iron deficiency, which suggest novel model to explain the sequential relationship of iron deficiency, microvascular alteration and development of RLS23.
Dopaminergic agents are widely used to improve the RLS. Levodopa treatment is associated with a higher prevalence of daytime augmentation, in which the symptoms worsen earlier in the day after administration of the drug,34 thus dopamine agonists were preferred now. As there is probably no degeneration of dopaminergic neurons in RLS patients, the mechanism of therapeutic effect from
15
dopaminergic agents were questionable. Even though hypodopaminergic pathology has long been suspected from these therapeutic effect of dopaminergic agents, but consistent dopamine deficiency has not been identified35. Dopamine is known to be associated with heart and vascular system and is an important regulator of systemic BP36. In vascular wall, dopamine induced vascular smooth muscle relaxation to reduce BP36. Also dopamine is reported to modulate functions of endothelial cells via vascular permeability factor/vascular endothelial growth factor37. From this study, we figured out that patients with good responsiveness with dopaminergic treatment showed poorer cerebral endothelial function than patients with no response to dopamine. We consider that vascular effect of dopaminergic therapy improves RLS symptoms, thus impairment of endothelial function is related with therapeutic response. Furthermore, this findings suggest the mechanism of how dopaminergic therapy improves the symptoms of RLS, in which not fully understood yet. Recently, Bauer et al identified that the number of blood pressure elevation events during polysomnography was significantly decreased after administration of dopamine agonist, which support our findings about vascular components in dopaminergic therapy25.
Our previous study suggested the impaired systemic endothelial function in RLS patients, assessed by FMD13. But the mechanism of cerebral endothelial
function is a bit differ from systemic endothelial system, as VMR represents cerebral autoregulation and cerebral vasodilation is mainly response to hypercapnia, and influence of NO is only partial38. Thus, in healthy subjects, they did not appear to correlation between VMR and FMD39. The assessment of cerebral endothelial dysfunction in RLS patients is meaningful, as the evidence to support the association with RLS and cerebrovascular events. Also, anatomical involvements of brain was reported, as iron deficiency or hypoxia inducible factor in substantia nigra, hence only association with peripheral microvascular hypoxia and sensory symptoms in legs is not enough to demonstrate the vascular-related pathophysiology of RLS. To
16
the best of our knowledge, this study is the first study applying VMR to evaluate cerebral endothelial dysfunction, and comparing it to systemic endothelial dysfunction via FMD.
There are several limitations in this study. First, despite age & sex matching between two groups and exclusion process for secondary RLS or RLS mimicking disease was done, other confounding factors on FMD, VMR and RLS such as dietary or lifestyle factors. Second, the cross-sectional design of the study and relatively small number of samples limit the generalization of these results of study to a direct causative effect between RLS and the impairment of FMD and VMR. Third, although sleep fragmentation and sympathetic hypersensitivity caused by PLMD may considered mechanism of association between RLS and vascular events, these were not assessed in this study due to lack of polysomnographic evaluation. Fourth, because patients were recruited from a tertiary referral hospital, patients with more severe RLS symptoms possibly tend to be enrolled in this study, which had been described in a study at a different tertiary hospital in korea40. Fifth, the responsiveness to dopaminergic agonist was evaluated by patients’ subjective feeling, thus objectively documented data or quantitative instruments were not used. IRLSS is commonly used to evaluate the severity of RLS, but there was no standard cut-off value to determine whether RLS is improved or not. Finally, because FMD follow standard circadian rhythms41, RLS symptoms were not actively ongoing around the
17
Ⅴ. Conclusion
This study demonstrated that RLS patients have poorer cerebral and systemic endothelial function than normal healthy subjects. This findings provide further evidence of a possible association between RLS and cardiovascular disease, including ischemic stroke, and also the role of vascular pathologies in the pathogenesis of the RLS. Further prospective, large-scale, randomized controlled studies about endothelial dysfunctions in RLS and the results of vascular-targeted therapy are needed.
18
References
1. Smith JE, Tolson JM. Recognition, diagnosis, and treatment of restless legs syndrome. J Am Acad Nurse Pract 2008;20:396-401.
2. Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol 2010;6:337-46.
3. Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord 2001;16:1159-63.
4. Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of
restless legs syndrome and mortality among men. Neurology 2013;81:52-9.
5. Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation 2012;126:1689-94.
6. Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology 2008;70:35-42.
7. Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 2009;32:589-97.
8. Lin CH, Sy HN, Chang HW, et al. Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur J Neurol 2015;22:142-9.
9. Ferri R, Cosentino FI, Moussouttas M, et al. Silent Cerebral Small Vessel Disease in Restless Legs Syndrome. Sleep 2016;39:1371-7.
10. Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol 1997;30:325-33.
11. Shimbo D, Grahame-Clarke C, Miyake Y, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis 2007;192:197-203.
19
12. Stout M. Flow-mediated dilatation: a review of techniques and applications. Echocardiography 2009;26:832-41.
13. Koh SY, Kim MS, Lee SM, Hong JM, Yoon JH. Impaired vascular endothelial function in patients with restless legs syndrome: a new aspect of the vascular pathophysiology. J Neurol Sci 2015;359:207-10.
14. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med 2014;15:860-73.
15. Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 2003;4:121-32. 16. Yoon JH, Lee JS, Yong SW, Hong JM, Lee PH. Endothelial dysfunction and hyperhomocysteinemia in Parkinson's disease: flow-mediated dilation study. Mov Disord 2014;29:1551-5.
17. Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001;32:1552-8.
18. Koo BB, Bagai K, Walters AS. Restless Legs Syndrome: Current Concepts about Disease Pathophysiology. Tremor Other Hyperkinet Mov (N Y) 2016;6:401.
19. Salminen AV, Rimpila V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease). Neurology 2014;82:1856-61.
20. Oskarsson E, Wahlin-Larsson B, Ulfberg J. Reduced daytime intramuscular blood flow in patients with restless legs syndrome/Willis-Ekbom disease. Psychiatry Clin Neurosci 2014;68:640-3.
21. Izzi F, Placidi F, Romigi A, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med 2014;15:1392-7.
22. Anderson KN, Di Maria C, Allen J. Novel assessment of microvascular changes in idiopathic restless legs syndrome (Willis-Ekbom disease). J Sleep Res 2013;22:315-21. 23. Patton SM, Ponnuru P, Snyder AM, Podskalny GD, Connor JR. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur J Neurol 2011;18:1329-35. 24. Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and
20
coronary heart disease in women. Arch Intern Med 2003;163:205-9.
25. Bauer A, Cassel W, Benes H, et al. Rotigotine's effect on PLM-associated blood pressure elevations in restless legs syndrome: An RCT. Neurology 2016;86:1785-93. 26. Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 2004;109:613-9.
27. Corti R, Fuster V, Badimon JJ. Pathogenetic concepts of acute coronary syndromes. J Am Coll Cardiol 2003;41:7S-14S.
28. Gonzalez-Quintanilla V, Toriello M, Palacio E, et al. Systemic and cerebral endothelial dysfunction in chronic migraine. A case-control study with an active comparator. Cephalalgia 2016;36:552-60.
29. Zupan M, Sabovic M, Zaletel M, Popovic KS, Zvan B. The presence of cerebral and/or systemic endothelial dysfunction in patients with leukoaraiosis--a case control pilot study. BMC Neurol 2015;15:158.
30. Rajan R, Khurana D, Lal V. Interictal cerebral and systemic endothelial dysfunction in patients with migraine: a case-control study. J Neurol Neurosurg Psychiatry 2015;86:1253-7.
31. Jimenez Caballero PE, Coloma Navarro R, Ayo Martin O, Segura Martin T. Cerebral hemodynamic changes at basilar artery in obstructive sleep apnea syndrome after continuous positive airway pressure treatment. J Stroke Cerebrovasc Dis 2013;22:e93-8. 32. Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart 2001;85:342-50.
33. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655-66.
34. Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep 1996;19:205-13.
35. Scholz H, Trenkwalder C, Kohnen R, Riemann D, Kriston L, Hornyak M. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev 2011:CD006009.
36. Zeng C, Zhang M, Asico LD, Eisner GM, Jose PA. The dopaminergic system in hypertension. Clin Sci (Lond) 2007;112:583-97.
21
37. Basu S, Nagy JA, Pal S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 2001;7:569-74.
38. Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol 1994;266:R546-52.
39. Palazzo P, Maggio P, Passarelli F, et al. Lack of correlation between cerebral vasomotor reactivity and flow-mediated dilation in subjects without vascular disease. Ultrasound Med Biol 2013;39:10-5.
40. Lim YM, Chang SE, Chung S, Kang BH, Kim KK. Small fiber function in drug
naive patients with idiopathic restless legs syndrome. J Clin Neurosci 2012;19:702-5. 41. Al Mheid I, Corrigan F, Shirazi F, et al. Circadian variation in vascular function and regenerative capacity in healthy humans. J Am Heart Assoc 2014;3:e000845.
22 - 국문요약 -
하지불안증후군 환자에서의 뇌혈관 및 말초혈관
내피세포의 기능 저하
서론: 하지불안 증후군은 수면 관련 운동질환으로써, 야간에 하지의 불편한 감각을 느끼는 증상을 특징으로 한다. 철결핍 및 도파민 시스템의 이상이 하지불안증후군에 주요한 기전으로 알려져있으나 그 정확한 생태병리학적 기전은 아직 완전히 밝혀져 있지 않다. 최근 심혈관질환과 하지불안증후군의 관련성이 지속적으로 보고되고 있으며 내피세포의 기능저하는 동맥경화나 심혈관질환의 초기단계에서 중요한 변화이다. 우리는 이러한 관련성에 주목하여, 내피세포의 기능저하가 하지불안증후군의 발병에 관련되어 있을 가능성을 고려하였고 FMD 및 VMR 검사를 통해 실제로 하지불안증후군 환자에서 뇌혈관 및 말초혈관의 내피세포의 기능저하 여부에 대해 알아보았다. 연구대상 및 방법: 본 연구에서는 2014 년 3 월부터 2017 년 4 월 사이에 아주대학교 신경과 파킨슨 및 이상운동 외래에 방문한 환자를 대상으로 하였다. 34 명의 하지불안증후군 환자와 36 명의 나이와 성별을 매칭한 대조군이 모집되었다. 모집된 참가자들을 대상으로 임상적인 문진과 신경학적 검사를 시행하였고 FMD, VMR 검사를 모두 시행하였다. 또한 내피세포의 기능저하 정도와 도파민 치료에 대한 반응성을 확인하기 위해 34 명의 하지불안증후군 환자에게 도파민 효능제를 복용한 후 증상의 호전 정도를 확인하여 내피세포 기능의 저하 정도와 비교하였다. 결과: 하지불안증후군 환자에서의 VMR 값은 중대뇌동맥과 기저동맥에서 모두 대조군에 비해 저하되었다 (좌측 중대뇌동맥 51.3±9.5%, vs 58.9±9.2%, p=0.003, 우측 중대뇌동맥 50.8±10.4%, vs 57.7±7.4%, p=0.006, 기저동맥 51.1±9.6%, vs 57.1±11.3%, p=0.030). FMD 값 또한 하지불안증후군 환자에서 유의미하게 낮은23 수치를 보였다 (7.1±1.5% vs 8.5±1.8%, p=0.006). 하지불안증후군 환자에서 도파민 효능제에 반응이 좋을수록 VMR 값이 더 낮은 결과를 보였다 (좋은 반응군 vs 부분적인 반응군 vs 반응없음 44.9±6.2% vs 49.9±11.3 vs 59.1±8.5%, p=0.004). 결론: 본 연구를 통해 하지불안증후군 환자들은 대뇌혈관과 말초혈관에서 내피세포의 기능저하가 있는 것을 확인하였다. 이 결과를 통해 하지불안증후군과 심혈관질환 사이에 병태생리학적인 연관성이 있으며 하지불안증후군의 발생에 혈관의 문제가 일부 기여할 수 있음을 시사한다. 핵심어 : 하지불안증후군, 내피세포 기능, 혈관운동 반응도 검사