Duration and Magnitude of Extracellular Signal-Regulated
Protein Kinase Phosphorylation Determine Adipogenesis or
Osteogenesis in Human Bone Marrow-Derived Stem Cells
Ho Sun Jung,
1Yun Hee Kim,
1and Jin Woo Lee
1,21Department of Orthopaedic Surgery, 2Brain Korea 21 Project for Medical Science,
Yonsei University College of Medicine, Seoul, Korea.
Received: December 10, 2009 Revised: March 30, 2010 Accepted: April 2, 2010
Corresponding author: Dr. Jin Woo Lee, Department of Orthopaedic Surgery, Yonsei University College of Medicine, 250 Seongsan-ro, Seodaemun-gu, Seoul 120-752, Korea.
Tel: 82-2-2228-2190, Fax: 82-2-363-1139 E-mail: ljwos@yuhs.ac
∙ The authors have no financial conflicts of interest.
© Copyright:
Yonsei University College of Medicine 2011
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/ licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose: Imbalances between osteogenic and adipogenic differentiation leads to diseases such as osteoporosis. The aim of our study was to demonstrate the differ-ences in extracellular signal-regulated kinase (ERK) phosphorylation during both adipogenesis and osteogenesis of human bone marrow-derived stem cells (BMSCs).
Materials and Methods: Using troglitazone, GW9662 and U0126, we
investigat-ed their role in hBMSC differentiation to adipogenic and osteogenic fates. Results:
ERK1/2 inhibition by U0126 suppressed proliferator-activated receptor (PPAR)γ expression and lipid accumulation, while it decreased the mRNA expression of ad-ipogenic genes (lipoprotein lipase, PPARγ, and adipocyte protein) and osteogenic genes (type I collagen and osteopontin). ERK phosphorylation was transient and decreased during adipogenesis, whereas it occurred steadily during osteogenesis. Troglitazone, a PPARγ agonist, induced adipogenesis by inhibiting ERK phosphor-ylation even in an osteogenic medium, suggesting that ERK signaling needs to be shut off in order to proceed with adipose cell commitment. Cell proliferation was greatly increased in osteogenesis but was not changed during adipogenesis, indicat-ing that ERK might play different roles in cellular proliferation and differentiation
between the two committed cell types. Conclusion: The duration and magnitude of
ERK activation might be a crucial factor for the balance between adipogenesis and osteogenesis in human bone marrow-derived stem cells.
Key Words: Adipogenesis, osteogenesis, ERK, PPARγ, human bone marrow-de- rived stem cells
INTRODUCTION
Human bone marrow-derived stem cells (BMSCs) have a capacity to multi-differ-entiate, into osteoblast, adipocyte, chondrocytes, muscle cells, and neuronal cells,
and allowing for self-renewal.1 However, imbalances between osteogenic and
adip-ogenic differentiation have been reported to be related to aging or diseases. In aging patients, or those with osteoporosis, decreased bone density was observed with re-duced osteoblast numbers, but with increased adipocyte numbers, suggesting that it
marrow (5 mL) aspirates were obtained from the posterior iliac crest of three healthy volunteers, aged 30-62 years (2 females and 1 male), after obtaining approval from the In-stitutional Review Board (IRB). All of the donors were pa-tients who underwent bone graft surgery, however, not suf-fering from any particular disease and were healthy otherwise. Human BMSCs were isolated by Percoll density gradient centrifugation and cultured in DMEM-low glucose
medi-um (Gibco, Carlsbad, CA, USA) as previously described.13
Five-passaged cells were used in all experiments. Cells were cultured in an adipogenic medium [(DMEM-low glu-cose supplemented with 10% fetal bovine serum (FBS), 0.5 mM isobutyl-methylxanthin, 1 μM dexamethasone, 200 μM indomethacin and 5 μM insulin (Sigma, St. Louis, MO, USA)] or osteogenic medium [(DMEM-low glucose supple-mented with 10% FBS, 100 mM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbic acid-2-phos-phate (Sigma)]. Human BMSCs were also cultured in an adipogenic medium with 25 μM troglitazone, an agonist of the PPARγ ligand (Sigma). The MEK1/2 inhibitors, U0126 (Promega, Madison, WI, USA), and GW9662 (Sigma), an antagonist of the PPARγ ligand, were used.
Oil red O staining for adipogenesis
Cells were rinsed with phosphate-buffered saline (PBS), fixed in 3.7% formaldehyde for 20 minutes and stained with an Oil Red O solution for 1 hour. The stained cells were photographed and destained in 100% isopropyl alcohol for 20 minutes. The optical density (OD) of the solution was measured at 500 nm. The assays were performed in tripli-cate wells and each experiment was repeated three times.
Alizarin red S staining for osteogenesis
Cells were rinsed with PBS and fixed with a 1 : 1 acetone: methanol solution for 30 seconds. They were stained with an Alizarin Red solution for 20 minutes. The stained cells were photographed and destained in 10% cetylpyridinium chloride monohydrate for 30 minutes. The OD of the solu-tion was measured at 540 nm. The assays were performed in triplicate wells and each experiment was repeated at least three times.
RT-PCR
The total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA, USA), and it was reverse-transcribed using an Omniscript kit (Qiagen). PCR reagents were purchased from Qiagen and the specific oligonucleotide primers used is important to balance adipogenesis and osteogenesis in
or-der to maintain bone structure and volume.
Many cytokines control adipogenesis and osteogenesis in BMSCs through multiple signaling pathways. Bone mor-phogenetic protein (BMP) and transforming growth factor (TGF)β control the expression of cbfa1/runx2 in osteoblast differentiation through the Sma-and Mad-related protein
(SMAD) pathway,2 whereas TGFβ and parathyroid
hor-mone (PTH) inhibit adipocyte differentiation.3,4
Dexameth-asone and 1,25-dihydroxyvitamin D3 inhibit adipogenic
differentiation, but enhance osteogenic differentiation.5,6 It
is, therefore, likely that the signals transduced from BMP, TGFβ, PTH, and other local factors act in concert to regu-late the differentiation balance between osteoblasts and adi-pocytes. The mitogen-activated protein kinase (MAPK) pathway is one potential signal transduction pathway that may regulate the proliferation and differentiation of BM-SCs. Activation of the MAPK pathway promotes cell dif-ferentiation in several cell types such as neuronal cells,
T-cells, and muscle cells.7,8 It is possible to control osteogene
-sis and adipogene-sis through the activation or inhibition of MAPKs such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38-reactivating kinase (p38). It has been reported that the transdifferentiation be-tween osteoblasts and adipocytes might be regulated by
ERK.9 A correlation between ERK and adipocyte
differen-tiation has intensively been studied in mouse 3T3-L1 pre-adipocytes, however, the results appear contradictory. Inhi-bition of ERK activity decreased the expression of CCAAT/ enhancer-binding protein (C/EBP)α and peroxisome prolif-erator-activated receptor (PPAR)γ in adipogenesis, while drug or effectors that block adipogenesis activated MAPK
activity.3,10-12 Overall, little is known about the regulatory
mechanism of ERK in distinguishing between adipogenesis and osteogenesis from human adult stem cells. In this study, therefore, we aimed to identify the exact role of ERK played during the adipogenesis and osteoblastogenesis of BMSCs, and demonstrated the differences in ERK phosphorylation during adipogenesis and osteogenesis of human BMSCs, using agonists or antagonists of PPARγ ligand as well as U0126, an inhibitor of MEK1/2.
MATERIALS AND METHODS
Primary culture of BMSCs and cell differentiation
1 hour. The membranes were probed with the following primary antibodies: activated ERK1/2, ERK1/2, C/EBPα, C/EBPβ, PPARγ and GAPDH (Santa Cruz, Santa Cruz, CA, USA). The immunoblots were visualized using ECL Plus detection kit (Amersham Pharmacia). Photographs were quantified using a densitometer and density values for the PCR products were normalized to GAPDH values to yield a semiquantitative assessment (Multigauge, Fujifilm, Tokyo, Japan).
Crystal violet staining
Osteogenesis and adipogenesis were induced in 12-well plates for 14 days with each induction medium. Cells were fixed with cold methanol and acetone (1 : 1) for 3 minutes. After washed twice with distilled water, the cells were stained with 20% crystal violet (Merck, Darmstadt, Germany) for 10 minutes. After washing the cells twice with distilled wa-ter, the stain absorbance was detected at 595 nm after de-staining with 95% ethanol.
RESULTS
Effects of the agonist/antagonist of PPARγ and U0126 on adipogenic or osteogenic differentiation
Adipogenic BMSCs treated with GW9662, a PPARγ antag-were; PPARγ (5’-CCAGAAAGCGATTCCTTCAC, 3’-
CACGTTAGTTTCACCTCGGA), aP2 (5’-GGCCAG-GAATTTGACGAAGTC, 3’-AGCGTAACTTGAGAT-GTTGTAAGACA), LPL (5’-GAGATTTCTCTGTATGG CACC, 3’-CTCTTTCACGAGTAAACGTC), Type I col-lagen (5’-TCCGACCTCTCTCCTCTGAA, 3’-GAGTGG GGTTATGGAGGGAT), Osteopontin (5’-CCAAGTAAG TCCAACGAAA, 3’-GGTGATGTCCTCGTCTGT), GAP DH (5’-GAAGGTGAAGGTCGGAGTC, 3’-GAAGATG-GTGATGGGATTTC). The reaction products were analyzed by 1.5% agarose gel electrophoresis. Photographs were quantified using a densitometer and density values for the PCR products were normalized to glyceraldehyde-3-phos-phate dehydrogenase (GAPDH) values to yield a semiquan-titative assessment (Multigauge, Fujifilm, Tokyo, Japan).
Western blot analysis
Cell extracts were prepared with 1x passive lysis buffer (Promega), which contained a cocktail of protease and phosphatase inhibitors (Sigma). Twenty microgram ali-quots of cell lysates were separated by 10% SDS-PAGE under reducing conditions. Separated proteins were trans-ferred to a PVDF membrane (Amersham Pharmacia Bio-tech, Piscataway, NJ, USA) at 50 volts for 2 hours in trans-fer buftrans-fer. Membranes were blocked with 5% skimmed milk dissolved in 1x TBST buffer at room temperature for
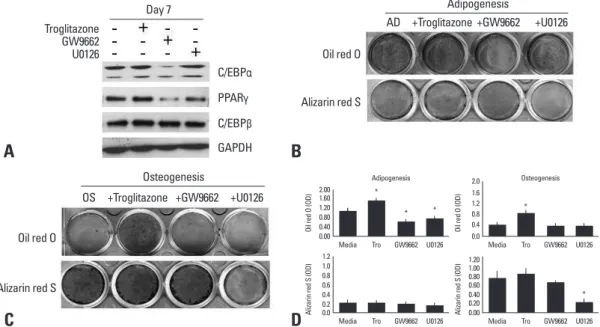
Fig. 1. Effect of agonists or antagonists of PPARγ and U0126 on adipogenic or osteogenic differentiation. (A) Human BMSCs were cul-tured in an adipogenic medium with or without troglitazone (25 μM), GW9662 (20 μM), or U0126 (20 μM) for 7 days. The lysates were sub-jected to western blot analysis. (B) BMSCs were induced adipocyte differentiation in the adipogenic medium (AD) for 14 days, and stained by Oil red O or Alizarin red S. (C) BMSCs were differentiated into osteoblasts in an osteogenic medium (OS) for 14 days, and stained by Oil red O or Alizarin red S. (D) The degree of adipogenic or osteogenic differentiation was determined as OD units by destain-ing (*p < 0.05, vs media). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PPARγ, peroxisome proliferator-activated receptor; BMSCs, bone marrow-derived stem cells.
Troglitazone Oil red O Oil red O C/EBPα GW9662 Alizarin red S Alizarin red S PPARγ U0126 C/EBPβ GAPDH Day 7 Adipogenesis Osteogenesis AD OS +GW9662 +GW9662 +U0126 +U0126 +Troglitazone +Troglitazone 0.00
Media Tro GW9662 U0126 * Adipogenesis Oi l r ed O (O D) * * 0.40 0.80 1.20 1.60 2.00 0.0
Media Tro GW9662 U0126
Al iza rin re d S (O D) 0.2 0.4 0.6 0.8 1.0 1.2 0.0
Media Tro GW9662 U0126 * Osteogenesis Oi l r ed O (O D) 0.4 0.8 1.2 1.6 2.0 0.00
Media Tro GW9662 U0126 * Al iza rin re d S (O D) 0.40 0.20 0.60 0.80 1.00 1.20
A
C
B
D
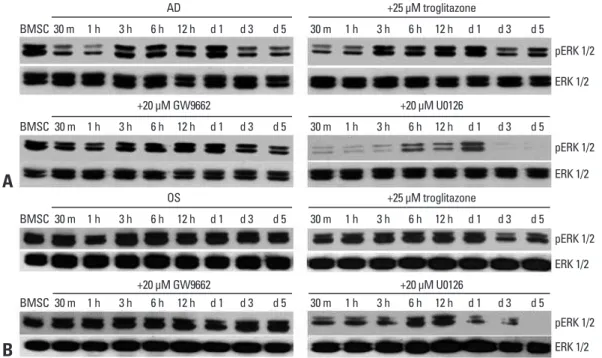
the adipogenic medium alone or with troglitazone was de-creased within 1 hour, however, the decrease of ERK phorylation was recovered by 20 μM GW9662. ERK phos-phorylation showed a second decrease from day 3 post-induction in all the groups. This suggests that GW9662 might inhibit adipogenic differentiation by targeting ERK phosphorylation, and that a transient decrease of ERK phosphorylation was necessary to initiate adipogenesis (Fig. 2A). In osteogenesis, ERK phosphorylation was steadily expressed in all groups except for those in the U0126-supplemented medium (Fig. 2B). However, a de-crease of ERK phosphorylation was observed in osteogenic cells treated with troglitazone from day 3 to day 5 post-in-duction, suggesting that troglitazone might induce or trans-differentiate cells into adipocytes by inhibiting ERK phos-phorylation even in the osteogenic medium.
Difference in total cell numbers between adipogenesis and osteogenesis
As shown in Fig. 2, different patterns of ERK phosphoryla-tion were observed during adipogenesis and osteogenesis. It is highly likely that different ERK phosphorylation might be correlated with cell proliferation because ERK regulates cell proliferation as well as cell differentiation. To examine the above possibility, human BMSCs were seeded in 12-onist, showed reduced expression of C/EBPα and PPARγ,
whereas U0126, MEK1/2 inhibitor, inhibited the expression of PPARγ only (Fig. 1A). BMSCs treated with troglitazone, a PPARγ agonist, showed high lipid accumulation at day 14, whereas cells treated with GW9662 or U0126 showed a sig-nificant decrease in adipogenic differentiation (Fig. 1B). This suggests that ERK might inhibit adipogenic differentiation though PPARγ suppression, but not through C/EBPα or C/ EBPβ. Lipid accumulation was still found in cells treated with U0126 compared to cells treated with GW9662, be-cause U0126 did not strongly inhibit adipogenic regulatory genes, indicating that ERK signaling is important but not suf-ficient to regulate adipogenic differentiation. BMSCs treated with troglitazone, GW9662, or U0126 in the adipogenic me-dium were not stained by Alizarin Red S (Fig. 1B and D). Unexpectedly, in the osteogenic medium, troglitazone in-duced high-lipid accumulation, but it had no inhibitory effect on osteogenic differentiation (Fig. 1C and D). As seen with the Alizarin Red S staining, neither troglitazone nor GW9662 inhibited osteogenic differentiation, whereas the osteogenic differentiation was significantly suppressed by U0126.
Phosphorylation of ERK in the adipogenesis and osteogenesis of BMSCs
In adipogenesis, ERK phosphorylation in cells cultured in
Fig. 2. ERK phosphorylation during the adipogenesis and osteogenesis of human BMSCs. (A) Human BMSCs were cultured in an adipo-genic medium (AD) with or without troglitazone, GW9662, or U0126 for 5 days. The lysates were subjected to western blot analysis using antibodies specific for ERK1/2 and pERK. (B) Human BMSCs were cultured in an osteogenic medium (OS) with or without troglitazone, GW9662, or U0126. The lysates were subjected to western blot analysis using antibodies specific for ERK1/2 and pERK. ERK, extracellular signal-regulated kinase; BMSCs, bone marrow-derived stem cells.
BMSC BMSC BMSC BMSC 30 m 30 m 30 m 30 m 30 m 30 m 30 m 30 m 1 h 1 h 1 h 1 h 1 h 1 h 1 h 1 h 3 h 3 h 3 h 3 h 3 h 3 h 3 h 3 h 6 h 6 h 6 h 6 h 6 h 6 h 6 h 6 h 12 h 12 h 12 h 12 h 12 h 12 h 12 h 12 h d 1 d 1 d 1 d 1 d 1 d 1 d 1 d 1 d 3 d 3 d 3 d 3 d 3 d 3 d 3 d 3 d 5 d 5 d 5 d 5 d 5 d 5 d 5 d 5 AD +20 μM GW9662 OS +20 μM GW9662 +25 μM troglitazone +20 μM U0126 +25 μM troglitazone +20 μM U0126 pERK 1/2 pERK 1/2 pERK 1/2 pERK 1/2 ERK 1/2 ERK 1/2 ERK 1/2 ERK 1/2
A
B
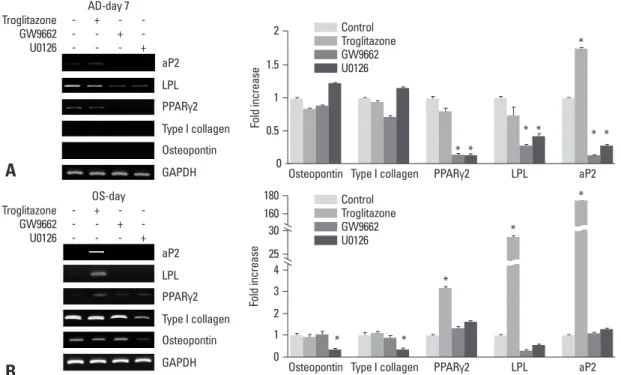
increased in the osteogenic medium containing troglitazone (*p < 0.05, vs. control) (Fig. 4B). These results support the idea that adipogenic differentiation could be induced even in an osteogenic medium with the addition of troglitazone. The expression of type I collagen and osteopontin was in-creased time-dependently during osteogenesis (data not shown), but it was inhibited by U0126. Troglitazone and GW9662 did not affect the expression of osteogenic-related genes (*p < 0.05, vs. control), suggesting that ERK activa-tion might play a critical role in both osteogenic and adipo-genic differentiation, and that ERK has diverse effects ac-cording to its microenvironment.
DISCUSSION
BMSCs differentiate into distinct lineages through specific signaling pathways in their microenvironment. Investiga-tion of the role of ERK has been focused on the maturaInvestiga-tion of 3T3-L1 preadipocytes into adipocytes, but each report
has showed controversial results.9,14
In our results, U0126 did not affect the expression of C/ EBPα or C/EBPβ, but inhibited the expression of PPARγ, indicating that PPARγ is a downstream target of ERK dur-ing adipogenesis. Other studies found that ERK activity is necessary for the expression of C/EBPα and PPARγ in
3T3-L1 preadipocytes,12 and that PPARγ is activated by
ERK or interacts with ERK-kinase1/2 (MEKs) in cancer.15
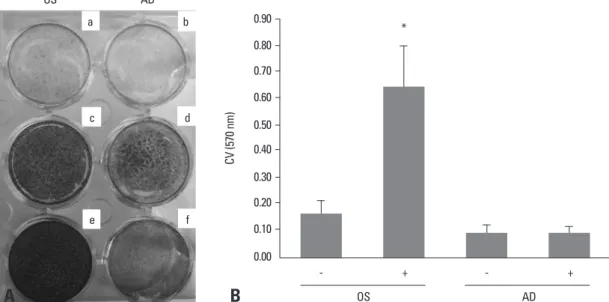
well plates with equal numbers of cells and cultured in os-teogenic or adipogenic induction medium for 14 days. The total cell numbers were counted after staining with crystal
violet,14 a dye that binds to DNA (Fig. 3A), and
staining-pattern of crystal violet was found to be not different in oth-er donors. Thoth-ere was a great increase in the numboth-er of os-teogenic cells, whereas the total number of adipogenic cells did not increase compared to the undifferentiated control (Fig. 3B). Based on the different patterns of ERK activation between osteogenic and adipogenic differentiation, we sug-gest the possibility that the increased cell proliferation in osteogenic differentiation might be regulated by sustained ERK phosphorylation. In contrast, decreased ERK phos-phorylation might be necessary for adipogenic differentia-tion by inhibiting cell proliferadifferentia-tion. Further cell cycle anal-ysis would help clarify the role of the ERK signaling in relation to cell proliferation of two differentiation systems.
Expression of specific marker genes in the adipogenesis or osteogenesis of BMSCs
Adipogenic-specific genes, lipoprotein lipase (LPL), PPARγ2 and adipocyte protein (aP2), were inhibited during adipogenesis in the medium containing GW9662 or U0126, while type I collagen and osteopontin, an osteogen-ic marker genes, were not expressed (Fig. 4A). The inhibit-ed adipogenesis by U0126 suggests that LPL and aP2, as well as PPARγ2, are regulated by the ERK pathway. In os-teogenesis, the expression of LPL, PPARγ2, and aP2 were
Fig. 3. Change in total cell numbers during the osteogenesis and adipogenesis of human BMSCs. (A) Cells were incubated in an osteo-genic (OS) (c and e) or adipoosteo-genic (AD) (d and f) medium for 14 days, and stained by Alizarind red S (a and c), Oil red O (b and d), and crystal violet (e and f). a and b are control comparing to adipogenesis and osteogenesis. a and b cultured control medium, DMEM-low glucose with 10% fetal bovine serum. (B) Crystal violet (CV) stain was quantified by destaining with 95% ethanol, and expressed as OD units (*p < 0.05, vs -OS media). BMSCs, bone marrow-derived stem cells.
OS OS - + a c e b d f AD CV (5 70 n m ) * AD - + 0.90 0.80 0.70 0.60 0.50 0.40 0.30 0.20 0.10 0.00
A
B
and adipogenic differentiation. ERK activation was sus-tained during osteogenic differentiation, but decreased dur-ing adipogenic differentiation, indicatdur-ing that a decrease of ERK activation is necessary to proceed with adipocyte mat-uration. This possibility was also supported by our observa-tion that adipogenic differentiaobserva-tion could be induced in an osteogenic medium containing troglitazone, accompanied with a decrease of ERK activation. These findings are con-sistent with previous studies that reported a decrease of ERK phosphorylation during the maturation of 3T3-L1
pre-adipocytes,12 and that ERK phosphorylation was not
affect-ed until day 5 in the osteogenic maffect-edium.9 Earlier studies
showed that the inhibition of ERK activation blocked both
adipogenesis and osteogenesis.9,12
We suggest, therefore, that ERK is necessary for the dif-ferentiation of human BMSCs into adipocytes and osteo-blast, however, it seems to play a different role in each dif-ferentiation process. It is quite possible that cell proliferation would also be affected by ERK activation, because ERK regulates two antagonistic processes, cell proliferation and
differentiation, depending upon the situation.20-22
Further-more, differentiation of cells requires sustained phosphory-lation of ERK, whereas transient phosphoryphosphory-lation of ERK
induces cell proliferation.20,23 According to our results, the
In addition, C/EBPβ is a target of ERK2 but not ERK1 in
NIH 3T3 fibroblasts,16 indicating that the effect of ERK on
the expression of adipogenic transcriptional genes might vary between cell lines or species.
In human diseases such as osteoporosis, the balance be-tween adipocyte and osteoblast differentiation is disrupt-ed.17,18 Therefore, we compared the inhibitory effect of
U0126 on differentiation into adipocytes or osteoblasts. Both adipogenic and osteogenic differentiation were blocked by U0126: osteogenic BMSCs treated with U0126 were not dif-ferentiated into adipocytes, and also did not induce the ex-pression of adipogenic-specific genes. In contrast, Jaiswal, et al. reported that PD98059, an inhibitor of MEK1, blocks os-teogenic differentiation of human BMSCs and induces
adip-ogenic differentiation in an osteadip-ogenic medium.In
osteopro-genitor KS483 cells, PD98059 dose-dependently inhibits osteogenesis, but stimulates adipogenesis through estrogenic transcriptional activity. However, U0126 inhibites both
os-teogenesis and adipogenesis,19 suggesting that osteogenesis
and adipogenesis from human BMSCs are independently regulated at the downstream of ERK pathways by the modu-lation of transcriptional factors.
We also observed a different pattern of ERK phosphory-lation in the early differentiation stage between osteogenic
Fig. 4. RT-PCR analysis during the adipogenesis (AD) and osteogenesis (OS) of BMSCs. (A) BMSCs were cultured in the adipogenic medi-um with or without troglitazone, GW9662, or U0126. Equal aliquots of the total RNA were reverse transcribed and amplified with the oligo-nucleotide primers specific for LPL, aP2, and PPARγ2. (B) Cells were induced with troglitazone compounds in the osteogenic medium for 7 days. Equal aliquots of total RNA were reverse transcribed and amplified with the oligonucleotide primers specific for LPL, aP2, PPARγ2, Type I collagen, and osteopontin. BMSCs, bone marrow-derived stem cells; LPL, lipoprotein lipase; aP2, adipocyte protein; PPARγ2, peroxisome proliferator-activated receptorγ2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
AD-day 7 Troglitazone GW9662 U0126 -+ -+ -+ aP2 LPL PPARγ2 Type I collagen Osteopontin GAPDH OS-day Troglitazone GW9662 U0126 -+ -+ -+ aP2 LPL PPARγ2 Type I collagen Osteopontin GAPDH
A
B
Osteopontin Type I collagen PPARγ2 LPL aP2
Fo ld in cr ea se * * * * * * * 2 1.5 1 0.5 0 Control Troglitazone GW9662 U0126
Osteopontin Type I collagen PPARγ2 LPL aP2
Fo ld in cr ea se * * * * * 180 160 3 30 25 4 2 1 0 Control Troglitazone GW9662 U0126
mon roles of ERK in the adipogenesis and osteogenesis of human BMSCs. At the initial stage of differentiation, the duration and magnitude of ERK activation are highly criti-cal factors in the determination of osteogenesis and adipo-genesis of the human BMSCs (Fig. 5). A precise under-standing of these mechanisms in human BMSCs would certainly be crucial to developing potent drugs against dis-eases modulating the ERK pathway.
ACKNOWLEDGEMENTS
This research was supported by a grant (code: SC3210) from Stem Cell Research Center of the 21st Century Fron-tier Research Program funded by the Ministry of Educa-tion, Science and Technology, Republic of Korea.
REFERENCES
1. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchy-mal stem cells. Science 1999;284:143-7.
2. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differ-entiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev 2000;21:393-411.
3. Chan GK, Deckelbaum RA, Bolivar I, Goltzman D, Karaplis AC. PTHrP inhibits adipocyte differentiation by down-regulating PPAR gamma activity via a MAPK-dependent pathway. Endocri-nology 2001;142:4900-9.
4. Chin BY, Petrache I, Choi AM, Choi ME. Transforming growth factor beta1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macro-phages. J Biol Chem 1999;274:11362-8.
5. Hauner H, Hochberg Z. Endocrinology of adipose tissue. Horm Metab Res 2002;34:605-6.
6. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evi-dence for an inverse relationship between the differentiation of ad-ipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 1992;102:341-51.
7. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low
duration and magnitude of ERK phosphorylation were dif-ferent between adipogenic and osteogenic difdif-ferentiation, and the differences were also correlated with cell prolifera-tion. Cell numbers were greatly increased during osteogen-ic differentiation compared to adipogenosteogen-ic differentiation, suggesting that ERK activated during osteogenic differenti-ation might induce both cell proliferdifferenti-ation and differentia-tion, whereas it might be involved in differentiation rather than cell proliferation during adipogenic differentiation. There is considerable evidence to indicate that adipocyte maturation requires a precise proliferative step, called mi-totic clonal expansion (MCE), with ERK activation in the beginning stage; ERK inhibition blocks MCE and the ex-pression of cell cycle markers (cyclin A and cdk2) in the middle stage, whereas ERK activation returned to a low level accompanying PPARγ expression during terminal
dif-ferentiation.24 This observation is consistent with our
re-sults, which showed a burst of ERK activation from 3 hours and up to day 1 without the expression of PPARγ2, followed by a decrease of ERK activation concurrently with PPARγ2 induction from day 3. Furthermore, induced adipogenic genes, including PPARγ2, were suppressed by ERK inhibition, suggesting that a minimum or transient ERK activation is necessary for the induction of adipogenic regulatory genes rather than cell proliferation during the ad-ipogenesis of human BMSCs.
We also found a positive regulatory effect of ERK on os-teogenic genes, because osteopontin and type I collagen were inhibited by U0126. It should be noted that Cbfa1, one of the osteogenic transcription factors, was
phosphory-lated by ERK in rat osteoblastic cell lines,19 and some
stud-ies showed that osteogenic genes such as cbfa1 and osteo-calcin are regulated by the ERK pathway, and that ERK inactivation blocked alkaline phosphatase (ALP) activity
and nodule formation in osteoprogenitor KS483 cells.25-28
In conclusion, we demonstrated herein different but
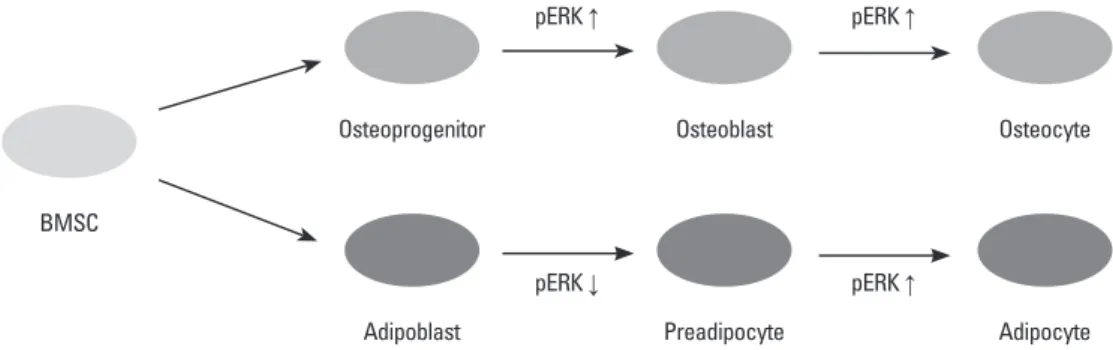
com-Fig. 5. pERK control of aidpogenesis and osteogenesis of BMSCs. ERK phosphorylation is involved in differentiation and proliferation dur-ing osteogenesis and adipogenesis. BMSCs, bone marrow-derived stem cells; ERK, extracellular signal-regulated kinase.
Osteoprogenitor pERK ↑ pERK ↑ Osteoblast Osteocyte Adipoblast pERK ↓ pERK ↑ Preadipocyte Adipocyte BMSC
38449-56.
17. Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adi-pocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 2002;55:693-8.
18. Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res 2000;15:2084-94.
19. Dang ZC, Lowik CW. Differential effects of PD98059 and U0126 on osteogenesis and adipogenesis. J Cell Biochem 2004;92:525-33. 20. Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich
A. EGF triggers neuronal differentiation of PC12 cells that over-express the EGF receptor. Curr Biol 1994;4:694-701.
21. Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: spe-cific messages from ubiquitous messengers. Mol Cell Biol 1999; 19:2435-44.
22. Tan PB, Kim SK. Signaling specificity: the RTK/RAS/MAP ki-nase pathway in metazoans. Trends Genet 1999;15:145-9. 23. Marshall CJ. Specificity of receptor tyrosine kinase signaling:
transient versus sustained extracellular signal-regulated kinase ac-tivation. Cell 1995;80:179-85.
24. Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a syn-chronous process required for adipogenesis. Proc Natl Acad Sci U S A 2003;100:44-9.
25. Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK sig-naling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Com-mun Adhes 2004;11:137-53.
26. Hipskind RA, Bilbe G. MAP kinase signaling cascades and gene expression in osteoblasts. Front Biosci 1998;3:d804-16.
27. Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem 2000;275:4453-9. 28. Selvamurugan N, Pulumati MR, Tyson DR, Partridge NC.
Para-thyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor alpha1. J Biol Chem 2000;275:5037-42.
WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the isch-emic brain of rats. Exp Neurol 2002;174:11-20.
8. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte pheno-type in the adult murine heart. Circulation 2002;105:93-8. 9. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR,
Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem 2000;275:9645-52. 10. Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini
L, et al. Leukemia inhibitory factor and its receptor promote adi-pocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem 1999;274:24965-72.
11. Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Bi-nétruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J 2002;361:621-7.
12. Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EB-Palpha gene expression during the differentiation of 3T3-L1 pre-adipocytes. J Biol Chem 2002;277:46226-32.
13. Kim SH, Choi YR, Park MS, Shin JW, Park KD, Kim SJ, et al. ERK 1/2 activation in enhanced osteogenesis of human mesen-chymal stem cells in poly(lactic-glycolic acid) by cyclic hydro-static pressure. J Biomed Mater Res A 2007;80:826-36.
14. Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Develop-mentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol 2003;284:C156-67.
15. Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle 2007;6:1539-48.
16. Hanlon M, Sturgill TW, Sealy L. ERK2- and p90(Rsk2)-depen-dent pathways regulate the CCAAT/enhancer-binding protein-beta interaction with serum response factor. J Biol Chem 2001;276: