저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Master’s Thesis in Sciences
Perturbation of MLKL-mediated
endosomal trafficking enhances TRAIL
signal to increase cancer cell death
Graduate School of Ajou University
Department of Biomedical Sciences
Perturbation of MLKL-mediated
endosomal trafficking enhances TRAIL
signal to increase cancer cell death
You-Sun Kim, Advisor
I submit this thesis as the Master’s thesis in Sciences.
August, 2019
Graduate School of Ajou University
Department of Biomedical Sciences
i
-ABSTRACT-
Perturbation of MLKL-mediated
endosomal trafficking enhances TRAIL signal
to increase cancer cell death
Mixed Lineage Kinase Domain-like (MLKL) is the essential molecule of programmed necrotic cell death, Necroptosis. To induce necroptosis, MLKL is phosphorylated by upstream partner, Receptor Interacting Protein Kinase-3 (RIPK3 or RIP3) and then translocate cellular membrane to disrupt membrane integrity. Recent data suggests that function of MLKL in RIP3-indendent manner in the effective generation of intraluminal and extracellular vesicles as well as in myelin sheath breakdown to promotes sciatic nerve regeneration. In this study, we investigated that whether MLKL play a role in link between TRAIL receptor endocytosis and apoptosis. Depletion of MLKL enhances TRAIL-induced cell death in various cancer cell lines and enhancement of cell death is RIP3-independent manner. TRAIL-induced cytotoxic signals are further increased by preventing endocytosis and MLKL depletion facilitates degradation of TRAIL-DR5. TRAIL-induced intracellular downstream signals were prolonged by depletion of MLKL and then prolonged cytotoxic signals promote cell death. Our data indicate that defect of the intracellular trafficking of TRAIL-DR5 by depletion of MLKL enhances cancer cell death. Based on our findings, it is tempting to speculate that to promote death receptor signals, it may effective to reduced MLKL expression and to prevent survival receptor signal, upregulated MLKL expression contribute to abolish the intracellular signal through the MLKL-dependent extracellular vesicles
ii
secretion. Although the processes that regulate these pathways require further elucidation for detail molecular mechanisms, both ways will be the potent therapeutic strategies to target cancer.
Keywords: MLKL, TRAIL, Endosomal trafficking
iii
TABLE OF CONTENTS
ABSTRACT --- i
TABLE OF CONTENTS --- iii
LIST OF FIGURES --- V
I. INTRODUCTION --- 1
II. MATERIALS AND METHODS --- 4
A. GST-TRAIL purification
---
4
B. Antibodies and chemical reagents
---
5
C. Cell culture
---
5
D. Lentiviral shRNA experiments
---
6
E. Cytotoxicity assay
---
6
F. Immunoblot analysis
--- 6
G. Ligands and Receptor uptake assays, and Immunofluorescence staining
---
6
H. Flow cytometry analysis
---
7
I. GST-pull down assay
---
7
J. Reverse transcription-PCR
--- 8
iv
A. Depletion of MLKL accelerates TRAIL-induced cancer cell death
--- 9
B. RIP3-independent enhancement of cell death by MLKL depletion in TRAIL-mediated cytotoxicity
--- 13
C. Depletion of MLKL caused defect on receptor-ligand endosomal trafficking
---
21
D. Endosomal trafficking is associated with MLKL-mediated sensitization of TRAIL-cytotoxicity
---
28
E. Prolonged death signal due to the defect of MLKL-mediated endosomal trafficking
--- 33
IV. DISCUSSION --- 36
V. REFERENCES --- 38
v
LIST OF FIGURES
Fig. 1. TRAIL-induced cell death was sensitized by MLKL depletion. --- 10 Fig. 2. TRAIL-induced signaling was enhanced by MLKL depletion --- 11 Fig. 3. Depletion of MLKL accelerates TRAIL-induced cell death in various cell lines. --- 12 Fig. 4. Depletion of MLKL accelerates TRAIL-induced death in RIP3-expressing cell line, HT-29. --- 15 Fig. 5. TRAIL-induced cell death was sensitized by MLKL depletion, independent RIP3. --- 16 Fig. 6. MLKL promotes cell death through activation of necroptosis pathway. --- 17 Fig. 7. Depletion of MLKL is response to TRAIL sensitivity, ectopically expressed RIP3. --- 18 Fig. 8. TRAIL-induced sensitization by MLKL depletion is typical apoptosis processes. --- 19 Fig. 9. TRAIL induced cell death and PARP cleavage were blocked by zVAD in RIP3 expressing cancer cells. --- 20 Fig. 10. Depletion of MLKL delays intracellular degradation of EGFR. --- 23 Fig. 11. Intracellular degradation of EGFR was delayed by MLKL depletion. --- 24 Fig. 12. Depletion of MLKL boosts EGF-induced signaling. --- 25 Fig. 13. Depletion of MLKL slowdowns intracellular degradation of Death Receptor 5. --- 26 Fig. 14. Depletion of MLKL did not altered both Protein and mRNA levels. --- 27 Fig. 15. TRAILinduced cell death was enhanced by blocking of DR5 endocytosis. --- 29
vi
Fig. 16. TRAIL-induced apoptotic signaling was enhanced by blocking of DR5 endocytosis. --- 30 Fig. 17. TRAIL-induced MAPK signaling was enhanced by blocking of DR5 endocytosis. --- 31 Fig. 18. TRAIL-induced MAPK signaling was enhanced by MLKL depletion. ---- 32 Fig. 19. Degradation of intracellular TRAIL delays in MLKL-depleted cells. --- 34 Fig. 20. DISC complex more recruits in MLKL depleted cells. --- 35
- 1 -
I. INTRODUCTION
Mixed Lineage Kinase Domain-like (MLKL) has been identified as a key molecule of necroptosis (Cai et al., 2014; Murphy & Vince, 2015; Sever, Muhlberg, & Schmid, 1999; Sun et al., 2012). MLKL is required to form necrosome complex which comprise Receptor Interacting Protein Kinase (RIPK, or RIP) 1 and 3, and RIP3-dependent plasma membrane localization of MLKL is necessary for programmed necrotic cell death to occur (Chen et al., 2014; Cho et al., 2009; Murphy et al., 2013; Wu et al., 2013; Zhao et al., 2012). The translocation of MLKL to the membrane has been alternately reported to leads to plasma membrane disruption by through its activation of ion channels or by directly permeabilizing the plasma membrane, perhaps through binding to phosphatidylinositol phosphates (Dondelinger et al., 2014; Wang et al., 2014).
Recent data suggests that MLKL functions RIP3-indendent manner. Yoon
et al., reported that MLKL affected endosomal transport independently of
RIP3 through the effective generation of intraluminal and extracellular vesicles (Yoon, Kovalenko, Bogdanov, & Wallach, 2017). In this report, they observed that effects of MLKL depletion on the rates of intracellular degradation of EGF and its receptor and suggested that possibility on enhancement of interference with endosomal trafficking by the role for MLKL. RIP3-independent contribution of MLKL was also observed in injury-induced activation signal (Ying et al., 2018). Sciatic nerve injury injury-induced MLKL expression in schwann cells and MLKL functions myelin sheath breakdown to promotes sciatic nerve regeneration in absence of RIP3.
- 2 -
TRAIL (TNF-Related Apoptosis-Inducing Ligand) is a member of the TNF superfamily and it induce apoptosis selectively in cancer cells, while having limited effect on normal cells (Ashkenazi et al., 1999; Walczak et al., 1999). TRAIL binding to its death receptors (DR4 and DR5, also named as TRAIL-R1 and TRAIL-R2) induces the formation of the death-inducing signaling complex (DISC) by the recruitment of the adaptor Fas-associated death domain (FADD) and the initiator caspase-8 (Juo, Kuo, Yuan, & Blenis, 1998; Kischkel et al., 2000; Slee, Adrain, & Martin, 2001). Binding of TRAIL to its apoptosis-inducing death receptors (DR4 and DR5) stimulates their internalization via clathrin-mediated endocytosis (CME) (Austin et al., 2006; Kohlhaas, Craxton, Sun, Pinkoski, & Cohen, 2007). Receptor-mediated endocytosis plays a critical role in regulating signaling (Di Fiore & von Zastrow, 2014; Sorkin & Von Zastrow, 2009), by either promoting rapid endocytosis of ligand–receptor complexes and attenuating cell-surface signaling, or by promoting the formation of endosomes that can serve as signaling platforms for these complexes (Platta & Stenmark, 2011; Sorkin & Von Zastrow, 2009). Link between TRAIL receptor endocytosis and apoptosis has been well established by demonstration of TRAIL receptor internalization by a clathrin-dependent mechanism. TRAIL-activated DRs trigger ryanodine receptor (RyR)-dependent Ca2+ release from endoplasmic reticulum (ER) stores, induce calcineurin-dependent dephosphorylation, and thereby activation of Dyn1, leading to ligand and receptor-selective uptake of DRs and the attenuation of their apoptotic signaling (Reis, Chen, Bendris, & Schmid, 2017).
In this study, we investigated the endosomal trafficking function of MLKL on TRAIL-induced cell death in cancer cells. We found that depletion of MLKL enhances TRAIL-induced cell death in various cancer cell lines and
- 3 -
enhancement of cell death is RIP3-independent contribution. TRAIL-induced cytotoxic signals by ligating DR5 is further increased by dynasore, which is a GTPase inhibitor that rapidly inhibits dynamin activity to prevents endocytosis. Depletion of MLKL interferes prolonged cytotoxic signals by defecting the intracellular trafficking of TRAIL-DR5 suggesting that MLKL facilitates endosomal trafficking.
- 4 -
II. MATERIALS AND METHODS
A. GST-TRAIL purification 1. Extraction, purification
For purifying GST-TRAIL, GST-TRAIL plasmid was transformed in E. coli strain BL21. After 16 hours, the picked colonies were incubated into 5mL LB with ampicillin at 37°C. After 18 hours, 2ml LB with bacteria add into 400mL LB and incubate for 30min and then add Isopropyl β-D-1-thiogalactopyranoside (IPTG) at the final concentration of 0.4mM and continue incubation for 3 hours at 37°C. The bacteria pellets were collected by ultracentrifuge. Pellets were lysed in lysis buffer (50mM pH 7.4 Tris, 50mM NaCl, 5mL EDTA, 1mM DTT, 0.2% Triton X-100, 1mL EGTA, 1X Lysozyme, 1X PMSF, 1X protein inhibitor in dH₂O) and sonicated. And then 10% Triton-X-100 was added and incubated for 2 hours at 4°C. Lysate was centrifuged at 12000 rpm for 10min at 4°C. Supernatant were incubated with 200uL GSH-bead (Glutathione Sepharose™ 4B; GE Healthcare) for 3 hours at 4°C. GSH-bead was equilibrated 3 times by lysis buffer. Beads were collected and washed 3 times in 2mL wash buffer I (50mM pH 7.4 Tris, 500mM NaCl, 1mL EGTA, 10% Glycerol, 0.1% Triton X-100, 0.1%
β-Mercaptoethanol, 1mL PMSF in dH₂O). This was repeated once with wash buffer II (50mM pH 7.5 HEPES, 100mM NaCl, 1mL EGTA, 10% Glycerol in
dH₂O). The GST-tagged proteins were eluted with elution buffer (50mM pH 7.5 HEPES, 100mM NaCl, 10% Glycerol, 40mM L-glutathione reduced, 0.03% Triton X-100 in dH₂O).
2. Dialysis
To dialysis, activate the dialysis membrane (Spectra/Por dialysis membrane; REPLIGEN) in boiling water for 5 min and allowed to cool. The purified
GST-- 5 GST--
TRAIL was put into an activated membrane and dialyzed in dialysis buffer (20mM pH7.5 Tris, 20% Glycerol, 100mM KCL, 0.2mM EDTA, 2mM DTT, 10mM β-glycerol phosphate in dH₂O) for overnight at 4°C.
After dialysis, run gel to check the purity and quantity of GST-TRAIL with Coomassie blue staining and comparing to known amount of BSA.
B. Antibodies and chemical reagents
Antibodies used in immunoblotting and immunofluorescence: anti-MLKL (Abcam, ab184718, 1:2000), anti-RIP3 (Cell Signaling Technology, 13526, 1:1000), ACTIN (Santa Cruz Biotechnology, 47778, 1:5000), anti-VINCULIN (Sigma-Aldrich, V9131, 1:5000), anti-PARP (Cell Signaling Technology, 9542, 1:1000), anti-Caspase-3 (Cell Signaling Technology, 9662, 1:1000), anti-Caspase-8 (Cell Signaling Technology, 9746, 1:1000), anti-FADD (BD biosciences, 610400, 1:1000), anti-DR5 (Abcam, ab199357, 1:1000), anti-EGFR (Abcam, ab2430, 1:1000), anti-p-ERK (Cell Signaling Technology, 9101, 1:1000), anti-p-AKT (Cell Signaling Technology, 9271, 1:1000), anti-p-p38 (Cell Signaling Technology, 9215, 1:1000) and anti-GST (Abcam, ab9085, 1:500). TNF-α and zVAD were purchased from R&D Systems. Etoposide and Dynasore were purchased from Sigma-Aldrich. SMAC mimetic (LCL-161) was purchased from Adooq Bioscience.
C. Cell culture
HeLa, HeLa (RIP3), HT-29, H2009, HEK293T cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). HCC4006, MDA-MB-231, A549 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 10%
- 6 -
FBS. To generate cell lines stably expressing the RIP3 construct, HeLa cells were infected with pLX303-hRIP3 lentivirus.
D. Lentiviral shRNA experiments
MISSION short-hairpin RNA (shRNA) plasmids targeting hMLKL mRNA (NM_152649), and non-targeting control sequences (NM_027088) were obtained from Sigma-Aldrich. Lentiviral plasmids were transfected into 293T cells (System Biosciences, LV900A-1) using Lipofectamine 2000 (Invitrogen, 11668019). Pseudoviral particles were collected 48 hours after the lentiviral plasmid transfection and infected into cells with polybrene (8 μg/mL). Infected cells were puromycin selected two days after infection, and knockdown was confirmed by immunoblotting.
E. Cytotoxicity assay
Representative images were taken by a phase-contrast microscope. Cell viability was determined using tetrazolium dye colorimetric tests (the MTT assay) read at 570 nm. The mean ± STDEV of duplicates is presented.
F. Immunoblot analysis
Cells were lysed in M2 buffer containing 20mM Tris at pH 7, 0.5% NP-40, 250mM NaCl, 3mM EDTA, 3mM EGTA, 2mM DTT, 0.5mM PMSF, 20mM β-glycerol phosphate, 1mM sodium vanadate, and 1 μg/mL leupeptin. Equal amounts of cell extracts were resolved by SDS-PAGE and analyzed by immunoblotting.
G. Ligands and Receptor uptake assays, and Immunofluorescence staining
- 7 -
1. Ligands and Receptor uptake assays
Before ligand treatment, the cells were incubated for 12 hours in serum-free media. GST-TRAIL and EGF were treated to the cells for 30min on ice in CO₂-independent media (Thermo Fisher Scientific), and rinsed in cold Dulbecco’s Phosphate-Buffered Saline (DPBS) and pre-warmed media (37°C) was added and incubated for the indicated time at 37°C.
2. Immunofluorescence staining
After the indicated time, cells were fixed in 4% paraformaldehyde for 10 min. Cells were permeabilized with 0.1% Triton X-100 for 15 min. After incubation in a blocking buffer (10% fetal bovine serum in DPBS) for 30 min, the primary antibody was incubated overnight at 4 °C and then FITC-conjugated secondary antibody (goat anti-rabbit IgG, 1:250, dilution, Invitrogen) was incubated for 1 hours at room temperature. A mounting medium containing DAPI (VECTASHIELD, Cat No. 94010, Vector Laboratories) was used for counterstaining.
H. Flow cytometry analysis
To stain DR5, the cells were harvested and centrifuged at 1000 rpm for 3min
at 4°C. The cells were rinsed in FACS buffer (0.5% BSA in DPBS) by 3 times. DR5 antibody conjugated FITC (MAB4505; Abnova) was added and for 1 hours on ice and then were rinsed in FACS buffer at 1000 rpm for 3min at 4°C.This process was repeated twice. Resuspend the cells in 200 μL of FACS buffer and analyze on flow cytometer. Annexin V staining was performed using the FITC Annexin V reagent (BD biosciences) under manufacturer’s instructions.
- 8 -
Cells were treated purified recombinant GST-TRAIL (5g/ml) for 20 and 40min. Total cell extract prepared in M2 lysis buffer and proteins immobilized on glutathione-Sepharose beads (GE Healtcare Life Sciences, no. 17075601)
for 3 h at 4°C. The beads were then washed extensively with M2 buffer and
the bound proteins were analyzed by western blotting.
J. Reverse transcription-PCR
Total RNA was extracted using the TRIzol reagent (Life Technologies), according to the manufacturer’s instructions. 1 μg of total RNA from each sample was altered to cDNA by MMLV reverse transcriptase (MGmed). Equal amounts of cDNA product were employed in reverse transcription-PCR conducted using the GoTaq® Green Master Mix (Promega). Amplification was executed using the following primers: DR5 forward GCCTCATGGACAATGAGATAAAGGTGGCT-3’), DR5 reverse CCAAATCTCAAAGTACGCACAAACGG-3’), beta-ACTIN forward (5’-GTGGGGCGCCCCAGGCA-3’), beta-ACTIN reverse (5’-CTCCTTAAT GTCACGCACGAT-3’)
- 9 -
III. RESULTS
A. Depletion of MLKL accelerates TRAIL-induced cancer
cell death
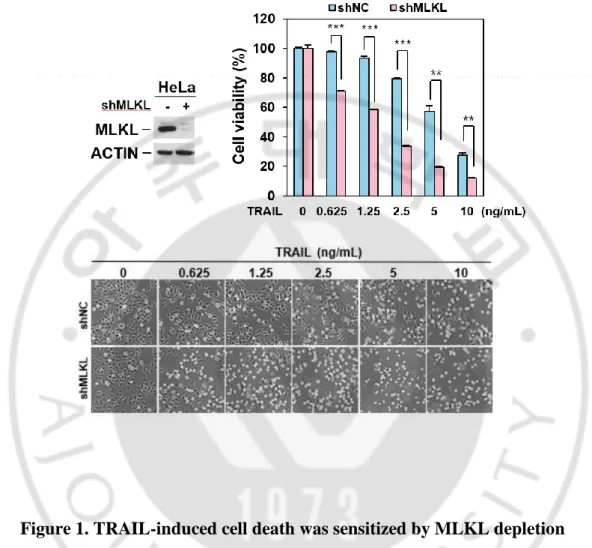
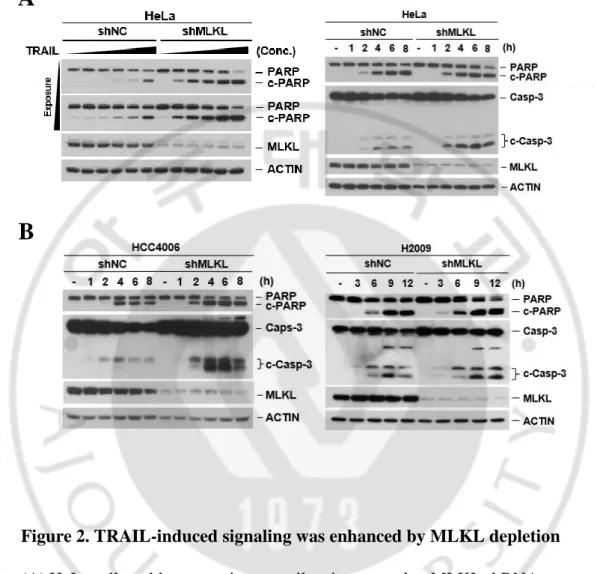
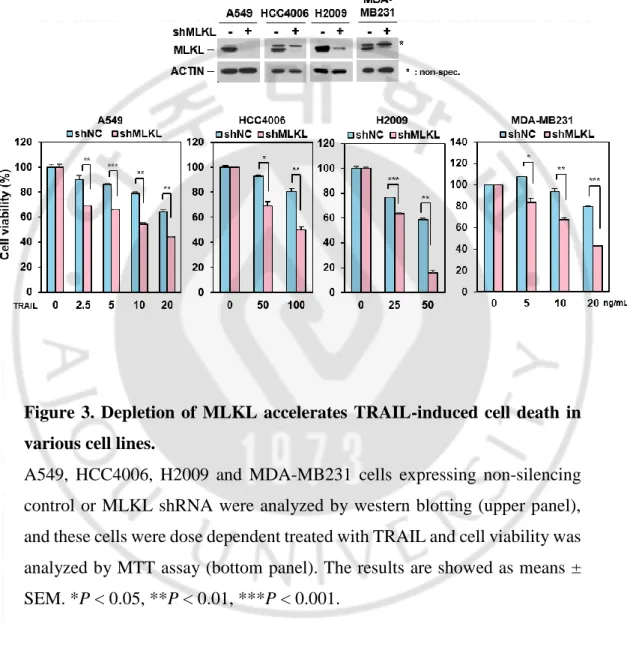
MLKL has been reported as a downstream molecule in RIP3-mediated necroptosis pathway but recently it was also reported that MLKL serves a non-deadly function. MLKL associates with the endosomes and helps for endosomal trafficking. Depletion of MLKL affected endosomal transport through the effective generation of intraluminal and extracellular vesicles (Yoon et al., 2017). It was demonstrated that TRAIL-induced cancer cell death was attenuated with defect of endocytosis and trafficking (Reis et al., 2017). Since MLKL is engaged in endosomal trafficking in receptor-ligand signals, we investigated the endosomal trafficking function of MLKL on TRAIL-induced cell death in cancer cells. To test this, we employed MLKL depleted HeLa cell by RNA interference (Figure 1) and treated cells with different concentration of TRAIL. MLKL depleted cells displayed relatively lower survival compared with the control cells (Figure 1) and difference of TRAIL-induced cell death was further confirmed by western blot showing the PARP and caspase 3 cleavage in does- and time-dependent manner (Figure 2) indicating that MLKL may involve TRAIL-induced cancer cell death. Consistently with this, in various cancer cell lines including the A549, HCC4006, H2009 (lung cancer cell lines) and MDA-MB231 (breast cancer cell line) cells, showed sensitization of TRAIL-induced cell death by depletion of MLKL (Figure 3).
- 10 -
Figure 1. TRAIL-induced cell death was sensitized by MLKL depletion
HeLa cells expressing non-silencing control or MLKL shRNA were analyzed by western blotting (upper left panel), and these cells were dose dependent treated with TRAIL for 12 hours and cell viability was analyzed by MTT assay (upper right panel) or phase-contrast microscopy (bottom panel). The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
- 11 -
Figure 2. TRAIL-induced signaling was enhanced by MLKL depletion
(A) HeLa cells stably expressing non-silencing control or MLKL shRNA were treated with TRAIL in dose dependent manner (left panel) or time dependent manner (right panel). The cells were harvested and lysates were analyzed by western blotting.
(B) HCC4006, H2009 cells stably expression non-silencing control or MLKL shRNA were treated with TRAIL in time dependent manner. Cell lysates were analyzed by western blotting.
A
- 12 -
Figure 3. Depletion of MLKL accelerates TRAIL-induced cell death in various cell lines.
A549, HCC4006, H2009 and MDA-MB231 cells expressing non-silencing control or MLKL shRNA were analyzed by western blotting (upper panel), and these cells were dose dependent treated with TRAIL and cell viability was analyzed by MTT assay (bottom panel). The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
- 13 -
B. RIP3-independent enhancement of cell death by MLKL
depletion in TRAIL-mediated cytotoxicity
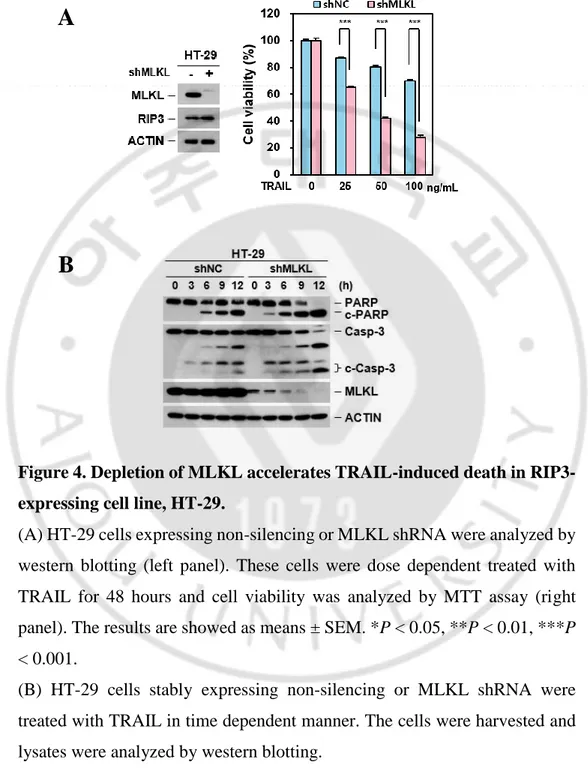
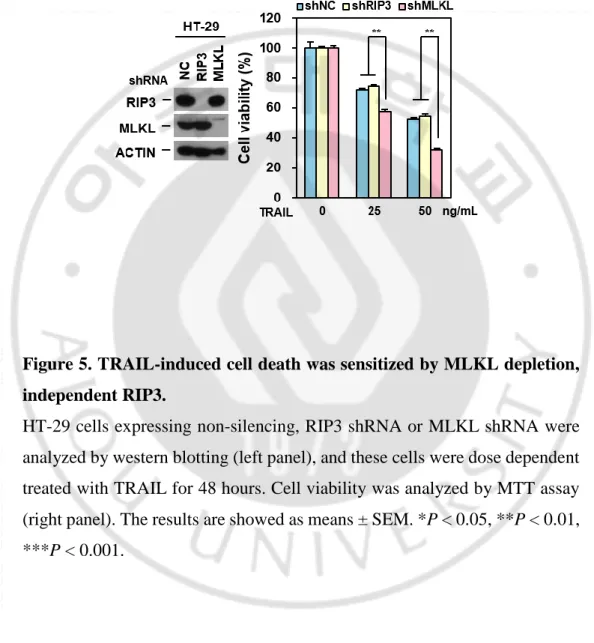
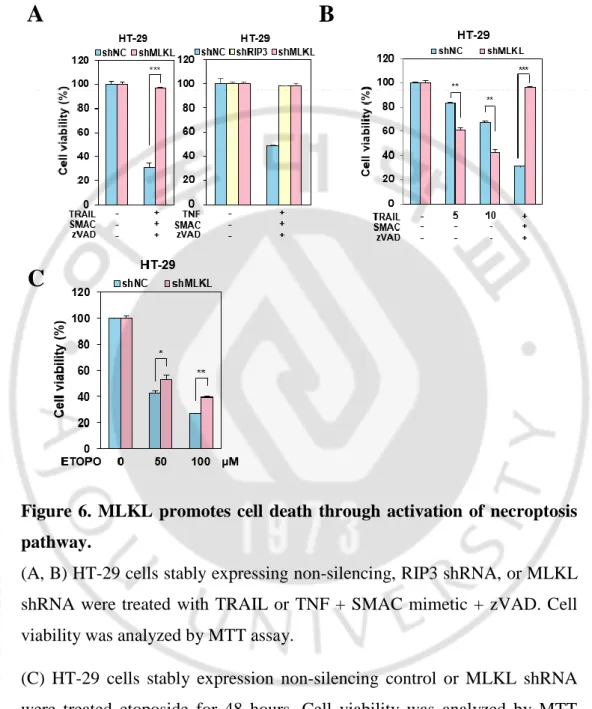
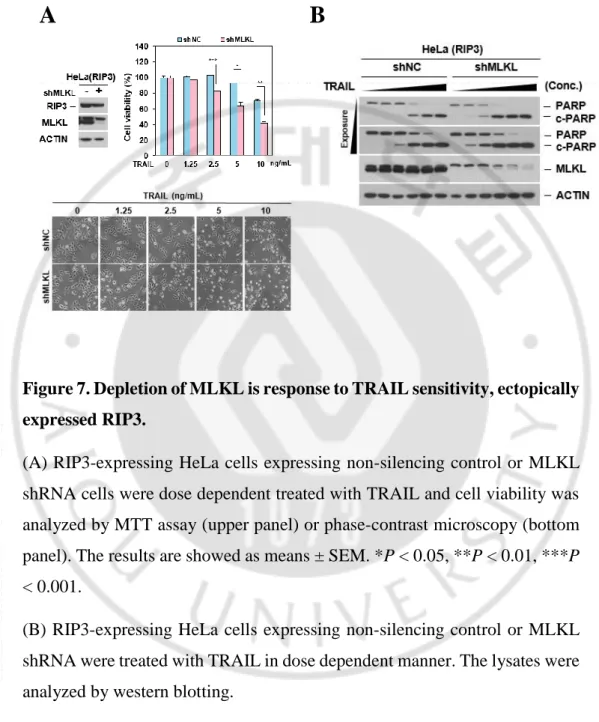
So far, we tested cancer cell lines which are not expressed RIP3 and we wonder whether this sensitization is related with RIP3 expression. To rule out the dependency, we took the HT-29 cells which is well studied in RIP3-dependent MLKL-mediated necroptotic cell death (S. He et al., 2009). HT-29 cells were depleted MLKL and treated with different concentration of TRAIL. Similar with cells lacking RIP3 expression, HT-29 cells were sensitized TRAIL-induced cell death (Figure 4A) and appeared fasten PARP / caspase 3 cleavage by MLKL depletion (Figure 4B) indicating RIP3-independent sensitization.It was further confirmed in RIP3 depleted HT-29 cells showing that RIP3 depleted HT-29 cells did not response to TRAIL-induced sensitization than HT-29 cells but still sensitive in MLKL depleted HT-29 cells indication that MLKL depletion may cause sensitization in TRAIL-induced cytotoxicity (Figure 5). Consistent with known RIP3 and MLKL function in necroptosis, these cells abolished TNF or TRAIL-induced RIP3-dependent necroptosis in both MLKL- or RIP3-depleted HT-29 cells (Figure 6A-B). We further examined MLKL dependency in necroptosis in response to chemotherapeutic agent, etoposide. Previous our report suggested that DNA damaging agents activate MLKL-dependent necroptosis and MLKL knockdown in HT-29 cells inhibited etoposide cytotoxicity (Koo et al., 2015). Consistent with this, MLKL-depleted HT-29 cells inhibited etoposide cytotoxicity (Figure 6C) suggesting that MLKL promotes cell death through activation of necroptosis pathway. In HeLa cells, when RIP3 is ectopically expressed, depletion of MLKL is response to TRAIL sensitivity (Figure 7A).
- 14 -
As expectedly, cells endogenously expressing MLKL acquired increased sensitivity to TRAIL when MLKL is knocked down by showing the PARP cleavage (Figure 7B). Taken together, these data suggested that depletion of MLKL enhances TRAIL-induced cell death in regardless of RIP3 status.
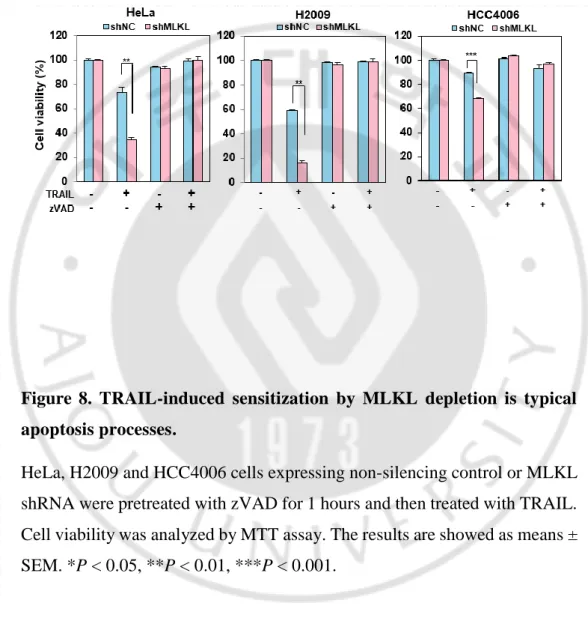
TRAIL binding to its death receptors DR4/DR5 induces the formation of DISC by the recruitment of FADD and 8 and then activated caspase-8 can further activate effector caspase-3 and -7, leading to cell-extrinsic apoptosis (Falschlehner, Emmerich, Gerlach, & Walczak, 2007). In the TRAIL-induced apoptosis signal, the functions of MLKL remain unclear. To verify the TRAIL-induced sensitization by MLKL depletion is typical apoptosis processes, we treated cells with pan caspase inhibitor zVAD which functions to inhibit apoptosis under TRAIL-induced cell death conditions. As shown in Figure 8, various cancer cells showed lost their sensitivity to TRAIL by zVAD treatment in absent or presence of MLKL. Same as this, zVAD completely abolished TRAIL induced cell death and PARP cleavage in RIP3 expressing cancer cells (Figure 9A-B) indicating MLKL contributes to apoptosis, as evidenced by the following: (1) MLKL depleting increases TRAIL-upregulated c-PARP /c-caspase 3 level (2) MLKL depleting remarkably increases cell death (3) MLKL depletion-mediated sensitization of TRAIL-cytotoxicity is reduced by zVAD treatment.
- 15 -
Figure 4. Depletion of MLKL accelerates TRAIL-induced death in RIP3-expressing cell line, HT-29.
(A) HT-29 cells expressing non-silencing or MLKL shRNA were analyzed by western blotting (left panel). These cells were dose dependent treated with TRAIL for 48 hours and cell viability was analyzed by MTT assay (right panel). The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
(B) HT-29 cells stably expressing non-silencing or MLKL shRNA were treated with TRAIL in time dependent manner. The cells were harvested and lysates were analyzed by western blotting.
A
- 16 -
Figure 5. TRAIL-induced cell death was sensitized by MLKL depletion, independent RIP3.
HT-29 cells expressing non-silencing, RIP3 shRNA or MLKL shRNA were analyzed by western blotting (left panel), and these cells were dose dependent treated with TRAIL for 48 hours. Cell viability was analyzed by MTT assay (right panel). The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
- 17 -
Figure 6. MLKL promotes cell death through activation of necroptosis pathway.
(A, B) HT-29 cells stably expressing non-silencing, RIP3 shRNA, or MLKL shRNA were treated with TRAIL or TNF + SMAC mimetic + zVAD. Cell viability was analyzed by MTT assay.
(C) HT-29 cells stably expression non-silencing control or MLKL shRNA were treated etoposide for 48 hours. Cell viability was analyzed by MTT assay.
The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
A
B
- 18 -
Figure 7. Depletion of MLKL is response to TRAIL sensitivity, ectopically expressed RIP3.
(A) RIP3-expressing HeLa cells expressing non-silencing control or MLKL shRNA cells were dose dependent treated with TRAIL and cell viability was analyzed by MTT assay (upper panel) or phase-contrast microscopy (bottom panel). The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
(B) RIP3-expressing HeLa cells expressing non-silencing control or MLKL shRNA were treated with TRAIL in dose dependent manner. The lysates were analyzed by western blotting.
- 19 -
Figure 8. TRAIL-induced sensitization by MLKL depletion is typical apoptosis processes.
HeLa, H2009 and HCC4006 cells expressing non-silencing control or MLKL shRNA were pretreated with zVAD for 1 hours and then treated with TRAIL. Cell viability was analyzed by MTT assay. The results are showed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
- 20 -
Figure 9. TRAIL induced cell death and PARP cleavage were blocked by zVAD in RIP3 expressing cancer cells.
(A) HT-29 and RIP3-expressing HeLa cells expressing MLKL shRNA, or non-silencing control were pretreated with zVAD (20 μM) for 1 hours and then treated with TRAIL. Cell viability was analyzed by MTT assay.
(B) RIP3-expressing HeLa cells expressing MLKL shRNA, or non-silencing control were pretreated with zVAD for 1 hours and then treated with TRAIL (5 ng/mL). Cell lysates were analyzed by western blotting.
A
- 21 -
C.
Depletion of MLKL caused defect on receptor-ligand
endosomal trafficking
Endocytosis is characterized by the internalization of molecules from the cell surface into internal membrane compartments, and after endocytosis, receptors enter the early endosome where they are sorted, and they are either recycled back to the plasma membrane or are degraded in the late endosome (Elkin, Lakoduk, & Schmid, 2016). Ligand internalization is a receptor-mediated endocytic process in which the cell will only take in an extracellular molecule if it binds to its specific receptor. Receptor internalization, or trafficking, is a part of cell signaling and MLKL has been suggested as a regulator for endosomal trafficking and extracellular vesicle generation. TRAIL receptor internalization by a clathrin-dependent mechanism has been shown the attenuation of apoptotic signaling (Reis et al., 2017). We sought to investigate the mechanism that MLKL silencing increases TRAIL-cytotoxicity. We first examined the impact of MLKL depletion on the cellular response to Epidermal Growth Factor (EGF). Treating cells with EGF leads to the rapid downregulation of the surface EGFR and by blocking cell-surface EGFR activation and EGFR recycling back to the cell cell-surface, internalized activated receptors have been shown to signal from endosomes and to promote cell survival (Tomas, Futter, & Eden, 2014). EGFR endocytosis decreased cell viability leading to the attenuation of downstream signaling of EGFR for the activation of molecules such as AKT in the ERK1/2 pathway for cell survival and proliferation (Ono et al., 2004). Consistent with the literature, knocked down of MLKL resulted in marked slowdown of the intracellular degradation of EGFR in western blotting (Figure
- 22 -
10A-B) and immunofluorescence staining (Figure 11). The slowdown of the intracellular degradation of EGFR by MLKL silencing resulted in boosting of several EGF-induced signaling activities (Figure 12). To determine whether the mechanism accounting for the observed slowdown also affects TRAIL, we examined the cellular response to TRAIL in MLKL-depleted cells. Like EGF, slowdown of degradation of DR5 by MLKL silencing in both MDA-MB231 and HCC4006 cells (Figure 13) suggesting that effects of depletion of MLKL imply a mechanism that exerts a general effect on receptor internalization and degradation. Defect of internalization or /and trafficking of TRAIL-TRAIL receptor by depletion of MLKL might resulted in enhancement of cell death. Sensitization of TRAIL-induced cell death is associated with increased expression of death receptor (Lee et al., 2013). To rule out that the impact of MLKL depletion on the cellular response to TRAIL-induced cytotoxicity, we checked DR5 expression pattern that whether MLKL silencing has any effect on DR5 expression. Depletion of MLKL did not altered any change of MLKL expression both in Protein and mRNA levels (Figure 14 A-C).
- 23 -
Figure 10. Depletion of MLKL delays intracellular degradation of EGFR.
(A, B) HeLa (A) and HCC4006 (B) cells stably expressing non-silencing control or MLKL shRNA were treated with EGF (100ng/mL) in time dependent manner. EGFR status was analyzed by western blotting (upper panel) and conducted using ImageJ quantification software to measure the relative band intensity (bottom panel).
- 24 -
Figure 11. Intracellular degradation of EGFR was delayed by MLKL depletion.
HeLa cells stably expressing non-silencing control or MLKL shRNA were treated with EGF in time dependent manner. Cells were stained with EGFR antibody and analyzed by confocal fluorescence microscopy (Green: EGFR, Blue: DAPI).
- 25 -
Figure 12. Depletion of MLKL boosts EGF-induced signaling.
(A, B) H2009, HeLa and HepG2 cells stably expressing non-silencing control or MLKL shRNA were treated with EGF in time dependent manner. Cell lysates were analyzed by western blotting.
- 26 -
Figure 13. Depletion of MLKL slowdowns intracellular degradation of Death Receptor 5.
(A, B) MDA-MB231 (A) and HCC4006 (B) cells stably expressing non-silencing control or MLKL shRNA were treated with TRAIL in time dependent manner. Death Receptor 5 (DR5) status was analyzed by western blotting (upper panel) and conducted using ImageJ quantification software to measure the relative band intensity (bottom panel).
- 27 -
Figure 14. Depletion of MLKL did not altered both Protein and mRNA levels.
(A) Death Receptor 5 expression pattern in MLKL depletion. Cell lysates were analyzed by FACS analysis. (H1299 - Red: Unstain, Sky blue: DR5 stain / HT-29 – Sky blue: Unstain, blue: DR5 stain)
(B) Death Receptor 5 expression pattern in MLKL depletion. Cell lysates were analyzed by western blotting.
(C) Death Receptor 5 mRNA level of various cancer cells in MLKL depletion.
A
- 28 -
D. Endosomal trafficking is associated with MLKL-mediated
sensitization of TRAIL-cytotoxicity
Since our data suggested that role for MLKL in maintaining the endosomal
trafficking and depletion of MLKL enhancement of TRAIL-induced apoptosis, we examined that whether interference with endosomal trafficking by blockage of internalization increases TRAIL-induced cell death. Dynamins are master regulators of Clathrin-Mediated Endocytosis (CME) and control earlier rate-limiting steps of clathrin-coated vesicle formation (Aguet, Antonescu, Mettlen, Schmid, & Danuser, 2013; Loerke et al., 2009; Sever et al., 1999). Using the dynamin inhibitor, dynasore, we prevented TRAIL-induced DR5 internalization in cells. As expected, TRAIL-induced cell death was boosted in the presence of dynasore by blockage of TRAIL-induced DR5 internalization in cancer cells (Figure 15) indicating that dynamin-dependent endocytosis of TRAIL-DR complex suppress apoptotic signaling (Figure 15, 16). It also resulted in some increase in TRAIL-induced signaling activities such as phosphorylation of ERK and p38 (Figure 17 A-B) in the presence of dynasore. Similar with the effect of dynasore, TRAIL-induced signaling activities was boosted in the absence of MLKL (Figure 18 A-B).
- 29 -
Figure 15. TRAIL-induced cell death was enhanced by blocking of DR5 endocytosis.
HCC4006 cells were pretreated with dynasore (80 μM) for 1hours and then treated with TRAIL (50ng/mL) in time dependent manner. Cell lysates were analyzed by western blotting (upper panel) and cell viability was analyzed by phase-contrast microscopy (bottom left panel) or FACS analysis after Annexin V staining (bottom right panel).
- 30 -
Figure 16. TRAIL-induced apoptotic signaling was enhanced by blocking of DR5 endocytosis.
MDA-MB231 and HeLa cells were pretreated with dynasore (80 μM) for 1 hours and then treated with TRAIL. Cell lysates were analyzed by western blotting (upper panel) and cell viability was analyzed by phase-contrast microscopy (bottom panel).
- 31 -
Figure 17. TRAIL-induced MAPK signaling was enhanced by blocking of DR5 endocytosis.
(A, B) HCC4006, MDA-MB231 and HeLa cells were pretreated with dynasore (80 μM) for 1hours and then treated with TRAIL. Cell lysates were analyzed by western blotting.
A
- 32 -
Figure 18. TRAIL-induced MAPK signaling was enhanced by MLKL depletion.
(A, B) HCC4006, H2009 cells stably expressing non-silencing control or MLKL shRNA were treated with TRAIL in time dependent manner. Cell lysates were analyzed by western blotting.
A
- 33 -
E. Prolonged death signal due to the defect of MLKL-mediated
e
ndosomal trafficking
Our data clearly imply that the link between MLKL-mediated endosomal trafficking and TRAIL receptor endocytosis contributes TRAIL-induced cytotoxicity. Immunofluorescence staining of the intracellular fate of the TRAIL in cells showed that it was bounded to cell surface at similar pattern in both MLKL-expressing cells and MLKL-depleted cells (Figure 19). However, at later time point, degradation of intracellular TRAIL was delayed/slowdowned in MLKL-depleted cells than MLKL-expressing cells (Figure 19). Although we failed to analyze for the intracellular fate of the DR5 in TRAIL-treated cells to show the subsequent translocation of the receptor from the cell membrane to the endosomes in both MLKL-expressing and MLKL-depleted cells, the amount of TRAIL in intracellular part imply that depletion of MLKL interferes the intracellular trafficking of TRAIL-DR5.
To further elucidate the effect of MLKL- mediated sensitization of TRAIL-cytotoxicity through the defect of endosomal trafficking, we examined whether MLKL depletion can facilitate the association/stabilization of components for DISC complex. A GST-pull down assay showed that the FADD and caspase-8 recruitment to TRAIL receptor were much higher in depleted HT-29 and H2009 cells (Figure 20) compared with MLKL-expressing control cell line. In addition, the activated form of caspase-8 was increased in MLKL depleted cells compared with control cells. These results demonstrate that MLKL depletion enhance enhancement of DISC complex formation and contribute to increased sensitivity to TRAIL-induced apoptosis.
- 34 -
Figure 19. Degradation of intracellular TRAIL delays in MLKL-depleted cells.
(A, B, C) HeLa, HCC4006, H1299 cells stably expressing non-silencing control or MLKL shRNA were treated with TRAIL in time dependent manner. Cells were stained with GST antibody and analyzed by confocal fluorescence microscopy (Green: GST, Blue: DAPI).
A
- 35 -
Figure 20. DISC complex more recruits in MLKL depleted cells.
(A, B) HT-29, H2009 cells stably expressing non-silencing control or MLKL shRNA were treated GST-TRAIL (5g/ml) for indicated time points and GST-TRAIL bound protein complexes were pulled down by glutathione-agarose beads and analyzed by western blotting.
- 36 -
IV. DISCUSSION
MLKL is a key executive effector of necroptosis and upon activation by phosphorylated RIP1, RIP3 will recruit and phosphorylate MLKL to execute necroptosis. For this, MLKL forms oligomer and translocate to the plasma membrane to cause membrane rupture. In addition to RIP3-dependent activation of MLKL, MLKL also functions for regulation in endosomal trafficking and extracellular vesicle generation via RIP3-inpendent manner. The observed effects of MLKL depletion on the rates of intracellular degradation of EGF and its receptor pointed to the possibility of a constitutive role for MLKL in maintaining the endosomal trafficking (Yoon et al., 2017).
Due to the signaling from activated receptor occurs mostly from the plasma membrane and endocytosis is thought to initiate termination of the signal, trafficking defects are associated with enhanced ligand-receptor signal. This point let us study that whether perturbation of MLKL-mediated endosomal trafficking enhances TRAIL-DR signal to increase cancer cell death. In this study, we found that depletion of MLKL accelerates TRAIL-induced cancer cell death. Enhancement of cell death by MLKL depletion in TRAIL-mediated cytotoxicity is RIP3-independent manner. Regardless of RIP3 expression in cancer cells, depletion of MLKL increases TRAIL-mediated cytotoxicity and it will be the potent strategies to sensitize cancer cell death by TRAIL treatment. Since the expression of RIP3 is lost/reduced in a large number of cancer cells/tumor tissues due to DNA methylation of the
RIPK3 genomic sequence (Koo et al., 2015), regulation of RIP3-independent
MLKL-mediated cancer cell death mechanism will be an effective therapeutic strategies.
- 37 -
trafficking and prolonged death signal due to the TRAIL-DR trafficking defect. Trafficking defect of TRAIL-DR was shown in depletion of MLKL, as
evidenced by the following: (1) plasma membrane bounded TRAIL degradation was delayed in MLKL-depleted cells (2) prolonged/enhanced intracellular signals such as p-ERK and p-p38 in MLKL-depleted cells (3) slowdown of degradation of DR5 in response to TRAIL by MLKL silencing. As noted generally, TRAIL’s ability to induced apoptosis in cancer cells, led to the clinical development of several agonists for TRAIL-TRAIL receptors. However, to date none of these TRAIL receptor agonists has produced a clinical benefit in cancer patients. One of reason for clinical failure is that lack of suitable biomarkers to identify patients who are more likely to respond to a TRAIL receptor agonist-comprising therapy (Ashkenazi, 2015). In this point, we could propose that patients who have a relatively low level of MLKL in tumor tissues, are more to respond to the TRAIL receptor agonist due to the perturbation in endosomal trafficking of TRAIL receptor agonist. Since abnormal expression of MLKL has been detected in many kinds of tumors, such as colon cancer, ovarian cancer and gastric cancer (Ertao et al., 2016; L. He, Peng, Liu, Xiong, & Zhu, 2013; Li et al., 2017) and recent studies also have revealed that MLKL could serve as a potential prognostic biomarker for patients with cancer (Colbert et al., 2013; Hu et al., 2018; Ruan, Mei, Zhu, Shi, & Wang, 2015). In these studies, the authors revealed that decreased expression of MLKL was significantly associated with poor overall survival in cancer patients suggesting that prognostic and clinicopathological significance of expression level of MLKL in cancer patients. Cancer patients who have decreased expression level of MLKL in tumor tissues will have clinical benefit from TRAIL receptor agonist-comprising therapy.
- 38 -
V. REFERENCES
Aguet, F., Antonescu, C. N., Mettlen, M., Schmid, S. L., & Danuser, G. (2013). Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint.
Developmental cell, 26(3), 279-291.
Ashkenazi, A. (2015). Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. The Journal of clinical
investigation, 125(2), 487-489.
Ashkenazi, A., Pai, R. C., Fong, S., Leung, S., Lawrence, D. A., Marsters, S. A., . . . Hebert, A. (1999). Safety and antitumor activity of recombinant soluble Apo2 ligand. The Journal of clinical investigation, 104(2), 155-162.
Austin, C. D., Lawrence, D. A., Peden, A. A., Varfolomeev, E. E., Totpal, K., De Mazière, A. M., . . . Scheller, R. H. (2006). Death-receptor activation halts clathrin-dependent endocytosis. Proceedings of the
National Academy of Sciences, 103(27), 10283-10288.
Cai, Z., Jitkaew, S., Zhao, J., Chiang, H.-C., Choksi, S., Liu, J., . . . Liu, Z.-G. (2014). Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nature cell biology, 16(1), 55.
Chen, X., Li, W., Ren, J., Huang, D., He, W.-t., Song, Y., . . . Chen, P. (2014). Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell research, 24(1), 105.
Cho, Y., Challa, S., Moquin, D., Genga, R., Ray, T. D., Guildford, M., & Chan, F. K.-M. (2009). Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell, 137(6), 1112-1123.
Colbert, L. E., Fisher, S. B., Hardy, C. W., Hall, W. A., Saka, B., Shelton, J. W., . . . Gandhi, K. (2013). Pronecrotic mixed lineage kinase domain‐ like protein expression is a prognostic biomarker in patients with early‐
- 39 -
stage resected pancreatic adenocarcinoma. Cancer, 119(17), 3148-3155.
Di Fiore, P. P., & von Zastrow, M. (2014). Endocytosis, signaling, and beyond. Cold Spring Harbor Perspectives in Biology, 6(8), a016865.
Dondelinger, Y., Declercq, W., Montessuit, S., Roelandt, R., Goncalves, A., Bruggeman, I., . . . Marquis, R. W. (2014). MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell reports, 7(4), 971-981.
Elkin, S. R., Lakoduk, A. M., & Schmid, S. L. (2016). Endocytic pathways and endosomal trafficking: a primer. Wiener Medizinische
Wochenschrift, 166(7-8), 196-204.
Ertao, Z., Jianhui, C., Kang, W., Zhijun, Y., Hui, W., Chuangqi, C., . . . Shirong, C. (2016). Prognostic value of mixed lineage kinase domain-like protein expression in the survival of patients with gastric caner.
Tumor Biology, 37(10), 13679-13685.
Falschlehner, C., Emmerich, C. H., Gerlach, B., & Walczak, H. (2007). TRAIL signalling: decisions between life and death. The international
journal of biochemistry & cell biology, 39(7-8), 1462-1475.
He, L., Peng, K., Liu, Y., Xiong, J., & Zhu, F.-f. (2013). Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. OncoTargets and therapy, 6, 1539.
He, S., Wang, L., Miao, L., Wang, T., Du, F., Zhao, L., & Wang, X. (2009). Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell, 137(6), 1100-1111.
Hu, B., Shi, D., Lv, X., Chen, S., Huang, Q., Xie, M., & Shao, Z. (2018). Prognostic and clinicopathological significance of MLKL expression in cancer patients: a meta-analysis. BMC cancer, 18(1), 736.
Juo, P., Kuo, C. J., Yuan, J., & Blenis, J. (1998). Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Current biology, 8(18), 1001-1008.
- 40 -
Kischkel, F. C., Lawrence, D. A., Chuntharapai, A., Schow, P., Kim, K. J., & Ashkenazi, A. (2000). Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity, 12(6), 611-620.
Kohlhaas, S. L., Craxton, A., Sun, X.-M., Pinkoski, M. J., & Cohen, G. M. (2007). Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Journal of biological Chemistry, 282(17), 12831-12841.
Koo, G.-B., Morgan, M. J., Lee, D.-G., Kim, W.-J., Yoon, J.-H., Koo, J. S., . . . Hong, S. S. (2015). Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell research, 25(6), 707.
Lee, J.-Y., Jung, K. H., Morgan, M. J., Kang, Y.-R., Lee, H.-S., Koo, G.-B., . . . Kim, Y.-S. (2013). Sensitization of TRAIL-induced cell death by 20 (S)-ginsenoside Rg3 via CHOP-mediated DR5 upregulation in human hepatocellular carcinoma cells. Molecular cancer therapeutics, 12(3), 274-285.
Li, X., Guo, J., Ding, A.-P., Qi, W.-W., Zhang, P.-H., Lv, J., . . . Sun, Z.-Q. (2017). Association of mixed lineage kinase domain-like protein expression with prognosis in patients with colon cancer. Technology
in cancer research & treatment, 16(4), 428-434.
Loerke, D., Mettlen, M., Yarar, D., Jaqaman, K., Jaqaman, H., Danuser, G., & Schmid, S. L. (2009). Cargo and dynamin regulate clathrin-coated pit maturation. PLoS biology, 7(3), e1000057.
Murphy, J. M., Czabotar, P. E., Hildebrand, J. M., Lucet, I. S., Zhang, J.-G., Alvarez-Diaz, S., . . . Webb, A. I. (2013). The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity,
39(3), 443-453.
Murphy, J. M., & Vince, J. E. (2015). Post-translational control of RIPK3 and MLKL mediated necroptotic cell death. F1000Research, 4.
Ono, M., Hirata, A., Kometani, T., Miyagawa, M., Ueda, S.-i., Kinoshita, H., . . . Kuwano, M. (2004). Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the
- 41 -
epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Molecular
cancer therapeutics, 3(4), 465-472.
Platta, H. W., & Stenmark, H. (2011). Endocytosis and signaling. Current
opinion in cell biology, 23(4), 393-403.
Reis, C. R., Chen, P.-H., Bendris, N., & Schmid, S. L. (2017). TRAIL-death receptor endocytosis and apoptosis are selectively regulated by dynamin-1 activation. Proceedings of the National Academy of
Sciences, 114(3), 504-509.
Ruan, J., Mei, L., Zhu, Q., Shi, G., & Wang, H. (2015). Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. International journal of clinical and experimental
pathology, 8(11), 15035.
Sever, S., Muhlberg, A. B., & Schmid, S. L. (1999). Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature,
398(6727), 481.
Slee, E. A., Adrain, C., & Martin, S. J. (2001). Executioner caspase-3,-6, and-7 perform distinct, non-redundant roles during the demolition phase of apoptosis. Journal of biological Chemistry, 276(10), 7320-7326.
Sorkin, A., & Von Zastrow, M. (2009). Endocytosis and signalling: intertwining molecular networks. Nature reviews Molecular cell
biology, 10(9), 609.
Sun, L., Wang, H., Wang, Z., He, S., Chen, S., Liao, D., . . . Lei, X. (2012). Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell, 148(1-2), 213-227.
Tomas, A., Futter, C. E., & Eden, E. R. (2014). EGF receptor trafficking: consequences for signaling and cancer. Trends in cell biology, 24(1), 26-34.
Walczak, H., Miller, R. E., Ariail, K., Gliniak, B., Griffith, T. S., Kubin, M., . . . Le, T. (1999). Tumoricidal activity of tumor necrosis factor–related apoptosis–inducing ligand in vivo. Nature medicine, 5(2), 157.
- 42 -
Wang, H., Sun, L., Su, L., Rizo, J., Liu, L., Wang, L.-F., . . . Wang, X. (2014). Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular cell,
54(1), 133-146.
Wu, J., Huang, Z., Ren, J., Zhang, Z., He, P., Li, Y., . . . Zhou, X. (2013). Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell research, 23(8), 994.
Ying, Z., Pan, C., Shao, T., Liu, L., Li, L., Guo, D., . . . Jiang, Z. (2018). Mixed lineage kinase domain-like protein MLKL breaks down myelin following nerve injury. Molecular cell, 72(3), 457-468. e455.
Yoon, S., Kovalenko, A., Bogdanov, K., & Wallach, D. (2017). MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity, 47(1), 51-65. e57.
Zhao, J., Jitkaew, S., Cai, Z., Choksi, S., Li, Q., Luo, J., & Liu, Z.-G. (2012). Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proceedings of
- 43 - - 국문요약 –
MLKL-매개 내막수송 결함에 의한
TRAIL-유도 암세포 사멸신호 증가에 대한 연구
아주대학교 대학원 의생명과학과 박 세 연 (지도교수: 김 유 선) 현재까지 보고에 의하면, MLKL이라는 단백질은 주로 세포사멸기작 중 하나인 Necroptosis에서 필수적인 작동인자의 하나로써 상위의 RIP3 단 백질에 의해 인산화되어 조절되는 것으로 잘 알려져 있다. 최근 보고에 의하면 RIP3-비의존적인 MLKL의 기능으로써 관내막(intraluminalvesicle)과 관외막(extraluminal vesicle)을 형성하여 내막수송
(endosomal trafficking)에 기여한다는 논문이 보고되었다. 하지만 이런 MLKL의 기능과 세포사멸에 대한 직접적인 연관이 보고된 바 없어 본 연구에서는 TRAIL(TNF-related apoptosis-induced ligand) 신호에서의 MLKL 기능에 대해서 연구하고자 한다. TRAIL은 암세포사멸을 일으키는 대표적인 리간드로 이의 수용체인 Death receptor 4/5(DR 4/5)에 결합하 여 내포작용(endocytosis)이 일어나 신호전달이 유도되고, 신호전달 이상 이 세포사멸에 영향을 미친다는 것이 보고된 바 있다. 이러한 내용을 토 대로 본 연구에서는 MLKL이 결실됨으로써 TRAIL에 의한 세포사멸과 세포신호가 증가됨을 확인하였고, 이는 MLKL이 TRAIL과 TRAIL 수용
- 44 -
체의 막 수송에 관여하여 나타나는 결과라는 것을 제시하고 있다.
핵심어: MLKL, TRAIL. 내막수송