이학 석사학위 논문
Human neural stem cells over-expressing
Akt1 provide cytoprotection and functional
recovery in mouse stroke model
아 주 대 학 교 대 학 원
의 학 과
Human neural stem cells over-expressing
Akt1 provide cytoprotection and functional
recovery in mouse stroke model
by
Kim Mi Kyung
A Dissertation Submitted to the Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the
Degree of
MASTER OF SCIENCE
Supervised by
Byung Gon Kim, M.D., Ph.D.
Department of Medical Sciences
The Graduate School, Ajou University
김미경의 이학 석사학위 논문을 인준함.
심사위원장 김 병 곤 (인)
심사위원 김 승 업 (인)
심사위원 서 해 영 (인)
아 주 대 학 교 대 학 원
2007년 6월 22일
감사의 글
조금은 긴 시간을 돌아, 드디어!! 졸업을 하게 되었습니다. 먼저 이렇게 부 족한 저를 끝까지 믿어주시고 이끌어주신 김승업 교수님께 깊은 감사의 말씀 을 드립니다. 마지막 한 학기, 많은 도움을 주신 김병곤 교수님께도 감사의 인사를 전하고 싶습니다. 또한 아낌없는 충고와 격려를 주신 서해영 교수님께 도 감사의 인사를 드립니다. 박인호 선생님, 정희 언니, 우경 언니, 지연 언니 께도 고맙다는 말씀 전해드리고 싶습니다. 실험이 잘 되지 않을 때마다 관심 을 가져주시고 많은 도움을 주신 광세 오빠, 그리고 곧 예쁜 아기 어머니가 되실 은정 언니, 비록 멀리 계시지만 정말 감사의 인사 전해드리고 싶습니다. 다시 한번 행복하게 오래오래 잘 사시길 기도드립니다^^. 또한 승임 언니, 동 훈 오빠, 혁민 오빠 그리고 막내 영미까지 다들 고맙습니다. 정말 좋은 추억 많이 안고 갑니다. 그리고 입학 때부터 지금까지 실험이 안 될 때마다, 안 좋 은 일이 있을 때마다 늘 저의 고민거리와 투정을 다 들어주시고 많은 조언을 해 주신 근우 오빠, 창미 언니 그리고 현정 언니 (저 드디어 졸업해요^^) 고 맙습니다, 많이 보고 싶을 꺼예요. 멀리 있는 소중한 친구 영은이 늘 힘이 되 어줘서 고맙고 다른 무엇보다 널 알게 되었다는 게 정말 감사하고 행복한 일 이었다는거 기억해주길…그리고, 늘 나의 투정 다 들어주고, 이해해주며 다독 여 준 은진이, 정은이 너무 고마웠어, 내 맘 알지? 지금은 졸업하고 자신의길을 잘 걸어가고 있는 입학 동기이자 사랑하는 동생들, 지선, 김유미, 허유미, 우희, 은양 모두 잊지 못 할 꺼야. 그리고 끝까지 이 실험을 함께 해 주시고 많은 도움을 주신, 이홍준 선생님께도 정말 감사의 인사를 올리고 싶습니다. 그리고 나의 영원한 친구들, 지난 3년 반 동안 힘들 때 함께 술잔을 기울 려 주고, 방황할 땐 진심 어린 충고와 격려 아끼지 않았던 사랑하는 우리 雨 酒會 친구들. 정말 너무너무 고맙다. 지난 8년여의 세월보다 더 길고 오래오 래 소중한 인연 이어갈 수 있으면 좋겠어. 물론 당연히 그럴꺼지만. 이렇게 좋은 날, 역시나 당신들이 많이 생각나.^^ 마지막으로 넉넉하지 않은 형편에도 공부를 하겠다는 저를, 믿어주시고 아 낌없이 응원해 주신 사랑하는 부모님 그리고 오빠, 재현이 정말 감사드립니다. 가족이란 이름만으로 정말 많은 힘이 되어주었습니다. 모두들 세상에서 제가 제일로 사랑 한다는 거 아시죠? 지면을 빌려 말로 표현하지 못할 만큼 정말 너무너무 감사드립니다. 더 큰 세상으로 나아가는 이 발걸음이 결코 가볍지만은 않지만, 욕심내지 않고 차분차분히 한걸음 한걸음 제 몫을 다 하며 열심히 사는 미경이가 되겠 습니다. 2007년 여름 미경 드림. p.s. : 실험을 위해 죽어간 많은 쥐들에게도 고개숙여 감사의 인사를…
- ABSTRACT -
Human Neural Stem cells over-expressing Akt1 provide
cytoprotection and functional recovery in mouse stroke model
Akt1 (protein kinase B), a serine/threonine kinase, has emerged as a critical molecule in signal transduction pathways involved in cell proliferation, growth, survival, apoptosis and glucose metabolism. A previous study has shown that transplanted human neural stem cells (hNSCs) selectively migrate to the brain and induce behavioral recovery in mouse intracerebral hemorrhage (ICH) stroke model. However, many of the grafted hNSCs did not survive the transplantation, which may have reduced therapeutic potential. In this study, we postulated that hNSCs over-expressing Akt1 transplanted into the lesion could improve survival of grafted hNSCs and behavioral recovery in mouse ICH model. To establish Akt1 over-expressing hNSC line, F3 hNSCs were transduced with retroviral vectors containing mouse Akt1 cDNA. We examined the viability of Akt1 over-expressing hNSCs against to the H2O2-induced cell death and oxygen glucose deprivation (OGD) condition in vitro.
F3.Akt1 cells were showed more resistance to the cell death stimuli. To investigate the therapeutic effects in vivo, ICH was induced in adult mice by unilateral injection of bacterial collagenase into striatum and then, we transplanted F3 and F3.Akt1 hNSCs into the animal brain. Transplantation of F3.Akt1 hNSCs increased survival of grafted cells by 0.5 – 2 fold at two weeks and eight weeks, and induced behavioral improvement in animals. These results demonstrate that the over expression of Akt1 supports the prolonged survival
of the engrafted cells and protects hNSCs from cell death by the inhibitory death machinery activation.
Key words: human neural stem cell, protein kinase Bα/Akt1, H2O2, oxygen glucose
TABLE OF CONTENTS
ABSTRACTS ··· i
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ···ⅴ LIST OF TABLES ··· ⅵ Ⅰ. INTRODUCTION ··· 1
Ⅱ. MATERIALS AND METHODS ··· 5
1. Cell culture ··· 5
2. Generation of human NSC F3.Akt1 cell line ··· 5
3. RT-PCR analysis ··· 6
4. Treatment of H2O2 ··· 6
5. Cell viability assay ··· 7
6. Western blot analysis ··· 7
7. Oxygen glucose deprivation (OGD) experiment ··· 8
8. Mouse Intracerebral Hemorrhage (ICH) model ··· 8
9. Cell transplantation ··· 9
10. Behavioral Testing ··· 9
11. Histology and immunohistochemistry ··· 10
12. Stereological cell counts ··· 11
13. Statistical analysis ··· 12
1. Stable human neural stem cell line over-expressing Akt1 ··· 13
2. F3.Akt1 cells prevent H2O2-Induced cell death ··· 16
3. Oxygen glucose deprivation (OGD) experiment ··· 18
4. Functional recovery in ICH animals by hNSC transplantation ··· 20
5. Transplanted hNSCs differentiate into neurons and astrocytes ··· 22
6. Survival of transplanted F3 and F3.Akt1 hNSCs in ICH brain ··· 25
Ⅳ. DISCUSSION ··· 27
Ⅴ. CONCLUSION ··· 32
REFERENCES ··· 33
LIST OF FIGURES
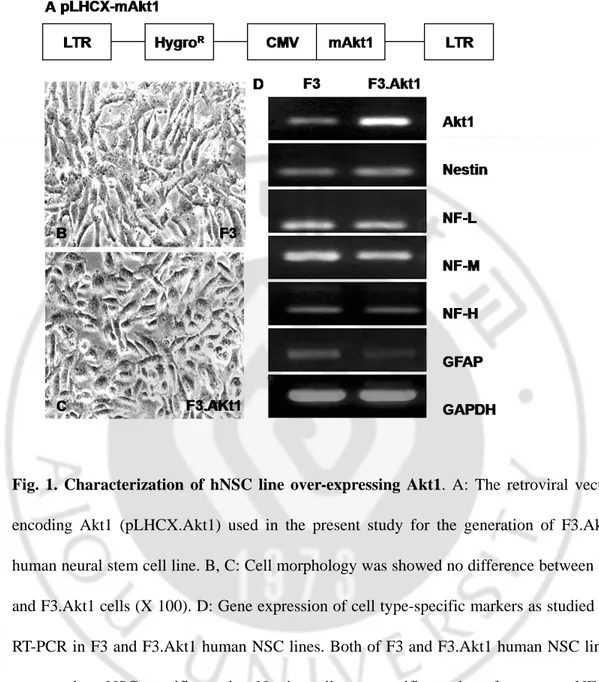
Fig. 1. Characterization of hNSC line over-expressing Akt1 ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 14
Fig. 2. Cell viability increases to H2O2–induced oxidative stress conditions and Akt1
phosphorylation in F3.Akt1 hNSCs ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 17
Fig. 3. Oxygen and glucose deprivation (OGD) to simulate ischemia ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 19
Fig. 4. Behavioral improvement demonstrated in mice with ICH transplanted with F3 or F3.Akt1 hNSCs ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 21
Fig. 5.F3.Akt1 hNSCs express Lac-Z-positive in ICH mouse at 2weeks after transplanted. ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 23
Fig. 6.Double immunofluorescent staining of engrafted F3.Akt1 cells in mouse brain 8 weeks post-transplantation ∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙ 24
Fig. 7. Survival of grafted F3 and F3.Akt1 hNSCs was demonstrated by
LIST OF TABLES
Ⅰ. INTRODUCTION
Two major types of stroke are ischemic and intracerebral hemorrhage (ICH) stroke. ICH represents at least 15% of all strokes in the western population (Qureshi et al., 2001), while in Asia including China, Japan and Korea ICH occupies considerably higher proportion at 50-60% (Inagawa, 2002). A previous epidemiological study has shown that the incidence of ICH in Korea is as high as 40-46% of the all stroke cases as compared to 30-35% of ischemic stroke (Lee et al., 1991).
Intracerebral hemorrhage is related with severe neurological deficits and an extensive mortality rate. In hemorrhage stroke, brain damage occurs through various mechanisms. In addition to mass effect, the hematoma induces pathological changes in its vicinity, including neuronal and glial cell death, vasogenic edema, and breakdown of the blood-brain barrier (Qureshi et al., 1999; Mutlu et al., 2001). Different forms of hemorrhagic insults are dependent on the amount and distribution of spared tissue as well as the type and severity of cell damage occurrence. Hemorrhagic injury causes severe neurodegeneration and consequently a loss of normal brain functions resulting from a complex sequence of pathophysiological events including excitotoxicity, inflammation (Wang and Tsirka, 2005a, b), and programmed cell death (Cho et al., 2004; Lee et al., 2005).
Since medical therapy against ICH such as mechanical removal of hematoma, prevention of edema formation by drugs and reduction of intracranial pressure shows only limited effectiveness, alternative approach is required (Gebel and Broderick, 2000; NINDS
ICH Workshop, 2005) such as stem cell-based therapy.
Recent progress in stem cell biology has opened up a way to therapeutic strategies to replace lost neural cells by transplantation of neural stem cells (NSCs) in CNS injury and disease (Makay, 1997; Gage, 2000; Lindvall et al., 2004). Previous studies have indicated that NSCs or neural progenitor cells engrafted in animal models of stroke survive and ameliorate neurological deficits in the animals (Jeong et al., 2003; Chu et al., 2003, 2004a, b; Ishibashi et al., 2004; Kelly et al., 2004). Among these studies, human neural progenitor cells isolated from fetal brain have been transplanted into the brain of stroke animal models and found to restore brain function. This approach, however, is not widely acceptable for stroke patients because of moral, religious and logistic problems associated with the use of human fetal tissues. In addition, primary human NSCs derived from fetal tissues can be provided for only a limited time before they undergo senescence, and it is difficult to secure sufficient numbers and homogeneous populations of human NSCs from fetal brain. These problems can be circumvented by the use of stable, permanent cell lines of human NSCs. Previous studies demonstrated human NSC line ameliorate neurological deficits in animal models of Parkinson disease (Kim et al., 2006), Huntington disease (Ryu et al., 2004; Lee et al., 2005) and lysosomal storage disease (Meng et al., 2003) following their transplantation into the brain. In stroke animal models, intravenously transplanted F3 human NSCs migrated selectively to the damaged brain sites caused by ischemia and ICH, differentiated into neurons and astrocytes, and promoted functional recovery in these animals. However, low survival rate of grafted F3 NSCs in ischemia and ICH rats in the previous studies is a grave concern; less than 50% of grafted NSCs survived in ICH mice
at 2 weeks post-transplantation (Lee et al., 2007).
One significant way to promote survival rate of transplanted NSCs in animal brain is to modulate properties of the NSCs, and in this study this might be accomplished by over-expressing Akt1 molecule which is known as a general mediator of survival signals.
Akt, also known as protein kinase B, is a serine/threonine kinase and plays a critical role in the modulation of cell development, growth, and survival. Akt is expressed in mammals, but can vary in the level of expression in a variety of tissues and cells. In the central nervous system, the expression of Akt1 can be observed at increased levels during development, but is gradually decreased during postnatal development (Owada et al., 1997). However, in the adult brain, expression of Akt1 is initially weak with a dramatic increase in the expression of Akt1 mRNA and Akt1 protein in cells that are subjected to injury (Owada et al.,1997; Kang et al., 2003), suggesting that Akt1 may play an important role during paradigms that involve cell injury. These cytoprotection by Akt1 is considered to be broad in central nervous system injuries that range from cerebral ischemia to Alzheimer's disease. It is also reported that the phospho-inositide 3-kinase [PI(3)K]-Akt pathway is critical for protection against neuronal cell death (Yao and Cooper, 1995; Dudek et al., 1997). Early studies have demonstrated that over-expression of Akt in cerebellar granule neurons prevents apoptosis during growth factor withdrawal (Dudek et al., 1997). Akt also promotes cell survival during free radical exposure in primary hippocampal neurons (Matsuzaki et al., 1999; Chong et al., 2003), neuronal cell lines (Kang et al., 2003a,b), and cerebral vascular endothelial cells (ECs) (Chong et al., 2005). Enhanced Akt activity can support cell survival against several other toxic insults that
include DNA damage (Henry et al., 2001; Chong et al., 2002; Kang et al., 2003), oxidative stress (Kang et al., 2003), and transforming growth factor-ß (TGF-ß) application (Conery et al., 2004). Importantly, the neuroprotective effects of active Akt have also been studied in culture systems with reactive oxygen species (ROS)-generating agents such as Aβ and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Martin et al., 2001; Salinas et al., 2001). Furthermore, Akt phosphorylation has been reported in a transient focal model of cerebral ischemia–reperfusion (Noshita et al., 2001; Taylor et al., 2005).
Moreover a recent study demonstrated that bone marrow-derived mesenchymal stem cells modified with Akt were more resistant to apoptosis, when injected into ischemic myocardium, prevent remodeling of infarcted hearts, repair infarcted myocardium and normalize cardiac performance (Abeel et al., 2003; Noiseux et al., 2006).
Considering evidence of functional recovery in stroke animals following brain transplantation of human neural stem cells (Jeong et al., 2003; Lee et al., 2007) and functions of Akt1 as mentioned above, this study was designed to investigate whether human NSCs over-expressing Akt1 can lead to the prolonged cell survival of grafted human NSCs and neurological recovery in the mouse ICH stroke model.
Ⅱ. MATERIALS AND METHODS
1. Cell culture
Immortalized human neural stem cell(NSC) line F3, was established as described previously (Kim SU, 2003), and was grown in Dulbecco’s modified Eagle’s medium(DEME) with high glucose (Hyclone, Logan, UT) containing 10% fetal bovine serum (Hyclone) with 10 ㎍/ml Gentamicin (Gibco, Grand Island, NY). These cells were subcultured once a week.
2. Generation of human NSC F3.Akt1 cell line
The retroviral vectors were constructed by ligating the cDNA of mouse Akt1 (was purchased from Upstate, Charlottesville, VA) into cloning site of pLHCX vector (BD Bioscience Clontech, Mountain View, CA). Before the ligation, mouse Akt1 cDNA was PCR-amplified by using forward primer 5’-CTAGTTAAGCTTATGGGGAGCAGC-3’; reverse primer 5’- GATATGATCGATTGATC AGAGGGTTTA -3’. PA317 mouse amphotrophic packaging cell line was infected with the pLHCX.Akt1 vector using Lipofectamine (Invitrogen, Carlsbad, CA) and successful infectants were selected by 100㎍ /ml hygromycin (Sigma, St.Louis, MO) treatment for 7 days. Six milliliters of culture supernatant from the packaging cell line and 8㎍/ml of polybrene (Sigma) were added to F3 cells and incubated for 4h at 37℃. The medium was then replaced with fresh growth medium; infection was repeated 24 hours later. Cells were allowed in normal media for 48
hours, selected by 200㎍/ml hygromycin for 8 days. Hygromycin-resistant F3.Akt1 clones were isolated, screened, and one of the clones F3.Akt1 was expanded, and used for the presentstudy.
3. RT-PCR analysis
F3 and F3.Akt1 hNSCs were collected by centrifugation and total RNA was isolated using Trizol reagent (Invitrogen), according to manufacture’s protocol. One ㎍ of total RNA was reverse-transcribed into first-strand cDNA using 0.5 ㎍ oligo-dT primer. Reverse transcription was performed with M-MLV reverse transcriptase (Promega, Madison, WI) for 1 hr at 42℃, inactivated for 15 min at 95℃ and cooled to 4℃. The cDNA one ㎕ was used in a PCR reaction containing 1X DNA polymerase buffer, 5 mM MgCl2, 1 mM each dNTPs, 10 pmol primers, 2.5 units of Taq polymerase (Promega). The
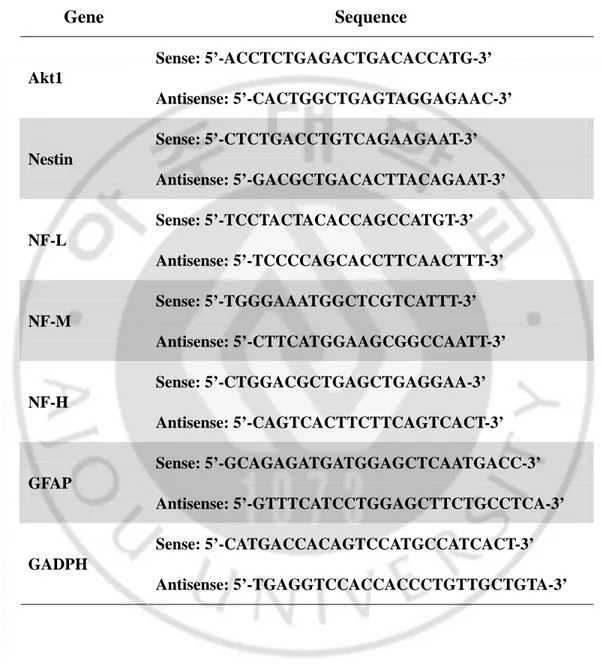
cDNA was amplified using 30 PCR cycles and RT-PCR products were separated electrophoretically on 1.2% agarose gel containing ethidium bromide and visualized under UV light. The primers used for the RT-PCR for nestin, neurofilament(NF)-L, NF-M, NF-H, glial fibrillary acidic protein (GFAP), GAPDH (all human) and Akt1 are listed in Table 1.
4. Treatment of H
2O
2F3 and F3.Akt1 cells were plated 1X104 cells per well in 96 well-plates (Falcon; Becton Dickinson, Franklin lakes, NJ) with 5% FBS containing DMEM and incubated for overnight. H2O2 (Sigma) was added to each F3 and F3.Akt1 cells well to give a final H2O2
alone. Cells were then left for 6h and 24h for cell viability assay and for western blot analysis.
5. Cell viability assay
Cell viability was determined by the conversion of MTT (3-(4,5-dimethylthiaxol-2-yl)-2,5 diphenyl tetrazolium bromide) to formazan utilizing NADH and NADPH pyridine nucleotide cofactors. After stimulation with H2O2 at different dose for 6 and 24 hours,
MTT [2 mg/ml in phosphate-buffered saline (PBS)] was added to each well and cells incubated for a further 2 hours. The media containing MTT was then removed, 100㎕ of dimethyl sulfoxide (DMSO, Sigma) added to each well and absorbances read at 570 nm. Each treated sample was compared with an untreated control.
6. Western blot analysis
Western blot analysis was performed with 50 ㎍ total protein extract separated on 10% SDS-PAGE gels that were subsequently transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). Blocking of membranes with 5% skim milk in TBST and washed in TBST, membranes were incubated with anti-phospho-Akt1-Thr 308 (1:500, Upstate), anti-caspase-3 (1:1000, Chemicon) and anti-beta-actin (1:10,000, Santa Cruz) antibody for overnight at 4℃. And membrane was washed in Tris-buffered saline with 0.05% tween 20 (TBST) for 1h at room temperature then secondary antibody [anti-rabbit and anti-mouse horseradish peroxidase-linked antibody] (GE healthcare, Anaheim, CA) incubation was performed for 2 hours at room temperature. Immunoreactive bands
were detected by chemiluminescence using ECL system. Western blots analyses were performed on samples from twice separate experiments.
7. Oxygen glucose deprivation (OGD) experiment
F3 and F3.Akt1 cells were seeded in plastic coverslips ( 4x104 cells/ml)and incubated in normal medium. At 24 hours the culture medium was replaced by no-glucose DMEM (Gibco) then the cells were placed for 1h in a chamber filled with 5% CO2 and 95% N2.
Control cells were incubated with no-glucose DMEM in a normoxic incubator for the same period. OGD was terminated by switching back to normal culture conditions. After 24h, cells were fixed 4% (w/v) paraformaldehyde for 10min at room temperature and stained by anti-phospho-Akt1, anti-caspase-3 and 4',6-diamidino-2-phenylindole (DAPI).
8. Mouse intracerebral hemorrhage (ICH) model
All experimental procedures were approved by the Animal Care Committee of the Ajou University Hospital. ICH was induced by stereotaxic, intrastriatal administration of bacterial collagenase by previously described methods (Lee et al., 2007). In brief, after an intraperitoneal injection of 1% ketamine (30 mg/kg) and xylazine hydrochloride (4 mg/kg), the mice were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). A burr hole was made, and a 30-gauge needle was inserted through the burr hole into the striatum (0.1 mm posterior, 4.0 mm ventral, and 2.0 mm lateral to the bregma). ICH was induced by the administration of collagenase type IV (0.5 ㎕ saline containing 0.078 U, Sigma) over a period of 5 min. After remaining in place for another 3 min, the needle was gently removed.
9. Cell transplantation
F3, F3.Akt1 hNSCs were dissociated into single cells by a brief trypsin treatment and suspended in PBS at 4x107 cells/0.1 ml and kept on ice until transplanted. Randomly selected ICH mice of one week after ICH surgery received 2 ㎕ (2x105 cells) of F3.Akt1 cell suspension (n=9), F3 cell suspension (n=9) and killed F3 cell suspension (n=10). F3 cells in glass tube were killed by placing the tube in boiling water for 1 min, injected slowly for 5 minutes into overlying cortex of the hemorrhage lesion (0.1 mm posterior, 2.0 mm ventral, and 2.0 mm lateral to the bregma). In another control group, 2 ㎕ of PBS was injected into the ICH mice (n=10). Immunosuppressant was not used in any of the animals. In order to identify the migration potential to the lesion site of grafted cells, F3.Akt1 hNSCs were infected with an adenovirus vector encoding LacZ gene (pAV.LacZ) in vitro at 100 MOI (PU/cell) for 24 hr before transplantation (n =2).
10. Behavioral Testing
Behavioral testing was performed weekly with the rotarod (Harvard Instrument) by 2 individuals blinded to mice treatment status (Lee et al., 2007). In the rotarod test, mice were placed on the rotarod cylinder, and the time the animals remained on the rotarod was recorded. The speed was slowly increased from 10 to 40 rpm within a period of 2 min. The trial was ended if the animal fell off the rungs or gripped the device and spun around for 2 consecutive revolutions. The animals were trained for 3 days before ICH operation. The maximum duration (in seconds) on the device was recorded with 3 rotarod measurements 1 day before ICH induction. Motor test data are presented as percentages of the maximal
duration compared with the internal baseline control (before ICH). The modified limb-placing test is a version of a test previously described (Lee et al., 2007). The test consists of 2 limb-placing tasks that assess the sensorimotor integration of the forelimb and the hind limb by checking responses to tactile and proprioceptive stimulation. First, the mouse is suspended 10 cm over a table, and the stretch of the forelimbs toward the table is observed and evaluated: normal stretch, 0 point; flexion with a delay (2 sec) and / or incomplete, 1 point; abnormal flexion, 2 point. Next, the mouse is positioned along the edge of the table, with its forelimbs suspended over the edge and allowed to move freely. Each forelimb (forelimb, second task; hind limb, third task) is gently pulled down, and retrieval and placement are checked. Finally, the mouse is placed toward the table edge to check for lateral placement of the forelimb. The 3 tasks are scored in the following manner: normal performance, 0 points; performance with a delay (2 sec) and/or incomplete, 1 point; no performance, 2 point. A total of 9 points means maximal neurological deficit, and 0 point means normal performance. Additionally, the body weights of all animals were checked weekly for 8 weeks.
11. Histology and immunohistochemistry
At the end of behavioral testing, each animal was anesthetized and perfused through the heart with cold saline followed by 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer. The brains were post-fixed in same fixative for 24 hr, followed with cryoprotection in 30% sucrose for 24 hr and then 30 ㎛ sections were prepared on a cryostat (Leica CM 3000). Three sections through the needle entry site, which was identifiable on the brain surface,
and sites 1.0 mm anterior and 1.0 mm posterior to plane were processed for X-gal staining to analyze the hemisphere area. These sections are representative of the core of the ICH lesion. The morphometric analyses involved computer-assisted hand delineation of the area of the striatum, cerebral cortex, and ventricles, as well as the whole hemisphere. Adjacent serial coronal sections were processed for double immunofluorescence staining of human nuclear matrix antigen (NuMA, 1:100, mouse monoclonal, Oncogene) and antibodies specific for cell type specific markers. Antibodies specific for neurofilament low molecular weight protein (NF-L, 1:1000, rabbit, Chemicon), neurofilament high molecular weight protein (NF-H, 1:1000, rabbit, Chemicon), microtubule associated protein-2 (MAP2, 1:500, rabbit, Chemicon), glial fibrillary acidic protein (GFAP, 1:1000, rabbit, DAKO, Carpinteria, CA), and phospho-Akt1 (1:100, rabbit, Upstate) were used for cell type identification of neurons and astrocytes. Brain sections were incubated in mixed solution of primary antibodies overnight at 4℃ as free floating sections, followed by mixed secondary antibodies of Alexa Fluor 488-conjugated anti-mouse IgG (1:1000, Molecular Probe, Eugene, OR) and Alexa Fluor 594-conjugated anti-rabbit IgG (1:1000, Molecular Probe) for 1 hr at RT. Negative control sections from each animal were prepared for immunohistochemical staining in an identical manner except the primary antibodies were omitted. Stained sections were then examined under an Olympus confocal laser scanning biological microscope (Olympus, Tokyo, Japan).
12. Stereological cell counts
brain sections from ICH animals was determined by stereological estimation. The sections used for counting covered the entire striatum with hemorrhage lesion and overlying cortex. This generally yielded six or seven sections in a series. Sampling was done using the Computer assisted stereological toolbox system, version 2.1.4 (Olympus), using an Olympus BX51 microscope, a motorized microscope stage (Prior Scientific, Rockland, NY) run by an IBM compatible computer, and a microcator (Heidenhain ND 281B, Schaumberg, IL) connected to the stage and feeding the computer with the distance information in the z-axis. The counting areas were delineated at a 1.25 x objective and generated counting areas of 150 x 150 ㎛. A counting frame (1612 ㎛2) was placed randomly on the first counting area and systemically moved through all counting areas until the entire delineated area was sampled. Actual counting was performed using a 100 x oil objective. Guard volumes (4 ㎛ from the top and 4–6 ㎛ from the bottom of the section) were excluded from both surfaces to avoid the problem of lost caps, and only the profiles that came into focus within the counting volume (with a depth of 10 ㎛) were counted. The estimate of the total number of hNuMA-positive F3 and F3.Akt1 hNSCs calculated according to the optical fractionator’s formula (West et al., 1991).
13. Statistical analysis
Data are presented as means±SEM. The statistical significance between group comparisons for behavioral data was determined by one-way ANOVA and two-way ANOVA. P values < 0.001 were considered to be statistically significant (version 12.0; SPSS Inc., Chicago, IL).
Ⅲ. Results
1. Stable human neural stem cell line over-expressing Akt1
F3 hNSC line was infected with a retroviral vector encoding mouse Akt1 gene (Fig. 1A) twice, clones resistant to hygromycin were selected and expanded. One of the clones was chosen and used in the present study. The morphology of the selected hNSC line, F3.Akt1 did not differ from the parental F3 hNSCs with bipolar or multipolar -morphology (Fig. 1B, C). Results of RT-PCR analysis of mRNAs isolated from F3 and F3.Akt1 hNSCs were shown in Figure 1D. Transcripts for nestin (an NSC specific marker), neurofilament triplet proteins (NF-L, NF-M and NF-H, cell type-specific markers for neurons), glial fibrillary acidic protein (GFAP, a specific marker for astrocytes) and Akt1 were all expressed by both F3 and F3.Akt1 cell lines. However, GFAP band at F3.Akt1 cells was detected just small quantity. In the results of RT-PCR analysis, we identified that there was no remarkable difference between the two hNSC lines in transcriptional level and introduced mouse Akt1 gene was over-expressed in F3.Akt1 cells.
Fig. 1. Characterization of hNSC line over-expressing Akt1. A: The retroviral vector encoding Akt1 (pLHCX.Akt1) used in the present study for the generation of F3.Akt1 human neural stem cell line. B, C: Cell morphology was showedno difference between F3 and F3.Akt1 cells (X 100). D: Gene expression of cell type-specific markers as studied by RT-PCR in F3 and F3.Akt1 human NSC lines. Both of F3 and F3.Akt1 human NSC lines expressed an NSC specific marker Nestin, cell type-specific markers for neuron, NF-L, NF-M, NF-H, a specific marker for astrocytes GFAP, and Akt1.
Table 1. PCR primer sequences for cell type-specific markers.
Gene Sequence
Sense: 5’-ACCTCTGAGACTGACACCATG-3’ Akt1 Antisense: 5’-CACTGGCTGAGTAGGAGAAC-3’ Sense: 5’-CTCTGACCTGTCAGAAGAAT-3’ Nestin Antisense: 5’-GACGCTGACACTTACAGAAT-3’ Sense: 5’-TCCTACTACACCAGCCATGT-3’ NF-L Antisense: 5’-TCCCCAGCACCTTCAACTTT-3’ Sense: 5’-TGGGAAATGGCTCGTCATTT-3’ NF-M Antisense: 5’-CTTCATGGAAGCGGCCAATT-3’ Sense: 5’-CTGGACGCTGAGCTGAGGAA-3’ NF-H Antisense: 5’-CAGTCACTTCTTCAGTCACT-3’ Sense: 5’-GCAGAGATGATGGAGCTCAATGACC-3’ GFAP Antisense: 5’-GTTTCATCCTGGAGCTTCTGCCTCA-3’ Sense: 5’-CATGACCACAGTCCATGCCATCACT-3’ GADPH Antisense: 5’-TGAGGTCCACCACCCTGTTGCTGTA-3’2. F3.Akt1 cells prevent H2O2-Induced cell death
H2O2 is a strong oxidant, and high concentrations can be toxic to cells. To investigate
whether H2O2-induced cell death is prevented in F3.Akt1 cells compared with F3 cells.
When F3 cells were exposed to varying H2O2 doses, cell viability was significantly
decreased but, F3.Akt1 cells were showed a resistance to H2O2 -induced cell death (Fig.
2A-C). To determine the protective effects of Akt1 on the H2O2-induced hNSCs death, we
examined the change of protein levels of hNSCs by H2O2 treatment. When F3 and F3.Akt1
were exposed to 0.5 mM H2O2 for 6h, phosphorylated form of Akt1and inactivated form
of caspase-3 were detected in F3.Akt1 cells (Fig. 2D), while F3 cells were showed the opposite results. The active fragment of caspase-3 (20 kDa) was seemed to increase in F3 cells. Therefore, we suggested the increase of cell viability in F3.Akt1 cells was caused by the Akt1 over expression and its phosphorylation. And phospho-Akt1 inhibits caspase-3 cleavage and activation so it provides protective effect to the hNSCs.
Fig. 2. Cell viability increases to H2O2–induced oxidative stress conditions and Akt1
phosphorylation in F3.Akt1 hNSC. A, B: F3.Akt1 cells we re showed more resistance to H2O2-induced cell death than F3 cells at different time 6 h (A) and 24 h (B) in MTT assay..
C: Phase contrast microscopy of H2O2-induced cell death was observed at 0.5mM
concentration for 6h in both F3 and F3.Akt1 hNSCs lines. Other than F3.Akt1 cells were lived more than F3 cells at that dose. D: These both of hNSCs were collected by PIRA
lysis buffer and performed SDS-PAGE to examine the phopho-Akt1 and caspase-3 protein level. This results demonstrated that F3.Akt1 cells showed the increased level of phosphorylation of Akt1 and decreased level of activation form of caspase-3 under the H2O2 treatment (* p<0.05, ** p<0.001).
3. Oxygen glucose deprivation (OGD) experiment
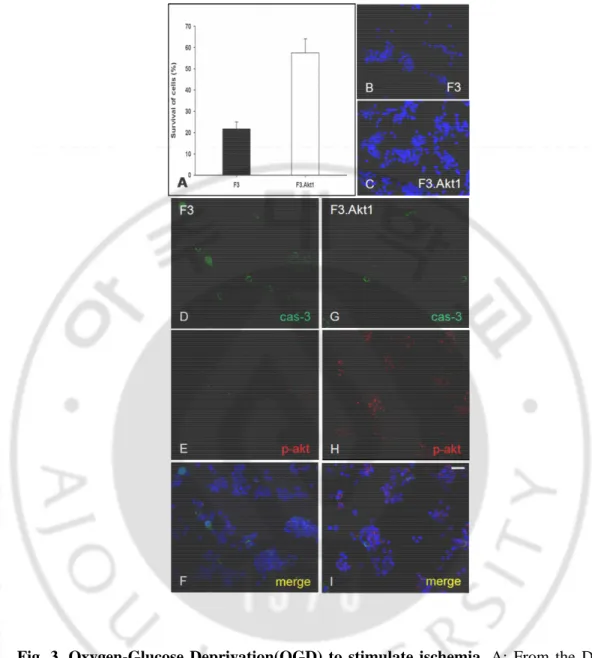
To investigate whether hypoxic condition triggers Akt1 phosphorylation and caspase-3 activation in F3 and F3.Akt1 hNSCs, we performed OGD experiment. Cultured F3 and F3.Akt1 cells were exposed to 1 h of OGD condition and assessed cell death 24 h later by nuclear staining with DAPI. Cell death was first observed 6 h after OGD; most dead cells had densely stained, shrunken, and compacted nuclei, which is consistent with morphological criteria for nuclear apoptosis. Like the H2O2-treated experiment, OGD
experiment also showed a similar result pattern. After 24 h, cells were fixed with 4% (w/v) paraformaldehyde and observed that by the fluorescence microscope, then survival cell numbers were counted using DAPI staining. F3.Akt1 cells showed a 2.5 fold increase in survival rate than F3 cells (Fig. 3A) and they did not illustrate chromatin condensation and fragmentation, the major apoptotic cell death features. Further, cells were incubated with the anti-phospho-Akt1 and anti-caspase-3 antibodies and then we identified the high amount of phospho-Akt1 expression and low level of caspase-3 in F3.Akt1 cells (Fig. 3G – I). In contrast, phopho-Akt1 is measured only a few level from F3 cells group (Fig. 3D–F). Therefore we confirmed again that phosphorylation of Akt1 was induced in a hypoxic condition and this phosphorylation of Akt1 was an important role in hNSCs survival.
Fig. 3. Oxygen-Glucose Deprivation(OGD) to stimulate ischemia. A: From the DAPI stain counting, cell viability was obtained. F3.Akt1 cells showed a 2.5 fold better survival rate than F3 cells. B, C: Most dead cells had densely stained, shrunken, and compacted nuclei, which is consistent with morphological criteria for nuclear apoptosis. D –I: Double immunostaining with phospho-Akt1 and caspase-3. F3.Akt1 cells expressed increased level of p-Akt1 and low level of activated cas-3. Bar indicates 20㎛. (* p<0.001)
4. Functional recovery in ICH animals by hNSC transplantation
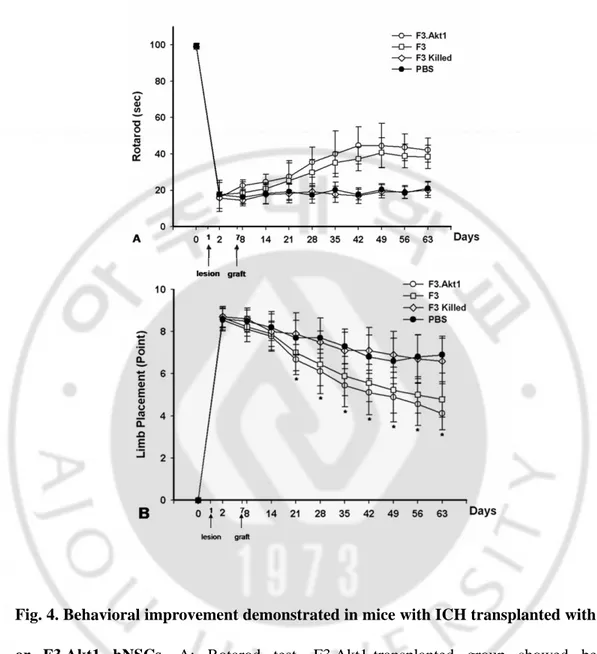
Motor performance of ICH animals receiving PBS, F3 Killed, F3 or F3 Akt1 hNSCs was determined by the rotarod and neurology score (Fig. 4A, B). The ICH mice receiving F3.Akt1 hNSCs showed improved behavioral performance on the rotarod compared with the PBS, F3.killed control groups, and the effect persisted for at least up to 8 weeks post-transplantation (the point at which animals were sacrificed) (Fig. 4A). Significant difference in rotarod test performance in F3.Akt1 vs F3 groups detected during the period of only 5 weeks post-transplantation (P<0.05). The F3.Akt1 transplantation group also showed marked improvement in the limb placement beginning 2 weeks post-transplantation and persisting for at least up to 8 weeks (Fig. 4B). No significant differences in behavioral performance in F3.Akt1 vs F3 groups detected during the period of 1~8weeks post-transplantation.
Fig. 4. Behavioral improvement demonstrated in mice with ICH transplanted with F3 or F3.Akt1 hNSCs. A: Rotarod test. F3.Akt1-transplanted group showed better performance than PBS controls 8 days on, and these benefits continued up to 8 weeks post-transplantation (* P<0.05). B: In the modified limb placement test, F3.Akt1-transplanted group showed better performance than PBS (* P<0.05).
5. Transplanted hNSCs differentiate into neurons and astrocytes
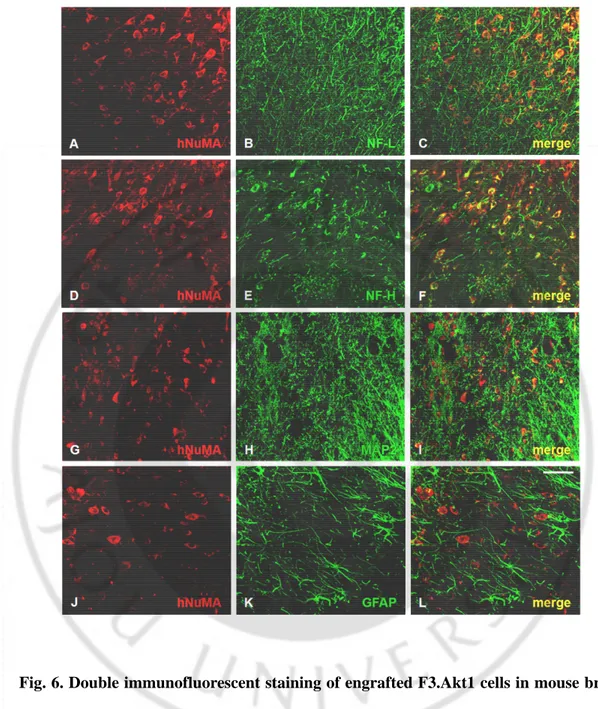
Following transplantation into ICH mouse cerebral cortex overlying hemorrhage lesion site, LacZ+ human NSCs migrated selectively to the hemorrhagic core and also located on the border of the lesion and further away from the injection sites (Fig.5). A large number of transplanted hNuMA+ F3.Akt1 cells (35 - 45%) differentiated into NF-L+/NF-H+ (Fig.6A-F) or MAP2+ (Fig.6G-I) neurons in the peri-hematomal sites. While only few proportion of the transplanted hNuMA+ F3.Akt1 cells (~4%) were GFAP+ astrocytes (Fig.6J-L ) and the hNuMA+/GFAP+ double-positive cells were found along the border of hemorrhagic core. These results indicate that a majority of grafted F3.Akt1 cells differentiate into either neurons or astrocytes in response to signals from the local microenvironment provided by the hemorrhagic lesion.
Fig. 5. F3.Akt1 hNSCs express LacZ-positive in ICH mouse at 2 weeks after transplantation. One week after an intracerebral hemorrhage lesion was produced by an intrastriatal injection of collagenase, AVV-LacZ (beta-galactosidase)-labeled F3.Akt1 hNSCs. Two weeks after the cell transplantation, LacZ-positive F3.Akt1 hNSCs found to migrate into the hemorrhage core and surrounding brain site (A: x 40, B: x 100).
Fig. 6.Double immunofluorescent staining of engrafted F3.Akt1 cells in mouse brain 8 weeks post-transplantation. F3.Akt1 human neural stem cells in the peri-hematomal sites are found to differentiate into neurons as shown by neuron specific markers neurofilament protein/ NF- L, H (A-F) and MAP2 (G-I) or into astrocytes as demonstrated by GFAP (J-L). Bar indicates 50μm.
6. Survival of transplanted F3 and F3.Akt1 hNSCs in ICH brain
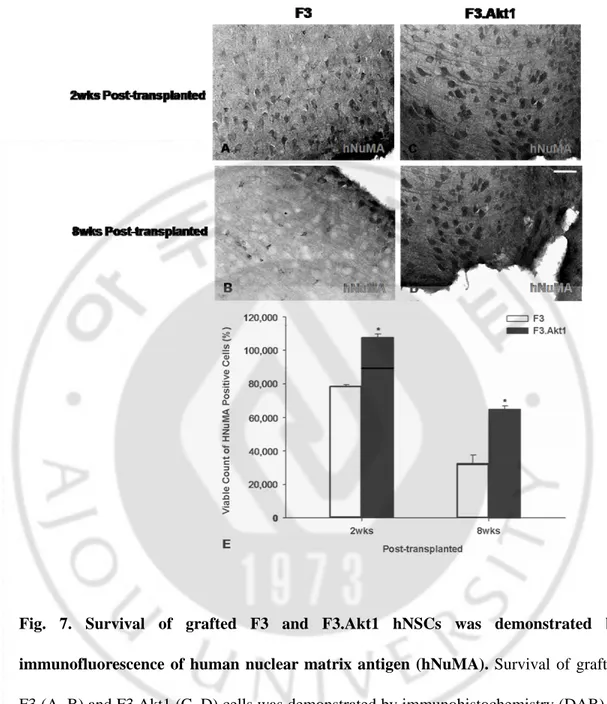
At 7 days after induction of experimental ICH, 2x105 cells of F3 or F3.Akt1 hNSCs were transplanted into ICH mouse cerebral cortex overlying hemorrhage lesion site, 2 mm cranial to the hemorrhagic lesion. Total numbers of hNuMA-positive F3 and F3.Akt1 hNSCs in the brain sections from ICH animals were determined by stereological estimation at 2- and 8-weeks post-transplantation. The results indicated that cell survival at 2-weeks post-transplantations is 107,765±2039 cells (53.9% of the initial population of 200,000 cells) and at 8 weeks post-transplantation the number is 64,892±1938 cells (32.5% of the initial population of 200,000 cells) (Fig. 7A–E) These results indicate that the survival rate of grafted F3.Akt1 NSCs in the brain of ICH animals is 53.9% at 2 weeks post-transplantation and 32.5% at 8 weeks post-transplantation. In ICH animals transplanted with F3 hNSCs, the number of surviving cells is 78,320±1250 cells (39.2% of the initial population of 200,000 cells) at 2-weeks post-transplantation and 32,538±4922 cells (16.3% of the initial population of 200,000 cells) at 8-weeks. These results indicate that Akt1 over expression in hNSCs resulted in a 0.5-fold increase in cell survival of transplanted hNSCs at 2 weeks transplantation and a 2-fold increase at 8 weeks post-transplantation. There was no sign of tissue distortion or tumor formation in brain of ICH animals grafted with F3 or F3.Akt1 hNSCs
Fig. 7. Survival of grafted F3 and F3.Akt1 hNSCs was demonstrated by immunofluorescence of human nuclear matrix antigen (hNuMA). Survival of grafted F3 (A, B) and F3.Akt1 (C, D) cells was demonstrated by immunohistochemistry (DAB) of hNuMA. (E) Number of surviving hNSCs expressing hNuMA (means土SEM) in ICH mouse brain at 2 and 8 weeks post-transplantation. Bar indicates 50㎛. (* P<0.05)
IV. DISCUSSION
In the present study, human NSCs over-expressing Akt1, F3.Akt1, showed strong resistant to the cell death stimuli than F3 cells in vitro and, F3.Akt1 cells transplanted into the brain of mouse ICH stroke model were found to increase survival of grafted cells, differentiate into the neuronal cells, and improve behavioral recovery in vivo.
The established F3.Akt1 cells showed normal growth pattern and morphology. They also expressed neural stem cell maker, nestin, and neuronal markers, NF-L, M and H but, F3.Akt1 cells did not showed a comparable level of GFAP, astrocytes marker, when compared to F3. In these results we confirm F3.Akt1 cells do not lose the properties of neural stem cells and still have the normal characteristics. And over expression of Akt1 gives cytoprotective effects to the hNSCs against cell death stimuli. In oxidative condition, F3.Akt1 cells showed increased survival rate by inducing phosphorylation of Akt1 and inhibition of caspase-3 cleavage. In this culture system, we could prove introducing Akt1 provides survival strength to the hNSCs.
Many of studies about neural transplantation, the aim of the cell transplantation is replacement or augmentation of losing cells. Selective neuronal loss such as in the case of Parkinson’s disease has already been considered a good candidate for neural replacement therapy. ICH is associated with considerable mechanical disruption of tissue in a large portion of the brain, and it was generally accepted that this disorder would be less likely to benefit from neural transplantation.
provide proof-of-principle that human NSCs over-expressing a survival factor Akt1 can be transplanted in the brain of animal models of neurological diseases, and produces beneficial effects of functional recovery and increased survival of grafted NSCs. Our results confirmed that the F3 hNSCs were transduced with Akt1 gene could survive well in the ICH animal brain following transplantation. From as early as 8 day post-transplantation to 8 weeks post-transplantation, F3.Akt1 cells provided functional recovery as determined by rotarod test and limb placement test and also induced an increased survival of transplanted NSCs in the host brain.
F3 hNSCs over-expressing Akt1 were able to survive much better than parental F3 NSCs, so that there were a 0.5-fold increase in cell survival of transplanted F3.Akt1 cells at 2 weeks post-transplantation and a 2-fold increase at 8 weeks, and a majority of grafted F3.Akt1 cells differentiated into neurons in the brain. These results indicate that F3.Akt1 NSCs at the ICH lesion sites are capable of preventing from the cell death and providing cytoprotective action in the ICH injury sites resulting in improved behavioral outcome.
But, for all the survival rate was increased in F3.Akt1 cells, there was no remarkable difference between F3 and F3.Akt1 groups in the behavioral test. In this study, we assessed in vivo test for 8 weeks so we suppose behavioral improvement may be different from the two hNSCs groups in more long-term period. We should do further study about this subject.
At 8 weeks post-transplanted, we found that the expression of the hNuMA+/GFAP cells of F3.Akt.1 was a small portion in ICH animals, which was not observed in transplantation of parental F3 cells. In the previous study using F3.VEGF cells in same animal models (Lee et al., 2007), they reported 55-65% of engrafted hNSCs were
differentiated into the astrocytes. A previous study reported Akt regulates the assembly and activity of basic helix-loop-helix (bHLH) transcription factor-coactivatior complexs to promote neuronal differentiation (Anne et al., 2003) and in fact, many transcription factors are known as the substrate of Akt1. We assumed that the activated phospho-Akt1 might translocate into the nucleus and it phosphorylates and activates transcriptional factors which then may regulate and determine the fate of engraft cells. However, despite we could not find significant differences in behavioral recovery between F3 and F3.Akt1 groups, we suggested that the extent of neuronal and glial differentiation may not affect the functional improvement.
As Akt is known to exert very strong control over cell survival and important influence on cell cycle control, it became a prime target in the search for cancer-related genes. In many studies have been reported that Akt gene is over expressed and constitutively active in many human cancers (Graff et al., 2000; .Brognard et al., 2001; Liu et al., 1999; Roy et al., 2002). We only focused on the survival effect of Akt1 in the present study so, we could not expect that what exactly happened in cellular level of the F3.Akt1 cell by the over expression of Akt1 and the result of the long-term survival in host brain. We were very concerned about the tumorigenesis in the F3.Akt1 cells. Because F3, parental hNSCs were genetically engineered with oncogene v-myc and we introduced another gene, also known as oncogene, Akt1 into the F3 cells in this study. So, from the beginning we considered the potential of tumorigenesis of F3.Akt1 cells. But, 6 months post transplantation of F3.Akt1 cells ICH mice were not showed any kinds of signs of tumor. We killed the mice and stained the brain slides by the hematoxylin and eosin. Also a
previous study reported about the v-myc expression was undetectable after F3 cell transplantation 24 or 48h in F3 cells (Flax et al., 1998). Therefore it might be concluded that introducing oncogenes were not induced tumorigenesis in F3.Akt1 cells and even though those genes were used to immortalize and establish the cell line, it means just immortalization not beyond the word.
In the present study we used hydrogen peroxide and OGD system in vitro and ICH animal model in vivo, so we concerned about the role of Akt1 in the oxidative stress condition. The PI3K-Akt signal is well-known pathway for the cell survival and it exhibits anti-apoptotic effects against oxidative stress-induced damage in various cell types, including neural progenitor cells (Franke et al., 1997; Elyaman et al.,2002). This pathway mediates cell survival by phosphorylation and therefore inactivating several pro-apoptotic factors. For example, activated Akt phosphorylates and releases pro-apoptotic FHKR transcription factor proteins from DNA. The free FHKR then binds to 14-3-3 proteins, which forms a complex that is transported out of the nucleus, thereby functionally inactivating the transcription factor. Activated Akt also phosphorylates and inactivates pro-apoptotic GSK-3β protein. Furthermore, when Akt phosphorylation is inhibited, FHKR phosphorylation is blocked and cell death is markedly increased. That is, inactivation of these proteins then prevents oxidant-mediated apoptotic cell death. Recently, some studies were showed antioxidant enzymes such as, glutathione peroxidase-1 (Gpx1), Cu/Zn-superoxide dismutase (Cu/Zn-SOD) and heme oxygenase-1 (HO-1) are became the new target substrate of activated Akt (Taylor et al., 2005; Rojo et al., 2004; Salinas et al., 2004). Even we have not testified these antioxidant enzymes in vitro and in vivo system, we
suggest that activated Akt1 in hNSCs might influence on these proteins and then modulate the redox system and reduced toxic levels of reactive oxygen species (ROS) in the cell. Further investigation is required to define the mechanism of F3.Akt1 cells’ survival effect and to widen understanding about the molecular signaling pathway.
V. CONCLUSION
As a potential therapeutic agent, Akt1as well as its downstream substrates, offer great promise for the development of therapeutics against a number of neurodegenerative disorders that may be acute in nature, such as stroke, or more sub-acute in duration, such as Alzheimer's disease. Initially believed to have cellular functions directed primarily toward cell survival and growth, Akt1 is now seen as a potential broad cytoprotective agent. Akt1 can offer cellular protection not only through the modulation of intrinsic apoptotic machinery, but also through the activation of survival signal pathways in the cells. Akt1 drives cellular survival through a series of distinct pathways that involve the Forkhead family of transcription factors, GSK-3ß, ß-catenin, eIF2B, c-Jun, CREB, Bad, IKK, p53, and JIPs. Yet, it is evident that further work that clarifies the cellular environment controlled by Akt1 will be of exceptional value to refine our knowledge of Akt1 and to maximize the potential of this protein as a therapeutic agent.
REFERENCES
1. 이학중, 위봉애, 박옥규, 강정채, 신영기, 이시래, 박요한, 박의현, 박영 춘, 유영상, 이 영, 유언호, 정문성, 지영구, 양인석, 김준욱, 박 원, 이홍 순. 문헌고찰과 아울러본 우리나라 뇌혈관 질환의 추이. J Korean Med Assoc. 34(7), 758-768. 1991
2. Abeel A Mangi, Nicolas Noiseux, Deling Kong, Huamei He, Mojgan Rezvani, Joanne S Ingwall and Victor J Dzau. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 9, 1195-1201. 2003
3. Ana I. Rojo, Marta Salinas, Daniel Martin, Rosario Perona, and Antonio Cuadrado. : Regulation of Cu/Zn-Superoxide Dismutase Expression via the Phosphatidylinositol 3 Kinase/Akt Pathway and Nuclear Factor-kB. J. Neurosci,
24, 7324 –7334. 2004
4. Anne B. Vojtek, Jennifer Taylor, Stacy L. DeRuiter, Jenn-Yah Yu, Claudia Figueroa, Roland P. S. Kwok, and David L. Turner. Akt Regulates Basic Helix-Loop-Helix Transcription Factor–Coactivator Complex Formation and Activity during Neuronal Differentiation. Mol Cel biol. 23; 4417-4427. 2003
5. Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 61, 3986-3997, 2001
6. Cheng G., Yu Z., Zhou D. and Mattson M. P. : Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Exp. Neurol. 175, 407–414. 2002
7. Chong Z.Z., Kang J.Q. and Maiese K.; Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation 106, 2973-2979. 2002
8. Chong Z.Z., Kang J.Q. and Maiese K.; Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br. J. Pharmacol. 138, 1107-1118. 2003
9. Chong ZZ, F. Li and K. Maiese: Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 20, 299-315. 2005
migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett 343: 129–133. 2003
11. Chu K, Kim M, Park KI, Jeong SW, Park HK, et al. Human neural stem cells improve sensorimotor deficits in the rat brain with experimental focal ischemia. Brain Res 1016: 145–153. 2004
12. Chu K, Kim M, Chae SH, Jeong SW, Kang KS, et al. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem cells in rats with focal cerebral ischemia. Neurosci Res 50: 459–465.2004
13. Conery A.R., Cao Y., Thompson E.A., Townsend C.M., Jr. Ko T.C. and Luo K.; Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat. Cell Biol. 6, 366-372. 2004
14. Dudek H., Datta S. R., Franke T. F., Birnbaum M. J., Yao R., Cooper G. M., Segal R. A., Kaplan D. R. and Greenberg M. E.: Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275, 661–665. 1997
15. Flax JD, Aurora S, Yang C, Simonin C, Wills AM, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nature Biotech 16: 1033–1039.1998
16. Gage FH. Mammalian neural stem cells. Science 287: 1433–1438. 2000
17. Gebel JM and Broderick JP: Intracerebral hemorrhage. Neurol Clin 18: 419–438. 2000
18. Graff JR, Konicek BW, Manulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. : Increased Akt activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 275, 24500-24505. 2000
19. Henry M.K., Lynch J.T., Eapen A.K. and Quelle F.W.; DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood 98, 834-841. 2001
20. Hayashi T, Abe K, Suzuki H, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial factor in rat brain after transient ischemia. J Cereb Blood Flow Metab 18: 887–895. 1998
21. Inagawa T.: What are the actual incidence and mortality rates of intracerebral hemorrhage? Neurosurg Rev 25: 237–246. 2002
22. Ishibashi S, Sakaguchi M, Kuroiwa T, Yamasaki M, Kanemura Y, et al.: Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res 78: 215–223. 2004
23. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 34: 2258–2263. 2003
24. Juliet M. Taylor, Ugiur Ali, Rocco C. Iannello, Paul Hertzog and Peter J. Crack : Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J. Neurochem. 92, 283–293. 2005
25. Kang J.Q., Chong Z.Z. and Maiese K.; Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J. Neurosci. Res. 74, 37-51. 2003a
26. Kang J.Q., Chong Z.Z. and Maiese K.; Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol. Pharmacol. 64, 557-569. 2003b
27. Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, et al.: Transplanted human fetal neural stem cells survive, migrate and differentiate in ischemic rat cerebral cortex. Proc Nat Acad Sci USA 101: 11839–11844. 2004
28. Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology 24: 159–171. 2004
29. Kim SU, Park IH, Kim TH, Kim KS, Choi HB, et al.: Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology 26: 129–140. 2006
30. Lee HJ, Kim KS, Park IH, Kim SU: Human Neural Stem Cells Over-Expressing VEGF Provide Neuroprotection, Angiogenesis and Functional Recovery in Mouse Stroke Model. PLoS ONE 2(1): e156. 2007
31. Lee ST, Chu K, Park JE, Lee K, Kang L, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res 52: 243–249. 2005
32. Lindvall O, Kokaia Z, Martinez-Serano A..: Stem cell therapy for human neurodegenerative disorders. Nature Med 10 suppl: S42–S50. 2004
33. Liu W, Li J, Roth RA.: Heregilin regulation of Akt/protein kinase B in breast cancer cells. Biochem Biophys Res Commun. 261, 897-903, 1999
34. Marta Salinas, Jinling Wang, Mar Rosa de Sagarra, Daniel Martin, Ana I. Rojo, Jorge Martin-Perez, Paul R. Ortiz de Montellano, Antonio Cuadrado.: Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 578, 90–94, 2004
35. Martin D., Salinas M., Lopez-Valdaliso R., Serrano E., Recuero M. and Cuadrado A. Effect of the Alzheimer amyloid fragment Ab(25–35) on Akt/PKB kinase and survival of PC12 cells. J. Neurochem. 78, 1000–1008. 2001
36. Mutlu N, Berry RG, Alpers BJ.: Massive cerebral hemorrhage: Clinical and pathological correlations. Arch Neurol. 8:644-661. 2001
37. Matsuzaki H., Tamatani M., Mitsuda N., Namikawa K., Kiyama H., Miyake S. and Tohyama M.; Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J. Neurochem. 73, 2037-2046. 1999
39. Mearow K. M., Dodge M. E., Rahimtula M. and Yegappan C. Stress-mediated signaling in PC12 cells – the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J. Neurochem. 83, 452–462. 2002
40. Meng XL, Shen JS, Ohashi T, Maeda H, Kim SU, et al. Brain transplantation of genetically engineered human neural stem cells globally corrects brain lesions in mucopolysacchridosis VII mouse. J Neurosci Res 74:266–277. 2003
41. NINDS ICH Workshop Participants: Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 36: 23–41. 2005
42. Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 14: 840-50. 2006
43. Owada Y., Utsunomiya A., Yoshimoto T. and Kondo H.; Expression of mRNA for Akt, serine-threonine protein kinase, in the brain during development and its transient enhancement following axotomy of hypoglossal nerve. J. Mol. Neurosci. 9, 27-33. 1997
44. Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. Pharmacologic reduction of mean arterial pressure does not adversely affect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit Care Med. 27:965-71. 1999
45. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 344: 1450–1460. 2001
46. Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashsk A, Lynch HT, Smyrk TC.: Akt proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 23, 201-205, 2002
47. Ryu JK, Kim J, Hong SH, Choi HB, Lee MC, et al. Proactive transplantation of human neural stem cells blocks neuronal cell death in rat model of Huntington disease. Neurobiol Disease 16: 68–77. 2004
48. Salinas M., Martin D., Alvarez A. and Cuadrado A.: Akt1/PKBa protects PC12 cells against the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium and reduces the levels of oxygenfree radicals. Mol. Cell Neurosci. 17, 67–77. 2001
49. Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, et al. Hypoxic preconditioning protects against Ischemic brain injury. NeuroRx 1: 26–35. 2004
50. Tamagno E., Robino G., Obbili A., Bardini P., Aragno M., Parola M. and Danni O.: H2O2 and 4-hydroxynonenal mediate amyloid b-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp. Neurol. 180, 144–155. 2003
51. Wang J, Tsirka SE.: Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 128:1622-1633. 2005a
52. Wang J, Tsirka SE.: Tuftsin fragment 1-3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 36:613-618. 2005b
53. Wang X., McCullough K. D., Franke T. F. and Holbrook N. J.: Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 275, 14 624–14 631. 2000
54. Yao R. and Cooper G. M.: Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267, 2003–2006. 1995
55. Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106: 829–838.
- 국문 요약 -
Akt1 과발현을 유도한 인간 신경줄기 세포주의
마우스 뇌출혈 뇌졸중 모델에서의 치료 효과
아주대학교 대학원 의학과
김 미 경
(지도교수 : 김 병 곤)
Akt1 분자는 serine/threonine 키나아제의 하나로, 세포의 성장과 증식, 생존 그리고 에너지 대사와 관련된 신호전달체계에서 중요한 역할을 하는 효소 중의 하나이다. 선행된 연구를 통해 래트 뇌출혈 뇌졸중 모델에 인간유래 신경세포줄기를 이식하여, 이식한 줄기세포들의 뇌 손상 조직으로의 선택적인 이동 능력과 모델 동물의 행동적 기능상의 회복을 확인 할 수 있었다. 하지만 이식 된 줄기세포의 낮은 생존율로 인해, 세포 이식 이후의 살아있는 세포에 의한 지속적인 효과에 관한 우려를 낳았다. 이에 본 연구에서는 대표적인 survival molecule 로 알려진 Akt1 유전자를 인간 신경줄기세포 내로 도입,과발현을 유도하여 이러한 세포를 이용한 질병 치료책에 있어서 이식된 세포의 장기간의 생존 효과를 보고자 하였다. 본 실험은 먼저 Akt1 과발현 신경줄기 세포주를 만들어 in vitro 상에서 과산화수소에 의한 세포사에 대한 저항성을 확인하고, 또한 뇌졸중 상태와 유사한 환경을 가지는 산소 및 글루코스 고갈 실험에서의 세포의 생존력을 비교해 보았다. 그 결과 Akt1 이 과발현 되는 세포주가 그렇지 않은 모세포주보다 강한 생존력을 가지고 있음을 알 수 있었다. 또한 마우스를 이용한 in vivo 동물 실험을 통해 확인을 해 본 결과, Akt1 이 과발현 되는 세포주가 그렇지 않은 대조군의 세포주에 비해 이식 후 일정 시간 후에 보다 더 오래 생존하고, 유지 되고 있음을 확인 할 수 있었다. 이렇듯 본 연구에서는 과발현 되는 Akt1 에 의해 이식하는 신경 줄기 세포의 생존율을 향상시키고, 이식한 세포의 장기간의 생존으로 인해 신경 줄기세포의 지속적인 neuroprotection 효과를 확인할 수 있었다. 핵심어 : 신경 줄기 세포, Akt1/단백질 키나아제 B, 산소-포도당 박탈, 뇌출혈, 세포 이식