저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The relationship between intravascular
ultrasound-derived percent total atheroma
volume and fractional flow reserve in the
intermediate stenosis of proximal or middle left
anterior descending coronary artery
by
Xiong Jie Jin
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
The relationship between intravascular
ultrasound-derived percent total atheroma
volume and fractional flow reserve in the
intermediate stenosis of proximal or middle left
anterior descending coronary artery
by
Xiong Jie Jin
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Master of Medicine
Supervised by
Seung-Jea Tahk, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Xiong Jie Jin is approved.
SUPERVISORY COMMITTEE
Seung-Jea Tahk
Myeong-Ho Yoon
Joon-Han Shin
Bon-Kwon Koo
Hong-Seok Lim
The Graduate School, Ajou University
June, 8th, 2015
Acknowledgement
꿈만 같았던 한국유학이 벌써 5 년이라는 시간이 흘렀습니다. 끝나지 않을 것 같던 5 년간의 한국유학생활을 돌이켜보면 힘든 시간도 많았지만, 새로운 지식을 배우고 많은 분들과 한 추억은 평생 잊지 못할 것 같습니다. 한국유학기간의 희로애락을 한 권의 논문으로 마무리 지으면서 저에게 많은 도움을 주시고 저에게 큰 힘이 되여 주신 여러분께 감사의 마음을 전합니다. 우선 많이 부족한 저를 받아주시고 임상과 연구방면에서 아낌없는 지도와 격려를 해주신 탁승제교수님께 진심으로 감사 드립니다. 그리고 바쁘신 와중에도 본 논문이 완성되기까지 세심한 지도와 많은 격려로 이끌어주신 양형모 교수님, 임홍석 교수님, 졸업논문심사를 해주신 신준한 교수님, 윤명호 교수님, 구본권 교수님께 깊은 감사를 드립니다. 또한 한국생활을 잘 적응하도록 많이 격려해주고 항상 열정적으로 수술을 가르쳐주신 최소연 교수님, 최병주 교수님, 황교승 교수님, 늘 따뜻한 마음으로 배려해주신 조대열 선생님, 서경우선생님, 박진선 선생님, 그리고 기쁠 때나 슬플 때 항상 저와 함께 해준 저의 친구들께 진심으로 감사드립니다. 마지막으로, 항상 저의 든든한 버팀목이 되어 저를 지켜봐 주시고 내 인생의 튼튼한 후원자가 되여 주신 부모님과 장인 장모님, 힘든 한국생활을 이겨낼수 있도록 변함없는 믿음과 사랑으로 저를 응원해준 안해와 사랑하는 딸 소연에게 진심으로 감사드립니다. 2015 년 6 월i
- ABSTRACT -
The relationship between intravascular ultrasound-derived percent
total atheroma volume and fractional flow reserve in the
intermediate stenosis of proximal or middle left anterior descending
coronary artery
It remains undefined whether the atherosclerotic disease extent of the conductive vessel (expressed as Intravascular ultrasound [IVUS]-derived percent total atheroma volume [%TAV]), correlates with functional severity of intermediate stenosis of left anterior descending artery (LAD). An IVUS study and fractional flow reserve (FFR) measurements performed in 130 patients with coronary angiographic intermediate stenosis of proximal or middle LAD. %TAV was calculated as the percentage of total vessel volume occupied by total atheroma volume on IVUS. A significant correlation was observed between %TAV and FFR (r=-0.71, p<0.001). Minimal lumen area (MLA) correlated moderately with FFR (r=0.54, p<0.001). The independent predictors of FFR<0.8 were %TAV (odds ratio [OR]:1.29, 95% confidence interval [CI]=1.18-1.40, p<0.001) and MLA (OR:0.37, 95%CI=0.16-0.85, p=0.019). A receiver-operating characteristic curve suggested %TAV≥39.0% (sensitivity 85%, specificity 83% and area under curve [AUC]=0.90) and
MLA≤2.6mm2 (sensitivity 72%, specificity 70% and AUC=0.75) as the best cut-off values
for FFR<0.8. Forty-eight point five (48.5%) of total studied lesions (63/130) showed %TAV≥39.0%. Eighty-four point four (84.4%) of lesions (38/45)
ii
with %TAV≥39.0% and MLA ≤2.6mm2, and 72.2% of lesions (13/18) with % TAV≥39.0%
and MLA>2.6 mm2, FFR was less than 0.8. Volumetric quantification of the atherosclerotic
disease extent of the coronary artery, expressed as IVUS-derived %TAV, showed a strong correlation with FFR. Not only the segmental luminal narrowing but also the total plaque burden of conductive artery are major determinants for the presence of myocardial ischemia in intermediate stenosis of LAD.
Key words:
Fractional flow reserve, Intravascular ultrasound, Percent total atheroma
volume
iii
TABLE OF CONTENTS
ABSTRACT ··· ⅰ
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· iv
LIST OF TABLES ··· v
Ⅰ. INTRODUCTION ··· 1
Ⅱ. MATERIALS AND METHODS ··· 2
1. Study population ··· 2
2. Quantitative coronary angiographic analysis ··· 2
3. IVUS imaging and analysis ··· 3
4. FFR measurement ··· 4
5. Statistical analysis ··· 5
Ⅲ. RESULTS ··· 6
1. Baseline characteristics ··· 6
2. FFR and QCA parameters ··· 7
3. FFR and IVUS parameters ··· 7
Ⅳ. DISCUSSION ··· 16
REFERENCES ··· 20
iv
LIST OF TABLES
Table 1. Baseline clinical characteristics of the patients ··· 6
Table 2. BCV and AUC in ROC curves of angiography and IVUS parameters in
prediction of functionally significant stenosis in the LAD ··· 9
Table 3. BCV and AUC in ROC curves of angiography and IVUS parameters in prediction of functionally significant stenosis according to lesion location in the LAD ··· 10
Table 4. Diagnostic accuracy of angiographic and IVUS parameters for
FFR<0.8 ··· 11
Table5. Multivariate analysis of independent factors for functionally significant stenosis (FFR<0.8) ··· 12
v
LIST OF FIGURES
Fig. 1. Relationship between FFR and angiographic parameters ··· 13
Fig. 2. Relationship between FFR and IVUS parameters ··· 14
Fig. 3. Comparison of ROC curves of IVUS parameters for functionally significant stenosis ··· 15
Fig. 4. Effect of MLA and %TAV on functionally significant stenosis
1
I. INTRODUCTION
Atherosclerosis is a diffuse systemic disease that often remains as an insignificant process on coronary angiography. Diffuse coronary atherosclerosis without visible stenosis on coronary angiography can significantly affect coronary flow hemodynamics by increasing the resistance of conductive vessels and contribute to myocardial ischemia (De Bruyne B, 2001).
Intravascular ultrasound (IVUS) provides not only the severity of segmental stenosis but also the diffuseness of the atherosclerotic process of the conductive vessel. However, it is practically difficult to represent the diffuseness of disease with one parameter. IVUS-derived minimum lumen area (MLA) has a moderate correlation with fractional flow reserve (FFR) (Koo BK et al., 2011; Kang SJ et al., 2011; Ben-Dor I et al., 2011; Kang SJ et al., 2012; Waksman R et al., 2013); however, it represents only the severity of segmental stenosis confined to the culprit site. Therefore, IVUS-MLA cannot accurately reflect the functional significance of the entire conductive vessel, and it may overestimate or underestimate the functional significance of the entire disease process and lead to erroneous revascularization decisions (Magni V et al., 2009; Nam CW et al., 2010).The correlation between the diffuseness of atherosclerotic disease of the conductive vessel and FFR in patients with intermediate stenosis remains undefined.
Therefore, we aimed to evaluate the relationship between the extent of atherosclerosis by IVUS, expressed as percent total atheroma volume (%TAV), which can be calculated from
2
the ratio of total atheroma volume to total vessel volume on an IVUS study, and FFR in the left anterior descending coronary artery (LAD) in patients with intermediate stenosis.
II. MATERIALS AND METHODS 1. Study Population
We selected 130 patients with single de novo intermediate stenosis from January 2010 to December 2012 (40–70% diameter stenosis by visual estimation) of the proximal or middle segment of the LAD on diagnostic coronary angiography, and who underwent an IVUS study and FFR measurements with informed consent. Exclusion criteria were multiple stenosis (>40% diameter stenosis by visual estimation) in the same vessel, left main stenosis, left ventricular ejection fraction < 40%, presence of a collateral vessel, contraindication to adenosine, previous coronary bypass surgery, and angiographically thrombi-containing lesions. In addition, patients with history and/or evidence of any myocardial infarction were excluded from the study. This study protocol was approved by the ethics committee of our institution.
2. Quantitative coronary angiographic analysis
The quantitative coronary angiographic (QCA) analysis was performed using the cardiovascular angiography analysis system II (CAAS II, pie Medical, Maastricht, Netherlands). Diameter stenosis (DS), minimum lumen diameter (MLD), reference vessel diameter, and lesion length were measured. Lesion length was calculated as the distance between the proximal and distal reference in the projection demonstrating the stenosis with the least foreshortening.
3
3. IVUS imaging and analysis
IVUS was performed using the Galaxy 2TM IVUS system (Boston Scientific Corporation,
Natick, MA, USA) after intracoronary bolus of 200 µg nitroglycerin. A 40-MHz coronary imaging IVUS catheter (Atlantis SR Pro, Natick, MA, USA) was pulled back from the distal LAD through the target stenosis to the ostium of the left main coronary artery using a motorized pullback device at a speed of 0.5 mm/s. All IVUS analyses were performed using computerized planimetry software (EchoPlaque 3.0, Indec Systems, Santa Clara, CA, USA) according to the American College of Cardiology Clinical Expert Consensus Document on Standards for acquisition, measurement and reporting of IVUS Studies (Mintz GS et al., 2001).We analyzed IVUS images spaced precisely 1 mm apart, with an average of 69.8 ± 14.9 mm per patient. When acoustic shadowing caused by lesion calcification made the identification of the external elastic membrane (EEM) difficult, circumferential or axial extrapolation was used (Mintz GS et al., 1995). Coronary arteries with calcium arc >90º in the studied segments were excluded. MLA and external elastic membrane (EEM) were measured at the narrowest luminal cross section of the target lesion and the reference area at the most normal looking cross section within 10 mm proximal and distal to the lesion without an intervening side branch. Lesion length was defined as the region around the MLA where the lumen area was <50% of the reference lumen area (Gonzalo N et al., 2012). Plaque burden at the narrowest site was calculated as [100 × (EEM area-MLA)/EEM area]. The atheroma area in each measured image was defined as the difference between the lumen area and the EEM area. Total atheroma volume (TAV) was calculated as the sum of plaque area and total vessel volume (TVV) as the summation of EEM area in all measured images
4
(Nicholls SJ et al., 2006).
TAV (mm3) = ∑ (EEM area − lumen area)
TVV (mm3) = ∑ (EEM area)
The extent of atherosclerosis was also expressed as the percent total atheroma volume (%TAV), calculated as the percentage of TVV occupied by TAV (Nicholls SJ et al., 2006).
TAV
%TAV (%) = —————— × 100 TVV
We randomly selected 10 cases to assess interobserver variability for re-measuring IVUS cross-sectional images by an independent investigator. The interobserver correlation coefficients for TVV, total lumen volume and TAV were 0.97, 0.95 and 0.98, respectively (Yamagishi M et al., 2002).
4. FFR measurement
After the IVUS assessment, FFR was measured using a 0.014-inch pressure guide wire (Pressure Wire, Radi Medical Systems, Uppsala, Sweden). If necessary, additional intracoronary nitroglycerin was administered to prevent coronary spasm. “Equalizing” was performed with the pressure sensor positioned at the guiding catheter tip. A pressure wire then was advanced across the target lesion, and the pressure-sensor was positioned at the same segment of the distal LAD where the IVUS pullback started. Then, the pressure wire was manually pulled back from the distal LAD through the target stenosis to the aorta to confirm the presence of pressure drift. FFR was calculated as the ratio of distal coronary pressure to aortic pressure during maximal hyperemia. Maximal hyperemia was induced by
5
continuous intracoronary infusion of adenosine (>360µg/min) via microcatheter for inducing more reliable sustained maximal hyperemia as described in our previous study (Yoon MH et al., 2009). FFR < 0.8 was considered functionally significant.
5. Statistical analysis
Data are expressed as means ± standard deviations for continuous variables and frequency (percentage) for categorical variables. Comparison of continuous variables was performed using Student’s t-test. The relationships between FFR and IVUS or QCA parameters were analyzed by Pearson’s correlation analysis. Multivariate logistic regression analysis was performed to determine independent predictors of FFR < 0.8. Receiver operating characteristic (ROC) curves were analyzed to assess the best cut-off values (BCV) of IVUS and QCA parameters to determine FFR < 0.8. All statistical analyses were performed using SPSS ver. 18.0, Medcalc (Medicalc Software, Antwerp Belgium), and a p-value <0.05 was considered significant.
6
III. RESULTS 1. Baseline characteristics
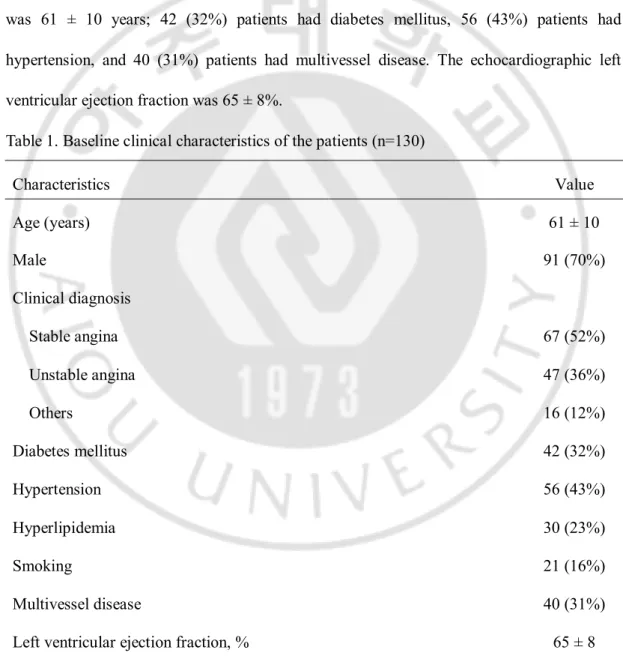
The baseline characteristics of the patients are summarized in Table 1. A total of 130 patients were analyzed. Sixty-seven (52%) of whom had stable angina, 47 (36%) had unstable angina, and the other 16 (12%) presented with atypical chest discomfort. Mean age was 61 ± 10 years; 42 (32%) patients had diabetes mellitus, 56 (43%) patients had hypertension, and 40 (31%) patients had multivessel disease. The echocardiographic left ventricular ejection fraction was 65 ± 8%.
Table 1. Baseline clinical characteristics of the patients (n=130)
Characteristics Value Age (years) 61 ± 10 Male 91 (70%) Clinical diagnosis Stable angina 67 (52%) Unstable angina 47 (36%) Others 16 (12%) Diabetes mellitus 42 (32%) Hypertension 56 (43%) Hyperlipidemia 30 (23%) Smoking 21 (16%) Multivessel disease 40 (31%) Left ventricular ejection fraction, % 65 ± 8 Values are mean ± SD or n (%)
7
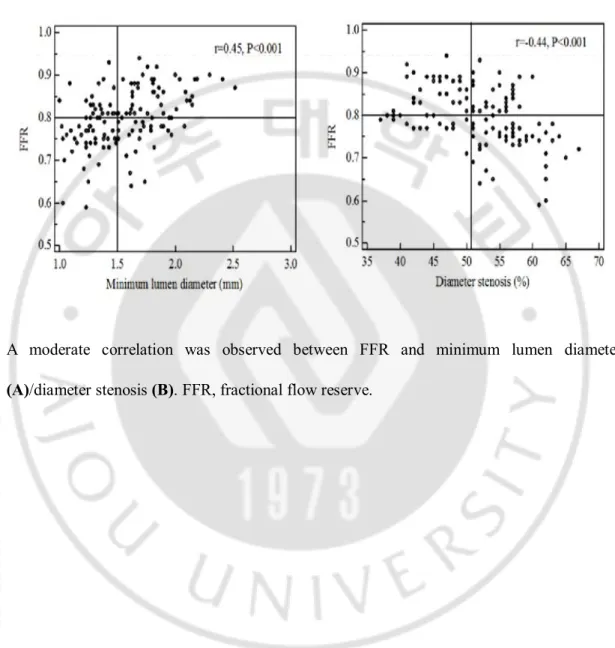
2. FFR and QCA parameters
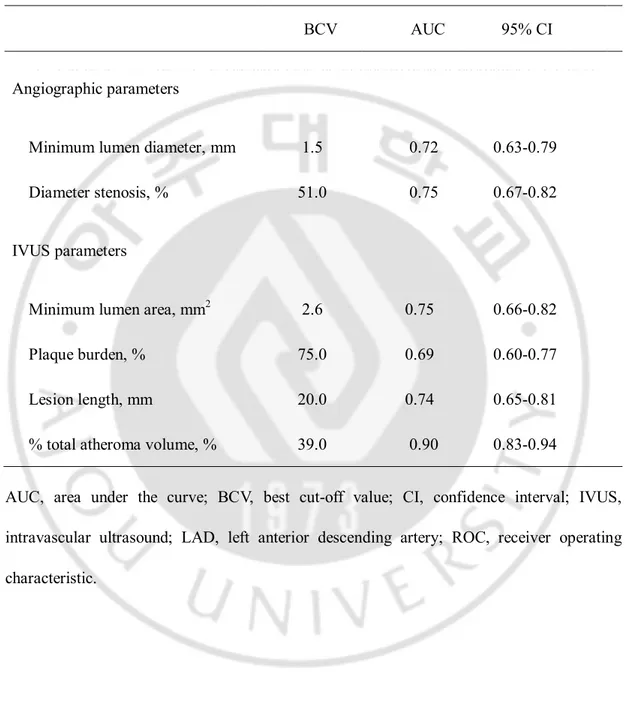
FFR showed moderate correlations with angiographic MLD (r = 0.45, p < 0.001) and DS (r = −0.44, p < 0.001) (Figure 1). The best cut-off value (BCV) to predict FFR<0.8 was 1.5mm for angiographic MLD (sensitivity 67%, specificity 64%, positive predictive value [PPV] 66%, negative predictive value [NPV] 69%, accuracy 65%, and area under curve [AUC]:0.72), and 51% for angiographic DS (sensitivity 70%, specificity 69%, PPV 69%, NPV 73%, accuracy 70%, and AUC:0.75) (Tables 2 and 4).
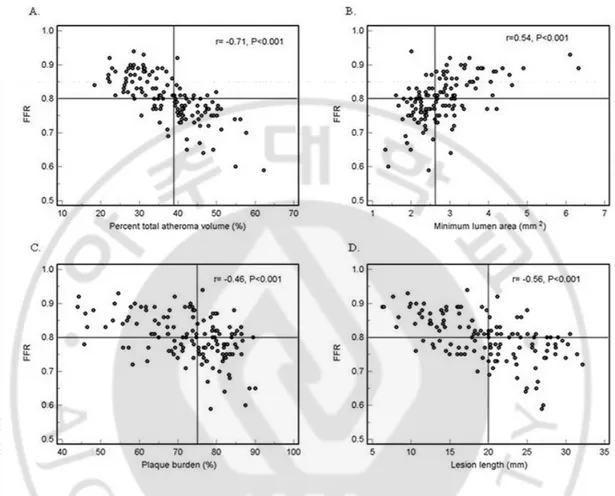
3. FFR and IVUS parameters
FFR correlated moderately with MLA (r = 0.54, p < 0.001), plaque burden at the MLA site (r = −0.46, p < 0.001), and lesion length (r = −0.56, p < 0.001). FFR showed the most significant correlation with %TAV (r = −0.71, p < 0.001) (Figure 2).
Multivariate logistic regression analysis demonstrated MLA (odds ratio [OR] = 0.37, 95% confidence interval [CI], 0.16–0.85, p = 0.019) and %TAV (OR = 1.29, 95% CI, 1.18–1.40, p < 0.001) as independent predictors of FFR < 0.80 (Table 5).
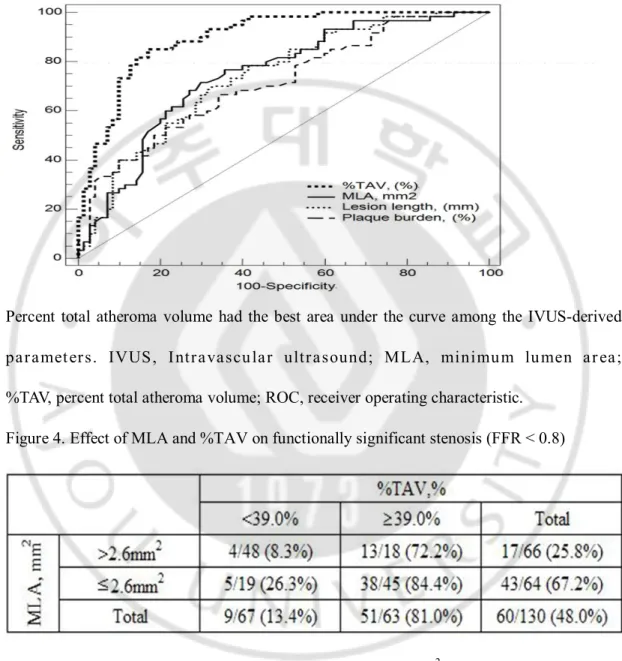
The BCV to predict FFR < 0.8 was 2.6mm2 for MLA (sensitivity 72%, specificity 70%,
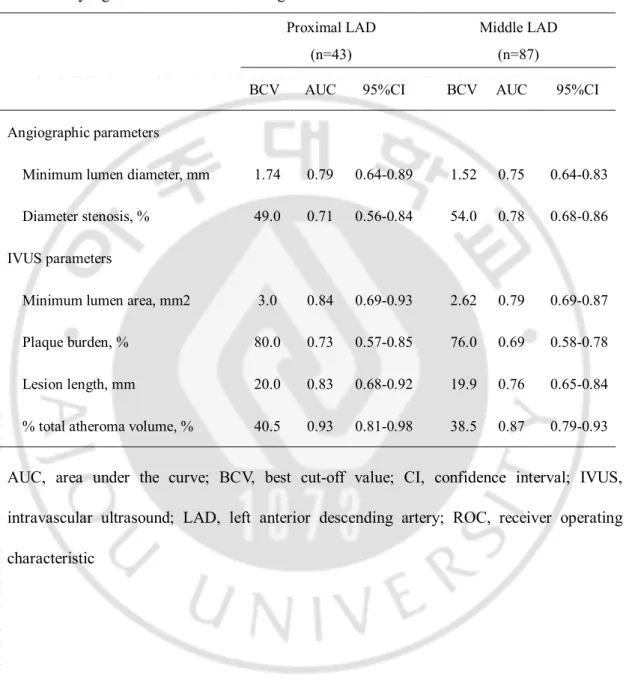
PPV 72%, NPV 74%, accuracy 71%, and AUC 0.75), 20.0mm for lesion length (sensitivity 70%, specificity 67%, PPV 70%, NPV 72%, accuracy 68%, and AUC 0.74), and 39.0% for %TAV (sensitivity 85%, specificity 83%, PPV 85%, NPV 87%, accuracy 84%, and AUC 0.90). (Tables 2 and 4, Figure 3). In a subgroup analysis of proximal and middle LAD, the BCV of MLA to predict FFR <0.8 was 3.0mm2 (AUC: 0.84, 95% CI: 0.69-0.93) in
8
BCV of %TAV to predict FFR <0.8 was 40.5% (AUC: 0.93, 95% CI: 0.81-0.98) in proximal LAD and it was 38.5% (AUC: 0.87, 95% CI: 0.79-0.93) in middle LAD (Table 3).
Forty-eight point five (48.5%) of total studied lesions (63/130) showed %TAV ≥39.0%.
Eighty-four point four (84.4%) of lesions (38/45) with % TAV≥39.0% and MLA ≤2.6mm2,
and 72.2% of lesions (13/18) with % TAV ≥39.0% and MLA >2.6 mm2, FFR was less than
0.8. In contrast, 8.3% of lesions (4/48) with % TAV <39.0% and MLA >2.6 mm2 showed
9
Table 2. BCV and AUC in ROC curves of angiographic and IVUS parameters in prediction
of functionally significant stenosis in the LAD BCV AUC 95% CI
Angiographic parameters
Minimum lumen diameter, mm 1.5 0.72 0.63-0.79 Diameter stenosis, % 51.0 0.75 0.67-0.82 IVUS parameters
Minimum lumen area, mm2 2.6 0.75 0.66-0.82
Plaque burden, % 75.0 0.69 0.60-0.77 Lesion length, mm 20.0 0.74 0.65-0.81 % total atheroma volume, % 39.0 0.90 0.83-0.94 AUC, area under the curve; BCV, best cut-off value; CI, confidence interval; IVUS,
intravascular ultrasound; LAD, left anterior descending artery; ROC, receiver operating characteristic.
10
Table 3. BCV and AUC in ROC curve of angiographic and IVUS parameters in prediction of functionally significant stenosis according to lesion location in the LAD
Proximal LAD Middle LAD (n=43) (n=87) BCV AUC 95%CI BCV AUC 95%CI Angiographic parameters Minimum lumen diameter, mm 1.74 0.79 0.64-0.89 1.52 0.75 0.64-0.83 Diameter stenosis, % 49.0 0.71 0.56-0.84 54.0 0.78 0.68-0.86 IVUS parameters Minimum lumen area, mm2 3.0 0.84 0.69-0.93 2.62 0.79 0.69-0.87 Plaque burden, % 80.0 0.73 0.57-0.85 76.0 0.69 0.58-0.78 Lesion length, mm 20.0 0.83 0.68-0.92 19.9 0.76 0.65-0.84 % total atheroma volume, % 40.5 0.93 0.81-0.98 38.5 0.87 0.79-0.93
AUC, area under the curve; BCV, best cut-off value; CI, confidence interval; IVUS, intravascular ultrasound; LAD, left anterior descending artery; ROC, receiver operating characteristic
11
Table 4. Diagnostic accuracy of angiographic and IVUS parameters for FFR<0.80
Sensitivity Specificity PPV NPV Accuracy
Angiographic parameters
Minimum lumen diameter 67% 64% 66% 69% 65%
Diameter stenosis 70% 69% 69% 73% 70%
IVUS parameters Minimum lumen area 72% 70% 72% 74% 71%
Plaque burden 68% 67% 68% 70% 66%
Lesion length 70% 67% 70% 72% 68%
% total atheroma volume 85% 83% 85% 87% 84% FFR, fractional flow reserve; IVUS, intravascular ultrasound; NPV, negative predictive value; PPV, positive predictive value.
12
Table 5. Multivariate analysis of independent factors for functionally significant stenosis (FFR<0.8)
OR 95% CI p value Minimum lumen area 0.37 0.16 - 0.85 0.019 % total atheroma volume 1.29 1.18 - 1.40 <0.001 CI, confidence interval; FFR, fractional flow reserve; OR, odds ratio.
Included variables: minimum lumen area, plaque burden, lesion length, and % total atheroma volume.
13
Figure 1. Relationship between FFR and angiographic parameters
A moderate correlation was observed between FFR and minimum lumen diameter (A)/diameter stenosis (B). FFR, fractional flow reserve.
14
Figure 2. Relationship between FFR and IVUS parameters
The correlation was most highly significant between FFR and percent total atheroma volume (A), A moderate correlation was observed between FFR and minimum lumen area (B), plaque burden (C), and lesion length (D). FFR, fractional flow reserve; IVUS, intravascular ultrasound.
15
Figure 3. Comparison of ROC curves of IVUS parameters for functionally significant stenosis
Percent total atheroma volume had the best area under the curve among the IVUS-derived paramet ers. IVUS, Intravascular ultrasound; MLA, minimum lumen ar ea; %TAV, percent total atheroma volume; ROC, receiver operating characteristic.
Figure 4. Effect of MLA and %TAV on functionally significant stenosis (FFR < 0.8)
In lesions with significant %TAV (≥39.0%) and MLA (≤2.6 mm2), 84.4% of lesions showed
FFR < 0.8. In contrast, when both %TAV (<39.0%) and MLA (>2.6 mm2) were not
significant, only 8.3% of lesions showed FFR< 0.8. FFR, fractional flow reserve; MLA, minimum lumen area; %TAV, percent total atheroma volume.
16
IV. DISCUSSION
The major findings of the present study are as follows: In intermediate stenosis of LAD, (1) the extent of diffuseness atherosclerosis of conductive vessel, expressed as IVUS-derived %TAV, showed the most significant correlation with FFR, (2) %TAV ≥ 39.0% and MLA ≤ 2.6 mm2 were the best cut-off values for FFR < 0.80, (3) 48.5% of total studied
lesions (63/130) showed %TAV ≥39.0%, which represents the diffuse atherosclerotic process limiting coronary blood flow.
IVUS is the most commonly used adjunctive procedure during percutaneous coronary intervention and provides comprehensive morphological information of the conductive vessel. IVUS-MLA has been most frequently used to represent the severity of coronary stenosis. However, MLA confined to the culprit site and does not represent the atherosclerotic severity of the whole conductive artery. IVUS-MLA was considered as parameter with cutoff values that vary according to vessel size and lesion location, and even subgroup-specific criteria were inaccurate in identifying ischemia inducible stenosis (Koo BK et al., 2011; Kang SJ et., 2011; Ben-Dor I et al., 2011; Kang SJ et al., 2012; Waksman R etal., 2013).
Previous pathological and IVUS studies have shown that visible angiographic segmental coronary stenosis is usually associated with diffuse atherosclerosis in the remainder of the coronary tree, although this is often not identified by coronary angiography (Glagov S et al., 1987; Nissen SE et al 1991; Mintz GS et al 1995). De Bruyne B et al demonstrated that approximately half of coronary arteries without angiographic focal stenosis have a graded, continuous decline in coronary pressure along their length, particularly during hyperemia
17
(De Bruyne B, 2001). Therefore, we supposed that the discrepancy between IVUS-MLA and FFR results mainly originates from the hemodynamic effects of diffuse coronary atherosclerosis in other segments of the conductive artery.
We traced the cross-sectional IVUS images of the conductive coronary vessel of interest using a pullback device at constant speed. Therefore, we could express the extent of diffuseness of the atherosclerotic process of nearly the entire conductive vessel using IVUS-derived %TAV. Percent total atheroma volume (%TAV) could represent not only the severity of segmental stenosis but also the extent of diffuseness of atherosclerosis, both of which may contribute to limit coronary flow.
We demonstrated that the diagnostic accuracy of %TAV was 84% for predicting functional significance (FFR<0.8) of intermediate coronary artery stenosis of LAD. The best cut-off value of %TAV was 39.0% with AUC of 0.90, and %TAV showed the best correlation with FFR among the IVUS-measured parameters that have been studied. As expected, %TAV was ≥ 39.0% in 48.5% of the patients, which means that about half of patients with intermediate stenosis of the LAD have diffuse disease that significantly influences the resistance of the conductive vessel. Additionally, MLA>2.6mm2 excluded the
possibility of FFR<0.80 with sensitivity of 72% and specificity of 70%. A moderate correlation was observed between MLA and FFR (r = 0.54, p <0.001). These results are consistent with those of other recently published studies (Koo BK et al., 2011; Kang SJ et.,
2011; Ben-Dor I et al., 2011; Kang SJ et al., 2012; Waksman R etal., 2013). Both of %TAV
(≥39.0%) and MLA (≤2.6mm2) were significant in 34.6% of the patients (45/130), and
18
were not significant in 36.9% of the patients (48/130), and only 8.3% of them (4/48) showed FFR<0.8.
The present data confirmed that the diffuse atherosclerotic process could significantly affect resistance of the conductive vessel and the amount of stress-induced maximal coronary flow regardless of segmental stenosis on angiography in patients with intermediate stenosis of LAD. Additionally, the degree of diffuseness, expressed by %TAV, showed an excellent correlation with FFR. Therefore, when evaluating the intermediate stenosis of the LAD by IVUS, we suggest that if diffuse coronary atherosclerosis exists in the entering vessel, regardless of whether MLA is significant or not, FFR should be measured to determine the presence of stress-induced myocardial ischemia.
Study limitations: First, the findings of this study were based on a single-center. Second, although intracoronary nitroglycerin was given before IVUS measurement, the possibility of catheter and guidewire induced spasm or bias cannot be excluded. Third, calculating the total plaque volume of conductive vessel by tracing all the IVUS pullback images was time-consuming and laborious. If the software for rapid automatic volumetric measurement is available, our study would be helpful in guiding the clinical decision process for equivocal cases, especially with anatomic and functional mismatch. Fourth, the results of this study would not be applicable for all types of coronary artery lesions. Validation of %TAV in the various types of lesions at various coronary artery sites is needed. Finally, although intravenous adenosine infusion has been known as standard method for inducing maximal coronary hyperemia for FFR measurement, this study adopted intracoronary continuous adenosine infusion via microcatheter. It has been validated as a safe and effective method in
19
inducing maximal hyperemia compared to standard intravenous infusion method (Yoon MH, 2009).
In conclusions, volumetric quantification of the atherosclerotic disease extent of the coronary artery, expressed as IVUS-derived %TAV, showed a strong correlation with FFR. Not only the segmental luminal narrowing but also the total plaque burden of conductive artery are major determinants for the presence of myocardial ischemia in intermediate stenosis of the LAD.
20
REFERENCES
De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “normal” coronary angiography. Cirulation
2001,104, 2401–6
Koo BK, Yang HM, Doh JH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenosis of different locations. JACC Cardiovasc Interv 2011, 4, 803–11
Kang SJ, Lee JY, Ahn JM, et al. Validation of intravascular ultrasound-derived Parameters with fractional flow reserve for assessment of coronary stenosis severity.
Circ Cardiovasc Interv 2011, 4, 65–71
Ben-Dor I, Torquson R, Gaglia MA Jr, et al. Correlation between fractional flow reserve and intravascular ultrasound lumen area in intermediate coronary artery stenosis. Eurointervention 2011, 7, 225–33
Kang SJ, Ahn JM, Song H, et al. Usefulness of minimal luminal coronary area determined by intravascular ultrasound to predict functional significance in stable and unstable angina pectoris. Am J Cardiol 2012, 109, 947-53
Waksman R, Legutko J, Singh J, et al. FIRST: Fractional Flow Reserve and Intravascular Ultrasound Relationship Study. J Am Coll Cardiol 2013, 61, 917-23
Magni V, Chieffo A,Colombo A. Evaluation of intermediate coronary stenosis with Intravascular ultrasound and fractional flow reserve: Its use and abuse. Catheter
Cardiovasc Interv 2009, 73, 441–8
21
in intermediate coronary artery disease. JACC Cardiovasc Interv 2010, 3, 812-7
Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS): A report of the American College of Cardiology Task Force on Clinical Expert consensus Documents. J Am Coll Cardiol 2001, 37,1478 –92
Mintz GS, Kovach JA, Javier SP, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty: an Intravascular ultrasound study. Circulation 1995, 92, 3408 –14
Gonzalo N, Escaned J, Alfonso F, et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol 2012, 59, 1080-9
Nicholls SJ, Sipahi I, Schoenhagen P, et al. Intravascular ultrasound assessment of novel antiatherosclerotic therapies: rationale and design of the Acyl-CoA: cholesterol acyltransferase Intravascular atherosclerosis treatment evaluation (ACTIV-ATE) study. Am Heart J 2006, 152, 67–74
Yamagishi M, Hosokawa H, Saito S, et al. Coronary disease morphology and distribution determined by quantitative angiography and Intravascular ultrasound: Re-evaluation in a Cooperative multicenter intravascular ultrasound study (COMIUS). Jpn Circ J 2002, 66, 735-40
22
Yoon MH, Tahk SJ, Yang HM, et al. Comparison of the intracoronary continuous Infusion method using a microcatheter and the intravenous continuous adenosine infusion method for inducing maximal hyperemia for fractional flow reserve measurement. Am Heart J 2009, 157, 1050-6
Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Koletitis GJ. Compensatory enlargement of human atherosclerotic coronary arteries.N Engl J Med 1987, 316, 1371-5 Nissen SE, Gurley JC, Grines CL, et al. Intravascular ultrasound assessment of lumen size
and wall morphology in normal subjects and patients with coronary artery disease. Circulation 1991, 84, 1087-99
Mintz GS, Painter JA, Pichard AD, et al. Atherosclerosis in angiographically "normal" coronary artery reference segments: an intravascular ultrasound study with
Clinical correlations. J Am Coll Cardiol 1995, 25, 1479-85
23
- 국문요약 –
좌전 하행지 관상동맥 중증도 협착 병변 에서 혈관내 초음파
이용하여
측정한 total plaque volume 과 심근분획
혈류
예비력 사이의 상관관계
본 연구 에서 는 좌전 하행 지 관상동맥 중증도 협착 병변 에서 혈관 내 초 음파를 이용하여 전체 혈관 내에서 측정한 동맥경화 의 정도를 percent total atheroma volume (%TAV) 로 표현하고 심근분획 혈류 예비력 (FFR) 과 의 상 관관계를 평가하고자 하였다. 2010 년 1 월부터 2012 년 12 월까지 좌전 하행 지 관상동맥 근 부위 혹은 중간부위에 중증도 협착 병변 이 있고 혈관 내 초음파 와 심근분획 혈류 예비력 검사를 시행한 130 명 환자를 대상으로 하였다. %TAV 는 total atheroma volume 과 total vessel volume 의 비율로 표시하고, 심근 허 혈 을 유발하는 관상동맥 내 협착과 관련 된 FFR 의 임계수치는 0.8 로 정의하 였다. 결과적으로 최소 혈관 내 면적 (MLA) 은 FFR 과 보통 상관관계를 가졌 고, %TAV 는 FFR 과 유의한 상관관계를 가졌다. MLA (교차비: 0.37, 95%신뢰 구간: 0.16-0.85, P<0.001) 과 %TAV (교차비: 1.29, 95%신뢰구간: 1.18-1.40, P<0.001) 는 FFR<0.8 를 예측하는 독립인자였다. ROC 분석결과 FFR<0.8 를 예 측하는 MLA 의 임계수치는 2.6mm2 (민감도: 72%, 특이도: 70%) 이고 %TAV 는 39% (민감도: 85%, 특이도: 83%) 였다. 본 연구에서 %TAV≥39.0% 병변 은 48.5% 이였고, %TAV≥39.0% 이면서 MLA ≤2.6mm2 병변에서 84.8% 병변이
24 FFR<0.8 이였고, TAV≥39.0% 이면서 MLA>2.6 mm2 병변에서는 72.2% 병변이 FFR<0.8 이였다. 결론적으로 관상동맥 내 동맥경화 질병 정도를 체적 정량화 하 여 %TAV 로 표현 하였을 때 심근분획 혈류 예비력 과 가장 유의한 상관관계를 보여주었고, 분절 협착 병변 뿐만 아니라 전체 혈관 내 에서의 total plaque burden 도 심근 허 혈을 일으키는 중요한 결정요소로 될 수 있다는 점을 알 수 있었다. 핵심어: 심근분획 혈류 예비력, 혈관내 초음파, 퍼센트 total atheroma volume