저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
醫學 博士學位 論文
Management of cartilage deficiency by applying
extracellular matrix membrane on bone
marrow stimulation technique and evaluating
the quality of repaired cartilage
By
Long Hao Jin
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Management of cartilage deficiency by applying
extracellular matrix membrane on bone marrow
stimulation technique and evaluating the quality of
repaired cartilage
by
Long Hao jin
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the Degree
of
Ph.D. in Medicine
Supervised by
Byoung-Hyun Min, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
i -ABSTRACT-
Management of cartilage deficiency by applying extracellular matrix membrane on bone marrow stimulation technique and evaluating the quality of repaired cartilage
Articular cartilage is a resilient load-bearing tissue that forms the articulating surface of diarthrodial joints and provides low friction, lubrication, and wearless characteristics necessary for repetitive gliding motion. However, because articular cartilage has neither vascular nor lymphatic channel supply and hence difficult access to stem cells, its self-healing potential is restricted. If left untreated, cartilage damage results in osteoarthritis (OA) and its endstage from, can only be managed by joint replacement surgery. We hereby provide means to repair cartilage using extracellular matrix (ECM) membrane on bone marrow stimulation (BMS) techic and development of a novel analytic method for detection of engineered cartilage.
Chapter Ⅰ: BMS has been regarded as a first line procedure for repair of articular cartilage. However, repaired cartilage from BMS is known to be unlike that of hyaline cartilage and its inner endurance is not guaranteed. The reason presumably came from a shortage of cartilage forming cells in blood clots derived by BMS. In order to increase repairable cellularity, the feasibility of autologous bone marrow-derived buffy coat transplantation in repair of large full-thickness cartilage defects was investigated in this study. Rabbits were divided into four groups: the defect remained untreated as a negative control; performance of BMS only (BMS group); BMS followed by supplementation of autologous bone marrow buffy coat (Buffy coat group); transplantation of autologous osteochondral transplantation (AOTS) as a positive control. Repair of cartilage defects in the Buffy coat group in a rabbit model was more effective than BMS alone and similar to AOTS. Gross findings, histological analysis, histological scoring, immunohistochemistry, and chemical assay demonstrated that supplementation of autologous bone marrow buffy coat after BMS arthroplasty effectively repaired cartilage defects in a rabbit model, and was more effective than BMS arthroplasty alone. Supplementation of autologous bone marrow-derived buffy coat in cases of BMS could be a useful clinical protocol for cartilage repair.
ii
Chapter Ⅱ: The recombinant human transforming growth factor beta-3 (rhTGF-β3) is
known as a key regulator of chondrogenesis of stem cells and cartilage formation. We have developed a novel drug delivery system that continuously releases rhTGF-β3 using an
extracellular matrix (ECM) membrane fabricated from cultured porcine chondrocytes. We hypothesized that rhTGF-β3-loaded ECM membrane could significantly enhance cartilage
regeneration, when it was applied on the osteochondral defect of rabbit articular cartilage treated with the bone marrow stimulation (BMS) technique. New Zealand white rabbits of 16 weeks old (an average weight of 3.0-3.5kg, n=78) were subjected to osteochondral defect on right knee joints and divided into four groups: The defect was (1) left untreated as a negative control (Control group), (2) covered with ECM membrane after BMS (Control-m), (3) treated with direct injection of rhTGF-β3,and covered with the ECM membrane after BMS
(TGF-i) and (4) covered with rhTGF-β3-loaded ECM membrane after BMS (TGF-m). The
results showed that repair of osteochondral defects was more efficient in the TGF-m group than the other groups including that with direct injection of rhTGF-β3 in gross findings,
histological analysis, histological scoring and chemical assays for GAGs, collagen and DNA contents. We speculate that the sustained release of rhTGF-β3 using the ECM membrane after BMS could be a useful clinical protocol for cartilage repair.
Chapter Ⅲ: In cartilage tissue engineering, it is very important to evaluate specifically and non-destructively the quality of engineered tissues in terms of matrix content and their three dimensional (3D) distribution. The objective of this study was to evaluate the feasibility of using microfocal computed tomography (µCT) with Hexabrix, a contrast agent, in examining the quality of engineered cartilage. Chondrocytes were isolated from the knee articular cartilage of 2- to 3-week-old rabbits. Cells at passage 2 (5.0 x 105/ml) were loaded dynamically on polyglycolic acid (PGA) scaffolds (2 mm in diameter and 2 mm in thickness), and cultivated in a chondrogenic defined medium for 7, 14, 21and 28 days in
vitro. The engineered cartilages were incubated with undiluted Hexabrix 320 for 20 min and
analyzed by µCT scanning. The scanning data were visualized by 2D and 3D images and quantified by counting the number of voxels. The results were validated by histological images of engineered cartilages by Safranin-O staining and proteoglycan content by
iii
biochemical analysis. The optimal threshold value for quantification was determined by regression analysis with the total contents of sulfated glycosaminoglycans (GAGs) from 14 individual samples. The biological effect of uCT scanning on the engineered cartilage was also examined for cell viability and total contents of sulfated GAGs and DNA. The 2D µCT image of an engineered cartilage was matching well with the histological image of corresponding section by Safranin-O staining. Quantitative data obtained by 3D µCT images of 14 engineered cartilages showed a strong correlation with sulfated GAGs contents obtained by biochemical analysis (R2=0.883, P<0.001). Repeated exposure of engineered cartilages to Hexabrix 320 and µCT scanning did not affect significantly cell viability, total DNA content and total sulfated GAGs content. µCT imaging using Hexabrix 320 provides high spatial resolution and sensitivity to assess sulfated GAGs content and 3D distribution in engineering cartilage. Therefore it could be a valuable tool to evaluate the quality of engineered cartilage and greatly benefit its developmental process.
Key Words: Cartilage repair, Bone marrow stimulation (BMS), Buffy coat, ECM membrane, rhTGF-β3, Micro-CT, Engineering cartilage
iv TABLE OF CONTENTS ABSTRACT ... ⅰ TABLE OF CONTENTS ··· ⅳ LIST OF FIGURES ... ⅵ LIST OF TABLES ... ⅵ GENERAL INTRODUCTION ... 1 CHAPTER Ⅰ ... 13 1.1 Introduction ... 14
1.2 Materials and Methods ··· 15
1.3 Results ··· 20
1.4 Discussion ··· 29
1.5 Conclusion ··· 32
CHAPTER Ⅱ ··· 33
2.1 Introduction ... 34
2.2 Materials and Methods ··· 35
2.3 Results ··· 38
2.4 Discussion ··· 43
2.5 Conclusion ··· 43
CHAPTER Ⅲ ··· 44
3.1 Introduction ... 45
3.2 Materials and Methods ··· 46
3.3 Results ··· 49
3.4 Discussion ··· 57
3.5 Conclusion ··· 59
v
vi LIST OF FIGURES
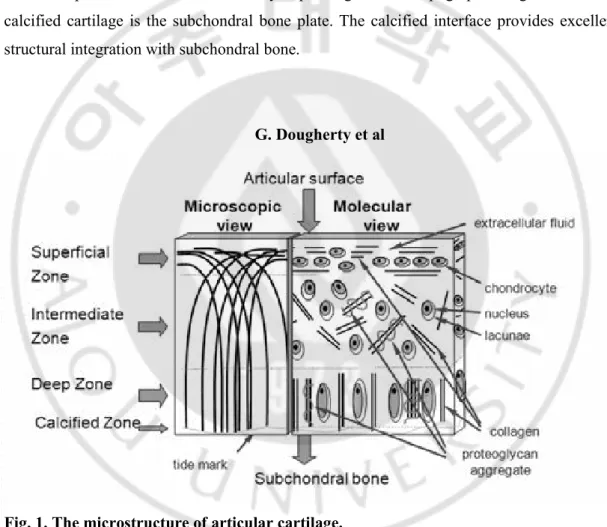
Figure. 1. The microstructure of articular cartilage ··· 2
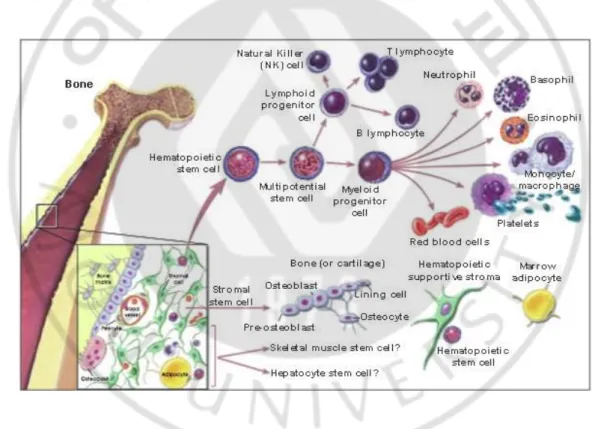
Figure. 2. Hematopoietic and stromal stem cell differentiation ··· 7
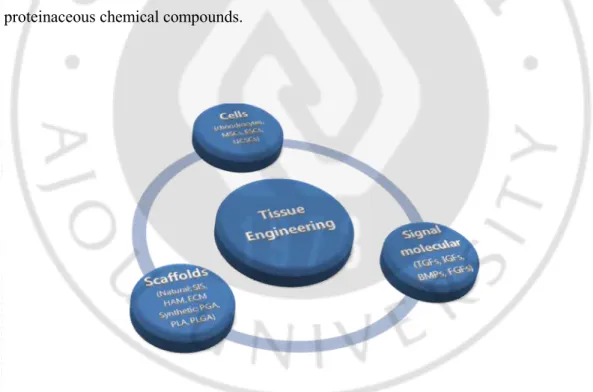
Figure. 3. Component of tissue engineering ··· 8
Figure. 1-1. Experimental design and surgery··· 17
Figure. 1-2. Gross findings and histological evaluation of cartilage defects··· 23
Figure. 1-3 Sirius red staining image ··· 25
Figure. 1-4. ICRS scores ··· 26
Figure. 1-5. Immunohistochemistry for collagen type II expression ··· 27
Figure. 1-6. Chemical assay of repaired tissue ··· 28
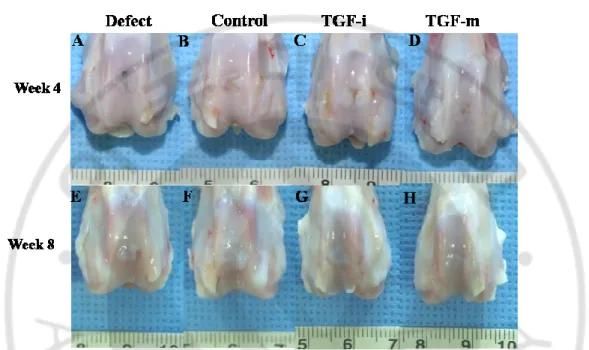
Figure. 2-1. Gross findings ··· 39
Figure. 2-2. Histological evaluation of cartilage defects ··· 40
Figure. 2-3 ICRS scores ··· 41
Figure. 2-4. Chemical assay of repaired tissue ··· 42
Figure. 3-1. Optimization of Hexabrix 320 treatment ··· 49
Figure. 3-2. Comparison between sulfated GAG distribution and µCT images ··· 51
Figure. 3-3. Observation and measurement of sulfated GAGs content ··· 53
Figure. 3-4. Observation of GAG in 3D image ··· 54
Figure. 3-5. Effect of Hexabrix 320 and µCT radiation on engineered cartilage ··· 56
LIST OF TABLES Table 1-1. Total MSC contents ··· 20
1
u General introduction
1. Articular cartilage
Gliding surfaces of synovial joint are covered with a specialized type of hyaline cartilage, call ‘articular cartilage’. Articular cartilage is a resilient load-bearing tissue that forms the articulating surfaces of diarthrodial joints and provides the low friction, lubrication, and wears characteristics required for repetitive gliding motion. It also absorbs mechanical shock and spreads the applied load onto subchondral bone. Human articular cartilage provides these essential biomechanical functions mostly for 8 decades or more. However, it has restricted self-healing potential. The reason for this low repair potential has been unknown, but articular cartilage has neither vascular supply nor easy access to stem cells (Hunziker, 2002; Prakash and Learmonth, 2002), and low cell mobility due to surrounding matrix, and a limited number of progenitor cells could be contributing factors (Tallheden et al., 2003). Until now much effort has been made to repair cartilage defects.
1-1 Microstructure
Articular cartilage has a simple architecture, both cells and matrix distribute within successive cartilage layers identified as superficial, intermediate, deep and calcified zones (Fig. 1). At its superficial surface, which is contacted by synovial fluid, the chondrocytes are flattened and aligned, and the extracellular matrix (ECM) is densely packed with collagen fibers running parallel to surface. Below the superficial zone is the middle zone where cell density is lower. The ultrastructure of the middle zone reveals morphologic features more typical of a hyaline cartilage with more rounded cells and an extensive extracellular matrix. Only few direct cell-cell interaction occur between the chondrocyte as the cell are relatively isolated, either individually or in small aggregates, in small cavities within the ECM called lacunae. The organization of the collagen fibers becomes more stochastic in the middle zone, and the proteoglycan content in the ECM increases. Between this middle zone and the layer of calcified cartilage is the deep zone, the chondrocyte are arranged in columns perpendicular to the surface and underlying bone. Collagen fibers in the deep zone are also organized in this manner. Aggrecan content and fibril diameter are maximal in the deep zone,
2
whereas collagen content is minimal. Below the articular cartilage, and separated from it, is a layer of calcified cartilage. The calcified cartilage is not very vascular normally, and the remodeling process is therefore not as effective as in vascularised locations. Cell density is lowest in this zone. The chondrocytes in this calcified zone usually express the hypertrophic phenotype, reaching a stage of differentiation that can also be found in fracture repair. The specific molecular composition of the ECM varies between the zones, with ECM molecules residing uniquely in some zones. Among the collagen, collagen type X is coupled with chondrocyte hypertrophy and is considered unique for the calcified zone that borders on the underlying bone. The cellular activity also differs between the topographical zones, and the metabolic potential of the cells can vary depending on their topographic origin. Below the calcified cartilage is the subchondral bone plate. The calcified interface provides excellent structural integration with subchondral bone.
G. Dougherty et al
3
1-2 Chondrocytes
Articular cartilage consists of a relatively sparse population of cells called chondrocyte. Chondrocytes are responsible for the majority of articular cartilage metabolic activity and are encased in the matrix, which are responsible for synthesizing and maintaining. In humans, chondrocytes represent only about 1% of the volume of hyaline cartilage (less than 10% of dry weight), but are essential since it is these cells that cells that replace degraded matrix molecules to maintain the correct size and mechanical properties of the tissue (Temenoff and Mikos, 2000).
Chondrocytes impose two main features. They constitute the cellular aspect of chondral tissue and they play a central role in bone formation through the process of endochondral ossification. The process of endochondral ossification is an example of the close functional relationship between osteoblasts and chondrocytes. These mesenchyumal condensations take on the shape of the skeletal elements for which they will serve as templates, and the precursor cells present in these dense cell masses then differentiate along an osteogenic pathway or a chondrogenic pathway. Cells that differentiate along the chondrogenic pathway initiate a specific genetic program in which they express a number of characteristic ECM components including type II collagen, type IX collagen, and type XI collagen. These cells progress through maturation to hypertrophy, when they express another cadre of ECM component including type X collagen. Hypertrophic chondrocytes have the ability to secrete matrix vesicles containing enzymes that actively degrade and mineralize their surrounding matrix. As these cells undergo apoptosis the tissue space is then filled by blood vessel, osteogenic cells and mesechymal precursors, finally leading to the formation of new trabecular bone.
1-3 Proteoglycan
These protein polysaccharide molecules form 10~20% wet weight. There are two major classes of proteoglycans found in articular cartilage, large aggregating proteoglycan monomers or aggrecans and small proteoglycans including decorin, biglycan and fibromodulin. Proteoglycans are composed of about 95% polysaccharide about 5% protein. The proteoglycans present in articular cartilage bind water and account for the swelling
4
pressure that makes it highly resilient to compressive loads. Negatively charged hydrophilic proteoglycan or aggrecan molecules are composed of glycosaminoglycan (GAG) attached to a linear core protein. Proteoglycans provide elasticity and resilience. GAG chains are unbranched polysaccharides made from disaccharides of an amino sugar and another sugar. At least one component of the disaccharide has a negatively charged sulfate or carboxyl group, so the GAGs tend to repel each other and other anions while attracting actions and facilitating interaction with water. Hyaluronic acid, chondroitin sulfate, keratan sulfate, dermatan sulfate and heparan sulfate are some of the GAGs generally found in articular cartilage (Wirth and Rudert, 1996; Cohen et al., 1998). There are both large aggregating monomers and smaller proteoglycans present in articular cartilage. The aggregating proteoglycans, or aggrecans, are composed of monomers with keratan sulfate and chondroitin sulfate GAGs attached to the protein core. In most aggrecan molecules, link proteins connect many (up to 300) of these monomers to a hyaluronic acid chain. Aggrecans fill most of the interfibrillar space of the ECM and are thought to be responsible for much of the resilience and stress distribution in articular cartilage through their ability to attract water. There are no chemical bonds between the proteoglycans and collagen fibers; aggregation prevents diffusion of the proteoglycans out of the matrix during joint loading (Wirth and Rudert, 1996; Buckwalter and Mankin, 1998; Cohen et al., 1998). The smaller proteoglycans include decorin, biglycan and fibromodulin. They have shorter protein cores and fewer GAG chains than their larger counterparts. Unlike aggrecans, these molecules do not affect physical properties of the tissue, but are thought to play a role in cell function and organization of the collagen matrix (Buckwalter and Mankin, 1998).
1-4 Collagen
Ninety percent of the collagen in articular cartilage is type II (10~20% of wet weight), which makes the cartilage resistant to shear and tension. Collagen types II, VI, IX, X and XI are found in articular cartilage, although type II accounts for 90~95% of the collagen in the matrix. Type II collagen fibers are predominantly responsible for the structure of hyaline cartilage with its strength and tensile stiffness. Types IX and XI, along with type II, form fibrils that interweave to form a mesh. This organization provides tensile strength as well as
5
physically entrapping other macromolecules. Although the exact function of types IX and XI are unknown, type IX has been observed to bind superficially to the fibers and extending into the inter-fiber space to interact with other type IX molecules, possibly acting to stabilize the mesh structure. Type X is found only near areas of the matrix that are calcified (Cohen et al., 1998).
1-5 Noncollagen protein
In contrast to proteoglycans, glydoproteins have only a small amount of oligosaccharide associated with the protein core. These polypeptides help to stabilize the ECM matrix and aid in chondrocyte-matrix interactions. Both anchorin CII and cartilage oligomeric protein anchor chondrocytes to the surrounding matrix. Other noncollagenous proteins commonly found in most tissues, such as fibronectin and tenascein, are also observed in articular artilage and believed to perform similar functions as the glycoproteins (Buckwalter and Mankin, 1998).
1-6 Water
Water comprises approximately 60~80% of the wet weight of articular cartilage and interacts with negatively charged GAG chains of proteoglycan, 65% in the deep zone. It allows load-dependent deformation of the cartilage.
2. Cartilage repair strategies
Human articular cartilage provides these essential biomechanical functions mostly for 8 decades or more. However, because articular cartilage has neither vascular nor lymphatic channel supply and hence difficult access to stem cells, its self-healing potential is restricted. The reason for this low repair potential has been unknown, but articular cartilage has neither vascular supply nor easy access to stem cells, and low cell mobility due to surrounding matrix, and a limited number of progenitor cells could be contributing factors. Until now much effort has been made to repair cartilage defects. For example, autologous chondrocyte transplantation, marrow-stimulation procedures like subchondral bone microfracture and autologous osteochondral graft (AOG) have been developed to restore normal structure and
6
function of cartilage. But, the reconstruction of hyaline cartilage and subchondral bone has not been fully realized. Autologous chondrocytes have significant limitations as a cell source for cartilage regeneration, such as the small number of cells available and their restricted proliferation capacity, Moreover they tend to dedifferentiate and gradually lose their specific phenotypes during monolayer in vitro culture. In the case of AOG, multiple osteochondral grafts are removed from non-weight bearing area of the joint surface and implanted into the defect area. However, the osteochondral grafts may exhibit fibrillation and degradation with time. The technique of autologous chondrocytes implantation (ACI) has been developed to allow the regeneration of hyaline like cartilage in order to delay and possibly prevent the onset of degenerative osteoarthritis. Articular chondrocytes are responsible for the unique features of articular cartilage; therefore, it seems rational to use committed chondrocytes to repair a cartilaginous defect. This initial report had a high impact on cartilage surgery and was regarded as a breakthrough for cellbased cartilage repair strategies. Furthermore, the size of the defect that can be repaired is still limited. Among these surgery techniques, bone marrow stimulation (BMS) technique has been widely used for the regeneration of articular cartilage defect, because it could provide a suitable environment for new tissue formation and it might utilize the natural healing power of the living body. BMS can facilitate the intrinsic healing response of MSCs or progenitor cells recruited by subchondral penetration to the bone marrow and can stimulate cartilage regeneration. But it was shown to regenerate fibrocartilage having poor mechanical properties when compared with the desired hyaline cartilage. And also the thickness of repaired tissue after BMS was usually thinner than that of the native articular cartilage.
3. Bone marrow cell
It is well known that bone marrow contains multipotent stem cells that can differentiate not only into haematopoietic cells (including B lymphocytes, T lymphocytes, neutrophils and macrophages) but also into nonhaematopoietic cells (including endothelial cells, pericytes, chondrocytes, osteoblasts, adipocytes, neural component cells, fibroblasts and probably keratinocytes) (Fig. 2). Azizi et al. reported that several investigators who are treating various intractable diseases (including ischemic myocardial diseases, osteogenesis imperfect,
7
Duchenne’s muscular dystrophy, and central nervous system disorders, such as Parkinson’s disease) are focusing on the use of bone marrow stem cells (Azizi et al., 1998). Many studies have reported on the therapeutic potential of bone marrow-derived mononuclear cells for regeneration of various types of tissues (Ohnishi et al., 2007). These cells have been used for treatment of non-traumatic avascular necrosis of the femoral head (Karatoprak et al., 2008), infarction of the myocardium (Yip et al., 2008), and cartilage damage (Chang et al., 2008) and have also been applied to a continuous spinal fusion mass (Viggeswarapu et al., 2001). In terms of cartilage repair, as bone marrow mononuclear cells (MNCs) can be fractionated to contain higher proportion of MSCs, they can potentially be used after microfracture to provide more MSCs in the defect area (Viggeswarapu et al., 2001; Chang et al., 2008). In fact, so far no perfect methods and results have been found to substitute the shapely articular cartilage normally present in the joint. Recently, the tissue engineering technology has been a strong candidate as regeneration for damaged articular cartilage.
8
4. Tissue engineering of cartilage
The tissue engineering technique first introduced in the late 1980s. It is a multidisciplinary research area that incorporates both biological and engineering principle for the purpose of generating new, living tissues to replace the diseased/damaged tissue and restore tissue/organ function. So far, tissue engineering technique was widely used in various filed of regenerative medicine. Among them, for successful articular cartilage tissue regeneration three tools are necessary (Fig. 3): 1. Tissue specific cell including stem cell (embryonic stem cell (ESC), mesenchymal stem cell (MSC), umbilical cord stem cell (UCSC)), chondrocytes, perichondrials cells, periosteum; 2. Biocompatible carrier scaffolds by which cells are supported and can develop. It is including natural materials such as small intestinal submucosa (SIS) De-mineralized bone matrix (DBM), human amniotic membrane (HAM), fibrin, collagen and synthetic materials such as polyglycolic acid (PGA), polylactic acid (PLA) and polylactic-glycolic acid (PLGA); 3. Signaling molecules including growth factors (tissue growth forming factors (TGFs), insulin-like growth factors (IGFs), bone morphogenetic proteins (BMPs), fibroblasts growth factors (FGFs)), cytokines and non-proteinaceous chemical compounds.
9
4-1 ECM membrane
Among these approaches, use of ECM materials is of particular interest. The ECM is thought to provide an optimal environment for differentiation of stem cells particularly into the cells of same origin as that of the ECM material. Thus, many studies attempted to employ tissue specific ECM materials for the regeneration of the tissue (Hudson et al., 2004). Scaffolds using ECM materials have been manufactured from many different tissues such as dermis, small intestine, urinary bladder, heart, trachea, and lung and from a variety of species including human, porcine, bovine, and equine (Cortiella et al., 2010; Ott et al., 2010). Li et al. reported a novel membrane type biomaterial made of cartilage ECM that is derived from porcine chondrocytes. The cartilage ECM membrane successfully protected the blood clots produced by BMS and enhanced the hyaline cartilage tissue formation in beagle models of cartilage defects. The cartilage ECM membrane not only has the appropriate biological properties that allow cell attachment and proliferation but also boasts suitable physical properties being semipermeable, biodegradable, and strong enough to protect blood clots. Considering that its components are similar to native cartilage and immuneprivileged on implantation, these physicochemical properties of the cartilage ECM membrane are believed to make it a better choice not only for BMS but also in other regenerative and reparative protocols of cartilage.
4-2 TGF-β
The TGF-β family has been identified as key regulators of mesenchymal-stem-cell (MSC) differentiation for chondrogenesis (Catelas et al., 2008). The TGF-β cytokines controls the production of extracellular matrix (ECM) by stimulating the synthesis of collagens, fibronectin and proteoglycans (Ignotz et al., 1987; Steinbrech et al., 2000). TGF-β is also appeared to have positive effects on cartilage differentiation and repair (Hunziker et al., 2001; Mierisch et al., 2002; Miura et al., 2002). On the contrary, TGF-β may exert detrimental effects on cartilage depending on the dose and length of exposure. For instance, long exposure to high doses of TGF-β increased osteophyte formation (Mierisch et al., 2002). Thus, the ability to release TGF-β in a controlled fashion is crucial to the optimal application of this growth factor for cartilage regeneration.
10
4-3 Drug delivery system (DDS)
The advantages of a controlled release system are that active ingredients are released at controlled rates over prolonged periods of time; that loss of ingredients during processing can be avoided or reduced; and that reactive or incompatible components could be separated. Sustained release of therapeutic drugs without degradation of the compounds, and biocompatibility of the delivery vehicle, are key factors in developing an efficient and applicable drug delivery system (DDS) with limited, adverse side-effects. Therefore, there are many reported efforts to control the release of conjugated drugs using various shapes (i.e., 3D porous scaffolds), hydrogels, and multilayer films (e.g., chitosan, PLGA, PLLA, alginate, and fibrin) (Sohier et al., 2007; Catelas et al., 2008; Kim et al., 2012). Unfortunately, most technologies are faced with the possible loss of biological activity of the drug loaded during the ‘growth factor-polymer formulation’ processes, which often include the use of heat, sonication, and organic solutions (Strobel et al., 2011).
The layer-by-layer (LbL) technique makes it possible to deposit functional polymer coatings on surfaces of variable chemistry and shape (Wang Y et al., 2008). Significant efforts have been made to design LbL structures as coatings to deliver small molecules, drugs and biomolecules from the surfaces of biomedical devices (Pavlukhina and Sukhishvili, 2011). The potential of ‘drug-loaded polyelectrolyte multilayers’ as antimicrobial, anti-inflammatory, and anticancer coatings has already been successfully demonstrated (Wang et al., 2009; Karlsson et al., 2010; Kittitheeranun et al., 2010).
5 Assessment of engineering cartilage
Distribution and quantity of these ECM molecules are generally examined by histological and biochemical analyses to evaluate the quality of native or tissue engineered cartilage (Cui et al., 2006; Cui JH et al., 2010). Histological analysis shows distribution of the ECM molecules, but only provides two-dimensional (2-D) information and cannot offer quantitative data. On the other hand, it is possible to operate quantitative measurement with biochemical analysis, but this analysis does not provide observation on distribution (Palmer et al., 2006). Additionally, longitudinal monitoring of ECM changes with time are
11
impossible because of the destructive nature of these histological and biochemical techniques (Palmer et al., 2006). Imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) could be non-destructive substitutes to evaluate the quality of native and engineered cartilage. Current MRI techniques, including delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), can produce three-dimensional (3-D) information of PG concentration and distribution in a cartilage (Burstein et al., 2001; Kurkijarvi et al., 2007; Trattnig et al., 2007). But they have limitations of high price and low resolution. It was also reported that they may have false positive or false negative results in the detection of the patellofemoral (PF) lesions (Collier et al., 1985; al-Janabi, 1994).
Microfocal computed tomography (µCT) using contrast agents are also widely investigated to observe cartilage (Cockman et al., 2006; Piscaer et al., 2008; Xie et al., 2009). µCT is an X-ray-based and high resolution imaging modality that enables 3D and quantitative morphological analysis at a micrometer-level voxel resolution. Recently, Equilibrium Partitioning of an Ionic Contrast agent via µCT (EPIC-µCT), a nondestructive imaging technique combining µCT with a charged X-ray-absorbing contrast agent, was shown to provide direct in situ visualization of articular cartilage morphology in a rabbit femur (Palmer et al., 2006). Hexabrax is a representative contrast medium and is sterile and immune-free diagnosis solution with high density. Mechanism on cartilage is not clearly understood yet, but it is widely known that negative charges on Hexabrix and sulfated glycosaminoglycans (GAGs) repulse each other and thus GAG-rich regions are not labeled with Hexabirx and negatively-charged in µCT analysis.
12
Aims of the study
Application of ECM membrane on bone marrow stimulation (BMS) technic enhances cartilage repair and development of a novel analytic method for detection of engineering cartilage.
Chapter I
Implantation of autologous uncultured bone marrow cells from buffy coat into a full-thickness articular cartilage defect would generate tissue that more closely resembles hyaline cartilage. ECM membrane was used to prevent leakage of injected cells and blood clots from the cartilage defect into the knee joint cavity.
Chapter II
rhTGF-β3-loaded ECM membrane could significantly enhance cartilage regeneration, when
it was applied on the osteochondral defect of articular cartilage treated with the bone marrow stimulation (BMS) technique.
Chapter III
Evaluate the feasibility of using µCT to nondestructively assess the sulfated GAG content and distribution in engineered cartilage.
13
u CHAPTER Ⅰ
Implantation of bone marrow-derived buffy coat can supplement
bone marrow stimulation for articular cartilage repair
14
1.1 INTRODUCTION
Articular cartilage is a resilient load-bearing tissue that forms the articulating surface of diarthrodial joints and provides low friction, lubrication, and wearless characteristics necessary for repetitive gliding motion. However, because articular cartilage has neither vascular nor lymphatic channel supply and hence difficult access to stem cells, its self-healing potential is restricted (Hunziker, 2002; Prakash and Learmonth, 2002). Additionally low cell mobility due to surrounding matrix and a limited number of progenitor cells could be another contributing factors (Tallheden et al., 2005). Until now, much effort has been placed on development of therapeutic approaches for cartilage repair. Among the various strategies at present, the bone marrow stimulation (BMS) technique is one that has widely been used for repair of articular cartilage defects. This approach is aimed at creation of a suitable environment for new tissue formation, and utilization of the intrinsic healing ability of the body. BMS recruits mesenchymal stem cells (MSCs) or progenitor cells via subchondral penetration to the bone marrow, thereby stimulating cartilage repair. However, it was observed that fibrocartilaginous tissue with poor mechanical properties was obtained instead of the desired hyaline cartilage. This observation is likely a result of excessive loading conditions and a limited number of reparative MSCs available due to loss and dilution of bone marrow originated blood clots from the BMS region caused by the synovial fluid. To overcome this problem, some approaches to prevent leakage of bone marrow stem cells from the defect have been investigated. Breinan et al. covered the cartilage defect with a collagen film after performance of the microfracture technique in order to minimize the flow of bone marrow into the joint cavity (Breinan et al., 2000). Consequently, cartilage repair was better in the group with the covered defect compared to the group with the defect left exposed. In another approach, Taner et al. covered the defective cartilage area with autologous periosteum membrane following microfracture, and fixed the leg with an assistive device for reduction of overloading (Gunes et al., 2006). Breinan reported that addition of cultured autologous chondrocytes results in the same tissue types as those yielded by microfracture treatment (Breinan et al., 2000). In our previous study, a larger number of MSC drained from bone marrow produced a better quality of repaired cartilage in the defect (B.H. Min et al., 2007). Therefore, the effort to enhance the intrinsic cells should be exerted
15
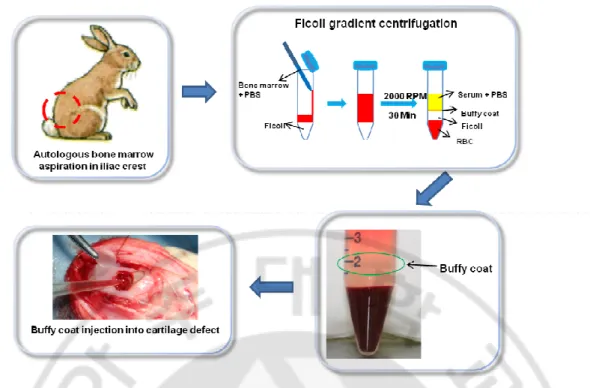
in order to obtain a more hyaline-like cartilage. Bone marrow is known to contain multipotent stem cells that can differentiate not only into hematopoietic cells, but also into mesenchymal tissue. Azizi et al. reported that several investigators who are treating various intractable diseases (including ischemic myocardial diseases, osteogenesis imperfect, Duchenne’s muscular dystrophy, and central nervous system disorders, such as Parkinson’s disease) are focusing on the use of bone marrow stem cells (Azizi et al., 1998). Many studies have reported on the therapeutic potential of bone marrow-derived mononuclear cells for regeneration of various types of tissues (Ohnishi et al., 2007). These cells have been used for treatment of non-traumatic avascular necrosis of the femoral head (Karatoprak et al., 2008), infarction of the myocardium (Yip et al., 2008), and cartilage damage (Chang et al., 2008) and have also been applied to a continuous spinal fusion mass (Viggeswarapu et al., 2001). In terms of cartilage repair, as bone marrow mononuclear cells (MNCs) can be fractionated to contain higher proportion of MSCs, they can potentially be used after microfracture to provide more MSCs in the defect area (Viggeswarapu et al., 2001; Chang et al., 2008). In this study, we hypothesized that implantation of autologous uncultured bone marrow cells from buffy coat into a full-thickness articular cartilage defect would generate tissue that more closely resembles hyaline cartilage. Extracellular matrix (ECM) membrane (ArtiFilm_, Regenprime Co., Ltd, Korea) was used to prevent leakage of injected cells and blood clots from the cartilage defect into the knee joint cavity.
1.2 MATERIALS AND METHODS
1.2.1 Experimental design and surgery
Use of animals in this experiment was approved by the Institutional Animal Experiment Committee of Ajou University. Seventy-two New Zealand white rabbits of 16 weeks old (an average weight of 3.0-3.5 kg) at which the growth plates are already closed were used in this study. Surgical procedures, including limb preparation and draping, were performed under general anesthesia with a mixture (0.2 ml/kg body weight) of Zoletil (50 mg/ml; Virbac Laoratoires-06516 Carros, France) and xylazine hydrochloride (Rompun; Bayer, Ansan, Korea) (Zoletil:Lompun 1:2). An arthrotomy was made through a midline longitudinal
16
incision on the medial parapatellar with the patella dislocated laterally to expose the femoral condyle. A drill 5 mmin diameter was used for creation of an osteochondral defect of 2 mmin depth in the patella groove (Jin et al., 2007c). BMS was performed using a 5 mm drill and 17-gauge needle.
For the in vivo study, we used a natural ECM membrane (ArtiFilm®, Regenprime Co., Ltd, Korea) derived from cultured young porcine chondrocytes to cover the cartilage defect (Fig. 1-1). ArtiFilm was fabricated by decellularizing a membrane-type cell/ECM composite produced by high density culture of porcine chondrocytes, and composed mainly of cartilage-enriched ECM components such as type II collagen and sulfated glycosaminoglycans (GAGs). The study group was divided at random into four groups (n=18/group): (1) the defect was left untreated as a negative control (Defect group), (2) treated with BMS using a 17-gauge needle after making the osteochondral defect using a 5 mm drill (BMS group), (3) supplemented concentrated autologous buffy coat was isolated from the iliac crest after BMS (Buffy coat group) and (4) implanted with an autologous osteochondral graft (AOTS) as a positive control. In this study, the AOTS group was set up by reinserting autologous osteochondral tissues isolated when making osteochondral defect using the AOTS system (Autograft diameter 6 mm, Arthrex, Germany) (Bottcher et al., 2010). Cartilage defects in the Defect, BMS, and Buffy coat groups were subsequently covered with an ECM membrane with fixation using the cross suture method. Each operated knee joint was immobilized with an assistive device postsurgery. Repair of cartilage was evaluated by macroscopic, histological and immunohistochemical analyses for repaired tissues at 4 and 8 weeks post-operation and additionally by biochemical analysis for repaired tissues at 8-week post-operation.
The description on the experimental design is based on Animals in Research: Reporting In vivo Experiments (ARRIVE) guidelines (Kilkenny et al., 2010).
17
Fig. 1-1. Experimental design and surgery. Procedure for implantation of autologous buffy coat. Bone marrow was aspirated from rabbit iliac crest. Then, 20 ml of buffy coat was separated from bone marrow using a Ficoll gradient centrifugation system and injected into the BMS treated area of articular cartilage, followed by ECM membrane covering.
18
1.2.2 Measurement of the number of MSC drained by the BMS technique and
harvested at the iliac crest
Colony forming unit-fibroblast (CFU-F) analysis was performed for measurement of the number of MSCs present in bone marrow isolated from the iliac crest or blood clots after BMS on the cartilage defect. Briefly, 4 ml of bone marrow blood was harvested from the iliac crest and 100 ml of blood was drained from cartilage defects by microfracture. The samples were diluted in PBS and the buffy coat was isolated by centrifugation using a Ficoll density gradient (Amersham Biosciences, 17-1440-02, Sweden) at 1,000g for 30 min (Yoon et al., 2007). A total of 1 ml of buffy coat in serum was isolated from this suspension, and then concentrated into 20 ml. The buffy coat was resuspended in MSC culture medium consisting of a-MEM supplemented with 10% FBS, and subsequently plated in 6-well culture dishes. Bone marrow harvested from the blood clot obtained after BMS was resuspended in the same manner. After 6 days, non-adherent cells were discarded and adherent cells were replenished with fresh medium after washing. The cells were then cultured for about 12 days, with replacement of the culture medium every 3 days, and stained with 5% crystal violet solution in 100% methanol for 10 min for the CFU-F assay . To minimize the effects of subjective bias three researchers counted the number of colonies greater than 2 mm in diameter in the blind manner.
1.2.3 Macroscopic and histological evaluations
At 4 and 8 weeks after surgery, the rabbits were euthanized by injection of an over-dose of pentobarbital. Femoral condyles were subsequently retrieved. Macroscopic images of the condyles were first observed; thesampleswere thenparaffin-embedded, sectioned, and processed for routine safranin-O, Sirius red staining and immunohistochemical analysis . For immunohistochemical analysis, the sectionswere incubated withmousemonoclonal anti-rabbit collagen type II antibody (Chemicon, Temecula, CA, USA) at 1:200 dilution for 1 h at room temperature. The sections were then incubated sequentially with biotinylated secondary antibody at 1:200 dilutions for 1 h and peroxidase-conjugated streptavidin solution for 30 min at room temperature (DAKO LSAB System, Carpinteria, CA, USA). After counterstaining with Mayer’s hematoxylin (Sigma, St Louis,MO, USA), the
19
sectionsweremounted with amount solution prior to microscopic observation (Nikon E600, Japan) (Cui et al., 2007; Jin et al., 2007a).
1.2.4 Histological scoring [International Cartilage Repair Society Score (ICRS)]
To minimize the effects of subjective bias three researchers independently evaluated the quality of the repaired articular cartilage in the defects, and a modified version of the histological grading scale system was used for the ICRS score (Wakitani et al., 1994; Cui et al., 2007; Jin et al., 2007a). The scale consists of seven categories and assigns a score ranging from 0 to 18.
1.2.5 Chemical assay of repaired tissue
For chemical assays of the repaired tissue, samples were harvested from the defect by surgical knife and curette. The harvested tissue was dried and digested in papain solution (5 mM L-cysteine, 100 mM Na2HPO4, pH 6.4, 5 mM EDTA, and 125 mg/ml papain type III) at 60_C for 24 h and then centrifuged at 12,000g for 10 min. For measurement of the GAG contents, the supernatant was subjected to 1,9-Dimethylmethylene Blue (DMB) colorimetric assay. DNA content was measured using a Qubit Fluorometer and the protocol given by the supplier (Invitrogen, Eugene, OR, USA) (Wakitani et al., 1994; Jin et al., 2007a; Sul et al., 2007). In all assays, normal cartilage from rabbit knee was used as a control. GAG contents were determined using chondroitin sulfate from shark cartilage (Sigma) as a standard .
1.2.6 Statistical analysis
Statistical analysis of the experimental data was carried out using one-way analysis of variance (one-way ANOVA) for multiple comparison and specific inter-data differences between mean values were identified using the Tukey-HSD test. P values less than 0.05 were regarded statistically significant and described in Results.
20
1.3 RESULTS
1.3.1 Colony-forming unit-fibroblasts (CFU-Fs) assay
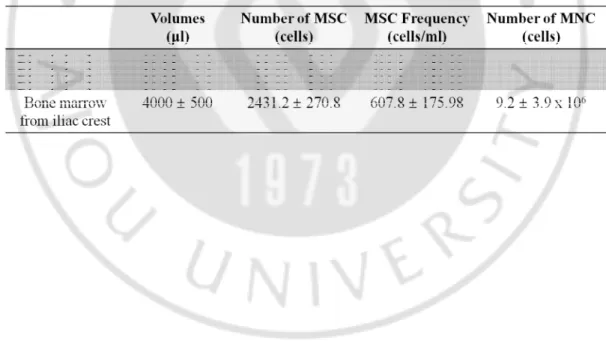
Crystal violet staining showed that there was no significant difference in the number of MSC colonies between the same volume of blood clot located in the BMS region and harvested from the iliac crest. Average blood volume and number of MSCs after BMS in the cartilage defect area were 39.25±9.8 µl and 23.88±5.96 MSCs, respectively. The mean volumes of bone marrow and number of MSCs aspirated from the iliac crest were 4 ml and 2431.2±270.8 MSCs, respectively (Table 1-1).
Table 1-1. Comparison of MSC frequencies between blood clot obtained from the BMS region and bone marrow obtained from the iliac crest. There was no difference in MSC frequency; however, as the achieved volume of bone marrow (4 ml) is bigger than the volume of the blood clot (40 μl) by 100 times, a centuple number of MSCs could be obtained from bone marrow through the buffy coat isolation method..
21
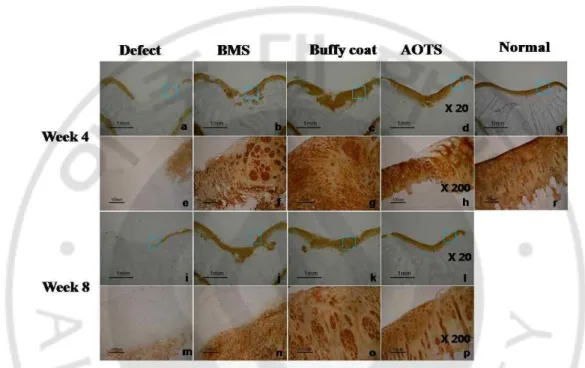
1.3.2 Gross findings and histological evaluation of cartilage defects
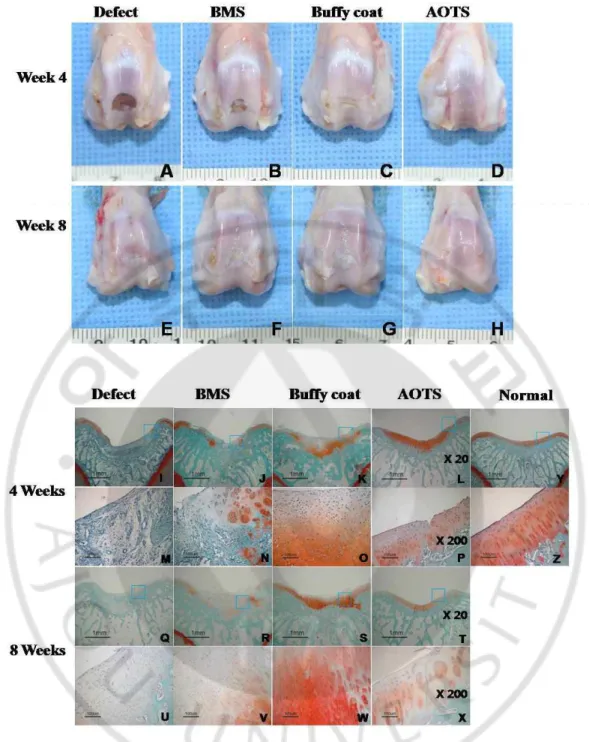
At week 4 after surgery, the defect area in the Defect group remained vacant while in the AOTS group, the defect was completely filled with smooth and glistening tissue. Repaired tissues in the BMS group partially filled the defect area and the extent of filling of the defect was much wider in the Buffy coat group, where the tissue looked a little whitish and was distinguished from the surrounding cartilage. At week 8, defects in all groups, except for the AOTS group, were observed to be filled with repaired tissue, but appeared different from the surrounding cartilage because of their white color. In the AOTS group, the defect area was filled with tissue that was indistinguishable from normal cartilage (Fig. 1-2A-H).
Safranin-O staining showed that defects in the Defect group were partially filled with fibrous tissue, while those in the AOTS group appeared to have normal cartilage, which had metachromatically stained ECM with well-aligned cells. However, graft tissue was not integrated with host tissue. In the BMS group, defects were filled with fibrous tissue minimally occupied with hyaline-like tissues at week 4. In contrast, most tissue, except for the surface layer, stained with safranin-O; however, its surface was quite rough and cells generally lacked orientation in the Buffy coat group. None of the groups, except for the AOTS group, showed subchondral bone remodeling. At week 8, fibrous tissue was filled in the Defect group, while the AOTS group showed good appearance of hyaline cartilage without evidence of degenerative change around the defect area. However, graft tissue still did not integrate with host tissue. Repaired tissue in the BMS group showed an increased area of metachromatic staining compared to that of 4 weeks; however, it was still occupied by much fibrous-like tissue covering the articular surface. In the Buffy coat group, repaired tissue was well organized with intense extracellular matrix; further, the cell distribution was composed of columnar and cluster cells with hyaline character, even though its surface and cartilage-bone were irregular. Except for the AOTS group, none of the groups showed the tide mark and columnar arrangement of cells (Fig. 1-2I-Z).
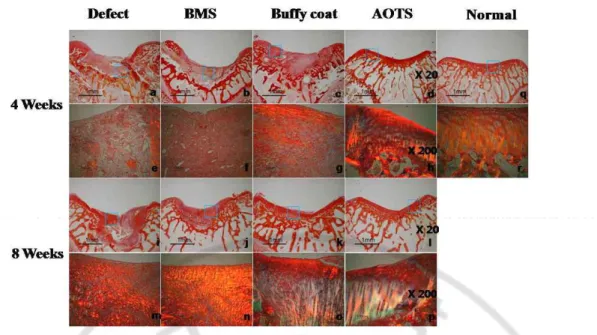
In Sirius red staining, collagen fiber was found only in small amounts in samples of the Defect and BMS groups at week 4, and no oriented pattern was observed. Collagen fibers were found in large amounts in the Buffy coat group. In the AOTS group, oriented collagen
22
fibers perpendicular to the cartilage surface were found; however, integration was not observed between implanted cartilage tissue and host cartilage. At week 8 after surgery, the amount of collagen fibers was increased in all groups; however, oriented arrangement of collagen fibers was not observed in the Defect and BMS groups. In the Buffy coat and AOTS groups, a well-oriented arrangement of collagen fibers similar to normal cartilage were found at 8 weeks; however, integration between implanted tissue and host tissue was also not observed (Fig. 1-3).
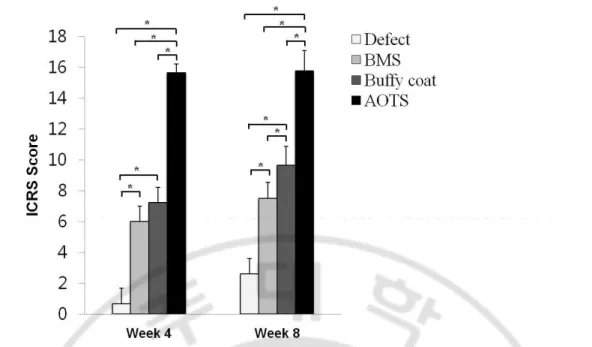
The ICRS histological score showed significant improvement with time in all but the AOTS group. It was not until 8 weeks that the Buffy coat group and the BMS group showed a difference in ICRS score (Number of femoral condyles (N) ≥5, p=0.018, p=0) (Fig. 1-4).
23
Fig. 1-2. Gross observation of the defect area of the cartilage at 4 weeks (A~D) and 8 weeks (E~H) after surgery. In the 4-week samples, C and D showed a smooth and glistening appearance, and continuity with the surrounding host cartilage tissue was also observed. In
24
contrast, the defect was not repaired in picture A and was filled partially with fibrous tissues in picture B. At 8 weeks after surgery, a white and glistening appearance of repaired tissues was shown in all groups. Histological observation of repaired cartilage in the defect area of the experimental groups at 4 weeks (I~P) and 8 weeks (Q~X) after surgery. And Safranin-O staining image of normal cartilage was also provided (Y). The defect was partially filled with fibrous tissues in the Defect and BMS groups at week 4. In contrast, hyaline cartilage-like tissues were observed partially in the Buffy coat group and completely in the AOTS group. At week 8, fibrous/hyaline cartilage was regenerated in the Defect and the BMS groups. In contrast, hyaline cartilage tissues with mature matrix and columnar organization of chondrocytes were observed in the Buffy coat and AOTS groups. Magnification was x20 (I~L, Q~T, Y), and x200 (M~P, U~Z).
25
Fig. 1-3. Sirius red staining image at 4 weeks (A~H) and 8 weeks (I~P) after surgery. An image of normal cartilage was also provided (Q). Magnification was at x100 (A~D, I~L) and x200 (E~H, M~P), respectively.
26
Fig. 1-4. ICRS scores at 4 weeks and 8 weeks after surgery. The total ICRS histological score increased significantly along with time in all groups. No significant difference was observed between the Buffy coat group and the BMS group at 4 weeks; however, at 8 weeks, a significant difference was shown in ICRS score. N≥5, p=0.018, p=0. (Error bars is standard deviation, *: p<0.05)
27
1.3.3 Expression of type II collagens
Expression of type II collagen increased gradually with time at the pericellular region in the repaired tissue of the BMS, Buffy coat, and AOTS groups. Collagen was diffusely distributed in territorial regions, as well as the pericellular area at week 4 in the BMS and Buffy groups. It was reorganized to converge into the pericellular area along with chondrocytes, similar to the AOT group at week 8. The Defect group showed minimal deposition of collagen, even at week 8 (Fig. 1-5).
Fig. 1-5. Immunohistochemistry for collagen type II expression. At 4 weeks (A~H) and 8weeks (I~P) after surgery. An image of normal cartilage was also provided (Q). (Magnification of x20: A~D, I~L and magnification of x200: E~H, M~P) Expression of type II collagen was gradually increased along with time at the pericellular region in repaired tissues of the BMS, Buffy coat, and AOTS groups. It was not significantly detected in the Defect group at both week 4 and 8. The most significant expression of type II collagen with dark brown color was observed in zonal-structure in the Buffy coat and AOTS groups at 8 weeks.
28
1.3.4 Chemical assay of repaired tissue
At 8 weeks post-surgery, the amount of GAG in the Buffy group was significantly higher than those in the Defect and BMS groups, but not as much as in the AOTS and Normal groups, while there was no statistically significant difference in the amount of GAG in the Buffy coat group, AOTS group, and normal cartilage. This finding suggested that GAG formation of the Buffy coat group approached that of normal cartilage (N≥5, p=0.015, p=0.047) (Fig. 1-6A). Lower levels of DNA content were detected in the Buffy coat group compared to the Defect and BMS groups. On the other hand, the AOTS and Normal groups did not show the significant difference. This finding means that repaired cartilage of the Buffy coat group was compatible to hyaline cartilage (N≥5, p=0.045, p=0) (Fig. 1-6B).
Fig. 1-6. (A): Changes and comparison of GAG contents among the experimental groups at 8 weeks after surgery. GAG contents of the Buffy coat group were higher than those of the Defect group and the BMS group. Moreover, they were significantly different in statistical analysis. However, the Buffy coat group showed no difference from those of the AOTS group and normal groups in statistical analysis. N≥5, p=0.015, p=0.047. (B): Changes and comparison of DNA contents among the experimental groups at 8 weeks after surgery. A lower level of DNA content was detected in the Buffy coat group compared with the Defect group and the BMS group. Moreover, they were significantly different in statistical analysis. However, the Buffy coat group showed no difference from those of the AOTS group and normal groups in statistical analysis. N≥5, p=0.045, p=0. (Error bars is standard deviation, *: p<0.05).
29
1.4 DISCUSSION
We hypothesized that implantation of buffy coat provides a large number of bone marrow stem cells and other biochemical factors, and, in turn, can significantly enhance repair of cartilage defects. Several aspects of the results from this study support the hypothesis. Gross morphology of repaired tissue in the autologous buffy coat-supplemented group (Buffy coat group) resembled that of normal cartilage, having a glistening appearance in the defect area. In addition, hyaline cartilage-like tissue with mature matrix and columnar organization of chondrocytes was observed by histological evaluation. Similarly, the chemical assay showed that the GAG component in repaired tissue is almost the same as that in normal cartilage.
The amount of blood clots is not much after BMS. In addition, most of the blood clots consisted of blood cells that were less likely related to tissue regeneration, and the number of stem cells was only hundreds per mm3 in defect area (B.H. Min et al., 2007). Given the fact that the number of chondrocytes in healthy cartilage is 100,000/mm3, the number of stem cells obtained through BMS is relatively low (Bobacz et al., 2004). The small number of MSCs may be a factor in the inclination of cartilage repair toward fibrocartilage after BMS. We found that an average of only 39.25 μl blood flowed out from the bone marrow through the BMS, which contained 23.88 MSCs (Table 1-1) while 4 ml of bone marrow blood could be obtained at our discretion from the iliac crest, of which buffy coat contained approximately 2431.2 MSCs (Table 1-1). In the rabbit bone marrow, the MSCs frequency of the ficoll-isolated buffy coat (2.43 per 104 MNCs) was similar to that of the human bone marrow ranging 1 per 103 ~ 105 MNCs (Zhiyong Zhang, March 2010). Resultantly, total number of MSCs in the defect area drastically increased with addition of buffy coat (approximately 2,400 MSCs). Therefore, a better result can be expected in cartilage repair if we supplement a larger number of cells. In the present study, we showed that implantation of autologous bone marrow buffy coat after BMS arthroplasty was more effective than the BMS alone and regenerated efficiently the cartilage defects in a rabbit model. Based upon the results of this study, the appropriate amount of buffy coat to be collected from the bone marrow when this technique is applied to a human subject can be approximated. In this study, the cartilage defect was 5 mm in diameter and 2 mm in depth, and its volume was
30
approximately 40 μl, while buffy coat was approximately 20 μl. In case, for a human cartilage defect that is 10 mm in diameter and 4 mm in depth, 157 μl of buffy coat from 31 ml of bone marrow seems appropriate for the therapy.
More MSCs may be transplanted by extracting a lot of blood from the bone marrow and concentrating it. Hernigou et al and Kasten et al gained 108 CFU-Fs/ml and 1480 CFU-Fs/ml in 306 ml and 297 ml aspirate, respectively, and finally concentrated them into 612 CFU-Fs/ml in 20 ml(4 times) and 2579 CFU-CFU-Fs/ml in 18 ml (14 times) by decreasing the volume (Hernigou et al., 2005; Kasten et al., 2008). It is of note that the concentrated bone marrow aspirates was shown to repair osteochondral defect in a large animal of equine model (Fortier et al., 2010). Transplant of buffy coat has some advantages compared to the transplant of cultivated MSC. Transplant of autologous cell requires 2 steps for harvesting the patient's cell or tissue and transplanting after cultivation. In contrast, the transplant of buffy coat can be finished at a time without cultivation as well as reduce chance of contamination derived from it. In addition, it will reduce the cost of cultivation drastically.
Many reports have shown that bone marrow-derived MSCs have a high potential for the repair of mesenchymal tissue (Pountos et al., 2007). However, Matsumoto et al. reported that the cultured MSCs transplanted in the cartilage defect regenerated it but formed fibrocartilage (Matsumoto et al., 2010). Besides, uncultured mononuclear cells (MNCs) that include both MSCs and HSCs, and the buffy coat have also been used widely as a cell source for cartilage regeneration. MNCs or buffy coat can be used immediately without any risk related to their culture in vitro. It is also believed that T cells, B cells, macrophages, and cytokines that are secreted by platelets in buffy coat may influence defect healing.
One possible reason for the occurrence of fibro-cartilage repair is that bone marrow and blood clots could have been washed away by fluid within the knee joint cavity. To overcome this problem, the ECM membrane (ArtiFilm) made by cultured porcine chondrocytes was used in this study. The ECM membrane is comprised mainly of cartilage-enriched ECM molecules such as collagen type II and sulfated GAGs, and previously shown to have high biocompatibility, biodegradability, and cartilage affinity (Jin, 2008). We speculate that the ECM membrane presumably can not only cover the cartilage defect to prevent loss of cells in the defect area, but can also interrupt influence from synovial fluid in order to prevent
31
differentiation of stem cells in an unwanted direction. Additionally it is very thin (approximately 30μm) not to be caught in articulation. Accordingly, it is regarded as a suitable biomaterial for cartilage repair. Similar to our study, a collagen type I/III membrane has been also used recently to protect autologous bone marrow and successfully repair the cartilage defect (de Girolamo et al., 2010).
It is widely accepted that scaffold biomaterials can also influence significantly the cartilage repair (Ishimoto et al., 2008). Because this study was designed to address the effect of BM-derived buffy coat, the ECM membrane was applied to all experimental groups to simply cover the defect area and prevent possible loss of MSCs. We speculate, however, that the ECM membrane could provide cells with a cartilage-like and favorable environment for cartilage repair. Although we have no direct results using the ECM membrane, a 3D scaffold made of the same ECM material was previously shown to be much superior to poly glycolic acid (PGA) scaffold in supporting cartilage tissue formation using chondrocytes and chondrogenesis of bone marrow MSCs in vitro (Choi et al., 2010). Therefore, the ECM membrane could be a better choice over other biomaterial scaffolds for the cartilage repair.
Though AOT has been shown to lead cartilage regeneration fairly well in human or animal models, transplanted tissues have still problems arisen from different thickness and collagen fiber direction from surrounding ones. In this study, we set up new AOT protocol by inserting the isolated osteochondral tissue from the osteochondral defect back into its own site. It is expected to be optimal since the thickness and direction of collagen fiber of a transplanted tissue fits well into the surrounding tissues.
A major limitation of this study is that we did not compare implantation of buffy coat only without performance of microfracture. Because the number of MSCs from buffy coat was overwhelming that by microfracture, the effect of microfracture may be abolished. However, we speculate that in order to prove this concept, we should design another set of experiments for evaluation of the effect of microfracture, that is to say, the role of subchondral bone as a holder of blood clot or foundation to support repair of cartilage.
32
1.5 CONCLUSION
We observe that implantation of autologous bone marrow buffy coat after BMS repair of the cartilage defect in a rabbit model is better than BMS alone. It is postulated that the implanted buffy coat provides a larger number of MSCs and plausible supporting humoral factors for repair of the defect and that the ECM membrane covering the defect played a role in prevention of the injected cells and blood clot from leaking into the joint fluid. As the method employed in this study is safe and easy, it is potentially feasible for clinical applications.
33
u CHAPTER Ⅱ
Effect of rhTGF-β 3 loaded ECM membrane on regeneration of
articular cartilage after bone marrow stimulation
34
2.1 INTRODUCTION
Growth factors are known to greatly contribute to tissue regeneration at different stages of cell proliferation and differentiation. For example, members of transforming growth factor beta (TGF-β) family have been identified as key regulators of MSCs differentiation into chondrocyte. It controls the production of extracellular matrices by stimulating the synthesis of collagens, fibronectin and proteoglycans (Salgado et al., 2004). Taken together, it appeared to have positive effects on cartilage differentiation and repair (Yamamoto et al., 1998; Muschler et al., 2004). However, tissue regeneration using growth factors has encountered for several problems (Anselme, 2000; Nakashima and Reddi, 2003; Saito and Takaoka, 2003), among which are growth factors’ excessively short half-life periods and their rapid clearance from the body, making it necessary to have an appropriate polymer matrix to achieve sustained release at the action site (Hori et al., 2007).
This necessitates the development of a controlled release system to provide sustained local delivery of growth factor in order to ensure therapeutic efficacy to cell. Advantages of controlled release system that active ingredients are released at controlled rates over prolonged periods of time are that loss of ingredients during processing could be avoid or reduce, and reactive or incompatible components could be separated. Sustained release of therapeutic drugs without degradation of compounds and biocompatibility of the delivery vehicle are key factors in developing an efficient and applicable drug delivery system (DDS) with diminishing adverse side effects. Therefore, many studies reported that controlled release of conjugated drug to various shape, such as 3D porous scaffold, hydrogel, multilayer films using a chitosan, PLGA, PLLA, alginate, fibrin etc. (Sohier et al., 2007). However, most technologies give rise to the loss of biological activity of loaded drug during the growth factor-polymer formulation process such as heat, sonication, and organic solutions (Abarrategi et al., 2008).
The layer-by-layer (LbL) technique, capable of depositing functional polymer coatings at surfaces of various chemistry and shape, is now viewed as a promising candidate for achieving a combination of these material properties.Significant efforts have been made to design LbL structures as coatings to deliver small molecules, drugs and biomolecules from
35
the surfaces of biomedical devices (Wang et al., 2009; Karlsson et al., 2010; Kittitheeranun et al., 2010). The potential of drug-loaded polyelectrolyte multilayers as antimicrobial, anti-inflammatory, and anticancer coatings has already been demonstrated. Previously, we have succeeded in manufacturing the extracellular matrix (ECM) biomaterial secreted by the porcine chondrocytes during cultivation. While ECM biomaterial can be formed into various shape depending on its application such as membrane, sponge and gel. we made a multilayered membrane type form porcine chondrocyte ECM, which was successfully applied to clinical use on microfracture for articular cartilage repair. This biomembrane is permeable and biodegradable in vivo (Jin et al., 2011b). This study, we developed novel systems consist of multiple layer of ECM membrane, and the rhTGF-β3 was inserted between each layer. When the drug is inserted in between the membranes, loaded drug will be released not only through membrane but by degradation of membrane in vivo. We hypothesized that rhTGF-β3 with being released in controlled fashion from the construct of membrane can activate chondrogenesis of stem cell and thus enhance healing potential when applied to endogenous stem cell inducing cartilage repair. We have developed a novel drug delivery system that continuously releases rhTGF-β3 using an extracellular matrix (ECM)
membrane fabricated from cultured porcine chondrocytes. We hypothesized that rhTGF-β3
-loaded ECM membrane could significantly enhance cartilage regeneration, when it was applied on the osteochondral defect of articular cartilage treated with the bone marrow stimulation (BMS) technique.
2.2 MATERIALS AND METHODS
2.2.1 In vivo implantation for articular cartilage repair
Use of animals in this experiment was approved by the Institutional Animal Experiment Committee of Ajou University. Seventy two New Zealand white rabbits of 16 weeks old (an average weight of 3.0-3.5 kg) at which the growth plates are already closed were used in this study. Surgical procedures, including limb preparation and draping, were performed under general anesthesia with a mixture (0.2 ml/kg body weight) of Zoletil (50 mg/ml; Virbac Laoratoires-06516 Carros, France) and xylazine hydrochloride (Rompun; Bayer, Ansan,