저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Doctoral Thesis in
Medical Sciences
Half-Field
Volumetric Modulated Arc
Therapy
for
Multileaf Collimator
Leakage
Reduction and Dosimetric Impact
in Large Irradiated Field
Graduate School of Ajou University
Department of Medical Sciences
Half-Field
Volumetric Modulated Arc
Therapy
for
Multileaf Collimator
Leakage
Reduction and Dosimetric Impact
in Large Irradiated Field
Young-Taek Oh, M.D., Ph.D., Advisor
I submit this thesis as the
Doctoral thesis in Medical Sciences.
February 2020
Graduate School of Ajou University
Department of Medical Sciences
The Doctoral thesis of Hyunsoo Jang in Medical Sciences
is hereby approved.
Thesis Defense Committee Chair
Mison Chun Seal
Member O Kyu Noh Seal
Member Hae-Jin Park Seal
Member Sunyoung Lee Seal
Member Young-Taek Oh Seal
Graduate School of Ajou University
-ABSTRACT-
Half-Field
Volumetric Modulated Arc Therapy
for
Multileaf
Collimator
Leakage Reduction and Dosimetric Impact
in Large Irradiated Field
Background:
Although there are some controversies regarding whole pelvic radiation
therapy (WPRT) due to its gastrointestinal toxicities, it is combined with other systemic
treatment to find an effective strategy for patients with gynecological, rectal, and prostate
cancer. To effectively spare organ at risk (OAR), doses using multileaf collimator’s optimal
segments and volumetric modulated arc therapy (VMAT) using a half-beam technique (HF)
were implemented for WPRT.
Methods and Materials:
Dosimetric benefits of HF-VMAT were presented with dose
conformity and dose volume parameters in terms of modulation complex score, as
compared to VMAT optimized using a fully opened field (FF).
The size of the FF was
decided to entirely include a planning target volume plus a 3-mm margin in all beam’s eye
view at arc angles. Half of the FF from the isocenter was opened for dose optimization in
HF-VMAT. Consequent normal tissue complication probabilities (NTCPs) by reduction of
the irradiated volumes and delivered doses for OAR were evaluated
Results: Compared to FF-VMAT, HF-VMAT showed superior conformal dose distribution
where the 75% isodose line compactly surrounded the separated regional lymph nodes.
Dose conformity was comparable between VMAT and FF-VMAT, however,
HF-VMAT used 60%-70% less intensity modulation than that used by FF-HF-VMAT. The small
intestine and colon showed noticeable reduction in the irradiated volumes of up to 35%
and 15%, respectively, at an intermediate dose of 20-45 Gy. The small intestine showed
statistically significant dose sparing at the volumes that received a dose every 5 Gy from
15 to 45 Gy. Such a dose reduction for the small intestine and colon in HF-VMAT
presented significant NTCP reduction than that in FF-VMAT.
of multileaf collimator segments to deliver conformal doses without excessive intensity
modulation. It achieved distinguishable dose sparing for gastrointestinal OAR in WPRT
without sacrificing the target dose conformity. HF-VMAT can be useful in reducing OAR
toxicities associated with WPRT.
Keyword: whole pelvic radiotherapy, volumetric modulated radiation therapy, half-beam
TABLE OF CONTENTS
ABSTRACT ···i
TABLE OF CONTENTS ···iii
LIST OF TABLES ···iv
LIST OF FIGURES ···v
I. INTRODUCTION ···1
II. MATERIALS AND METHODS ···3
A. Patient selection and simulation
···3
B. Target delineation
···
5
C. Volumetric modulated arc therapy planning
···5
D. Analysis of dosimetric factors
···7
E. Beam complexity of volumetric modulated arc therapy plans
···9
F. Dose homogeneity evaluation
···10
G. Statistical analysis
···10
III.RESULTS ···11
A. Normal organ dosimetry ···11
1. Small bowel
···12
2. Bladder
···14
3. Colon
···16
4. Rectum
···18
B.
Normal tissue complication probability
···20
C. Target coverage ···22
D. Modulation index ···24
IV. Discussion
···25
REFERENCES
···28
LIST OF TABLES
Table 1. Characteristics of patients, tumors, and treatment regimen selected for whole
pelvic radiation therapy. ···4
Table 2. Radiobiological parameters to calculate equivalent uniform dose and normal tissue
complicatin probabilities for organs at risk in whole pelvic radiation therapy···8
Table 3. Comparison of dose-volume parameters for small bowel in VMAT plans using
three different techniques. ···13
Table 4. Comparison of dose-volume parameters for bladder in VMAT plans using three
different techniques. ···15
Table 5. Comparison of dose-volume parameters for colon in VMAT plans using three
different techniques. ···17
Table 6. Comparison of dose-volume parameters for rectum in VMAT plans using three
different techniques. ···19
Table 7. Evaluation of normal tissue complication probabilities and equivalent-uniform
dose (EUD) with statistical significance. ···21
Table 8. Mean conformity number (CN) and homogeneity index (HI) according to three
plans. ···22
LIST OF FIGURES
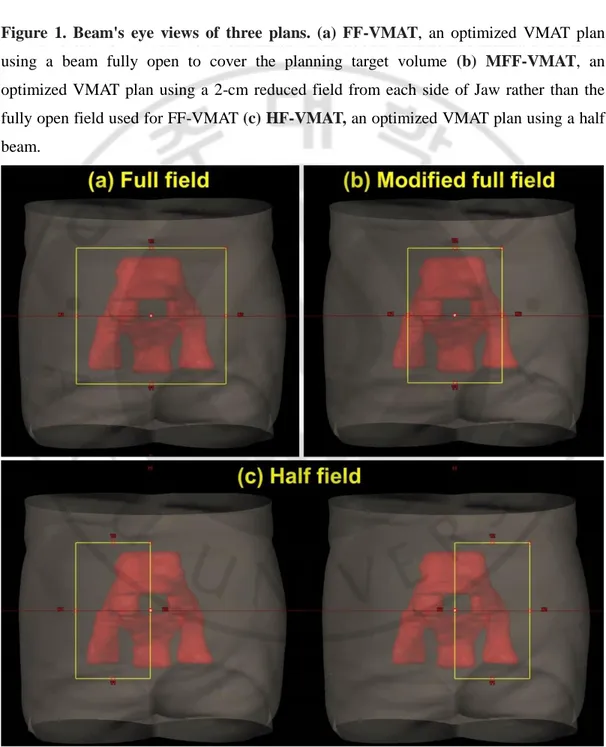
Figure 1. Beam's eye views of three plans. (a) FF-VMAT (b) MFF-VMAT (c) HF-VMAT.
···6
Figure 2. Comparison of irradiated isodose distributions according to three plans. (a)
FF-VMAT (b) MFF-FF-VMAT (c) HF-FF-VMAT.
···11
Figure 3. The mean dose-volume histogram of small bowel.···12
Figure 4. The mean dose-volume histogram of bladder. ···14
I. Introduction
Radiation therapy (RT) is an important part of cancer treatment and is applied to various
organs and cancers (1-3). However, during RT, normal organs are exposed to radiation,
which causes various radiation induced toxicity (4). Because the side effects of RT can
cause not only the toxicity but also the total dose reduction, a delicate RT plan is needed.
Especially, more careful attention is required in the abdominal RT planning due there are
many sensitive organs in abdomen. Of abdominal organs, the radiation tolerance of small
bowel is lower than the other organs and the toxicity is fatal (5, 6). So, the dosimetry of
small bowel is important part in abdominal RT planning.
Colon and bladder have higher
radiation tolerance than small bowel. However, due to the individual difference of
radiosensitivity, toxicity can be induced at lower radiation doses (7, 8).
The whole pelvic radiotherapy (WPRT) including pelvic lymph node irradiation is
regarded as a standard treatment regimen for locally advanced gastrointestinal and
gynecological cancers (9, 10). Although WPRT can bring out clinical merits (11-15), acute
and late intestinal and urinary complications have been significant concerns (16-18).
Recently, the trend of RT is rapidly changing due to the advancement of radiation delivery
and patient fixation techniques. In particular, the utilization of
intensity modulated
radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) is greatly increasing,
and these techniques are actively used in wide field irradiation such as WPRT (19, 20).
The important point of IMRT and VMAT techniques are dynamic movements of multileaf
collimator (MLC). And, It makes optimal dose distribution depending on gantry movement
and dose rate change. To achieve the improved organ at risk (OAR) in large and
complicated anatomical geometries, the VMAT is the useful radiation delivery method to
provide conformal dose distribution (21-23). Optimal fluence, which is created by MLC
segments at individual numerous beam angles going through the arc trajectories in VMAT,
can effectively reduce the normal organ doses.
However, when the irradiation range is widened, the MLC movement is restricted. This
leads to MLC leakage and non-blocking phenomenon, and degrades the efficiency of
IMRT and VMAT plan. The MLC leaves move in a horizontal direction when a collimator
angle is 0° in a beam’s eye view. If the OAR are placed between the separated target
volumes including lymph nodes in the same direction with the MLC movement,
suboptimal MLC segments can be created to meet the mechanical restrictions such as
gantry and MLC speed with varying dose rate (24-26). For example, one side of the MLC
can reach the target volume, however the other side cannot properly shield OAR exposure
or opens outside of the planning target volume (PTV) to deliver the prescribed dose to the
both of the two separated targets. Half field technique is possible to supplement these
problems. The use of half field in VMAT plan has attempted in RT of breast cancer, and the
dosimetric benefit was proved (27). The half field technique has also been applied to
improve lower neck coverage in head and neck cancer (28). However, there are rare studies
about the half field technique, and no study has yet been conducted in abdominal
irradiation. In present study, a VMAT planning method using a half-beam technique was
devised to provide superior normal tissue dose sparing, as well as to achieve dose
conformity for target volumes in WPRT.
II. Materials and methods
A. Patient selection and simulation
To evaluate the large and complicated irradiated field, patients with inguinal lymph node
in the irradiation field were enrolled in present study. A total of ten patients with a
diagnosis of cervical, anal, and vaginal cancer was selected.
Cervical, anal, and vaginal
cancer were 4, 4, and 2, respectively. The four patients with cervical cancer were involved
to lower 1/3 vagina. Treatment decision and radiotherapy protocol reflected
National
Comprehensive Cancer Network guidelines (29, 30). The patient and tumor characteristics
were summarized in Table 1. For cervical and vaginal cancer patients, WPRT was followed
by high-dose rate brachytherapy.
All patients underwent a computed tomography simulation in a supine position with
arms on the chest. This retrospective dosimetric study was approved by the Institutional
Review Board of the Dongguk University Medical Center (110757-201711-HR-02-01),
and informed consent in writing was obtained from individual patient. All information was
anonymous prior to analysis.
Table 1. Characteristics of patients, tumors, and treatment regimen selected for
whole pelvic radiation therapy.
No Sex/Age Origin Stage Pathology Treatment aim WPRT Prescribed Dose (Gy) Chemotherapy 1 M/59 Anus T2N0 SCC Adjuvant 50 -
2 F/75 Anus Recurrent SCC Adjuvant 50 -
3 F/78 Anus T2N1 SCC Definitive 50 FMC
4 M/88 Anus T2N0 SCC Definitive 41.4 FMC
5 F/51 Vagina T2N0 AC Definitive 45 -
6 F/61 Vagina T1N0 SCC Definitive 45 -
7 F/67 Cervix T3aN0 SCC Definitive 45 WC
8 F/71 Cervix T3bN0 SCC Definitive 45 WC
9 F/89 Cervix T3aN0 AC Definitive 45 -
10 F/86 Cervix T3aN1 SCC Definitive 45 -
WPRT, whole pelvis radiotherapy; SCC, squamous cell carcinoma; FMC, 5-fluorouracil,
mitomycin C; AC, adenocarcinoma; WC,
weekly cisplatin.
B. Target delineation
Clinical target volume (CTV) and pelvic lymph nodes were delineated according to the
consensus guidelines (31-33). Treatment volume for gynecological cancer patients includes
the tumor involving the lower third of the vagina as well as tumor bed, parametria,
uterosacral ligaments, and pelvic lymph nodes. Obturator, presacral, and internal iliac node
chains come under the pelvic lymph nodes in all cases. External and perirectal nodes are
added for gynecological and anal cancer patients, respectively. The PTV was created by
adding a 5 mm margin to the CTV. Small bowel, bladder, rectum, and femur heads, were
contoured as OAR. Each PTV received a prescribed dose of 50 Gy in 25 fractions. Dose
constraints were applied for OAR as per Radiation Therapy Oncology Group (RTOG)
protocols for each treatment site (9, 10, 32).
C. Volumetric modulated arc therapy
planning
All VMAT plans consisted of four full arcs alternately rotating clockwise and
counterclockwise in Eclipse (ver. 10.0, Varian Medical Systems, Palo Alto, CA) and the
normal tissue optimization. The collimator angles less than ± 40° were used for each arc to
improve dose conformity as well as to minimize the interleaf leakage and the
tongue-and-groove effect in VMAT. Meanwhile, dose distributions of the VMAT plans were optimized
by using different field sizes. As shown in figure 1, an optimized VMAT plan (FF-VMAT)
is created with a field size (FF) which is enough to fully cover an entire PTV, but less than
15 cm with X-jaws in consideration of the maximum leaf span of the Millenium MLC. The
opening of the FF in a superior-inferior direction was adjusted to sufficiently include the
PTV plus a margin less than 1cm. The field size was reviewed using beam’s eye views at
all different gantry angles of the arcs. A new VMAT plan (HF-VMAT) is optimized with
half size of the FF. The optimized dose was delivered by opening the half and the other
half of the FF for two arcs rotating clockwise and counter clockwise, respectively, as in
Fig.1(c). In addition, a modified field size (MFF) 2 cm reduced from the each X-jaw of the
FF is employed for a VMAT plan (MFF-VMAT) to evaluate dose conformity depending on
MLC segments and sequences. Dosimetric benefits among three VMAT plans were
analyzed, when a 95% of the PTV was covered by the prescribed dose with the same dose
constraints applied for OAR. The dose distributions for each treatment site were calculated
by Analytical Anisotropic Algorithm (ver. 10.0, Varian Medical Systems) and progressive
resolution optimizer (ver. 10.0, Varian Medical Systems).
Figure 1. Beam's eye views of three plans. (a) FF-VMAT
, an optimized VMAT plan
using a beam fully open to cover the planning target volume
(b) MFF-VMAT
, an
optimized VMAT plan using a 2-cm reduced field from each side of Jaw rather than the
fully open field used for FF-VMAT
(c) HF-VMAT,
an optimized VMAT plan using a half
beam.
D. Analysis of dosimetric factors
As the dose conformity for the PTV are comparable among the VMAT plans optimized
with different field sizes, dose sparing for OAR was evaluated with dose-volume
histogram (DVH) as well as relevant dose-volume parameters associated with estimating
complications for individual OAR.
The normal organ dosimetric parameters for small
bowel, bladder, rectum and femur head were compared according to radiotherapy planning
technique. The DVH of all plans obtained, and dosimetric parameters, such as, V
doseand
mean dose, were calculated from DVH. The V
dosewas defined as the percentage volume
that received at least the dose.
Maximum (D
max) and mean (D
mean) doses, and the dose (D
2cc)
delivered to the most irradiated 2cc volume were analyzed for OAR. For small bowel and
colon, each volume receiving the doses from 45 Gy to 5 Gy at a dose interval of 10 Gy was
evaluated, especially including the V
15Gyregarded as an important parameter to predict
gastrointestinal toxicities (34).
Because rectum is included in the treatment volume of anal
cancer, rectal dose was analyzed in 6 patients except anal cancer.
In addition, to evaluate
the potential radiobiological impacts on OAR depending on dose distributions in WPRT,
equivalent uniform dose (EUD) and
normal tissue complication probability (NTCP) were
calculated using Emami-Burman parameters. To present the sensitivities and variability of
NTCP in terms of analytic models, Lyman-Kutcher-Burman (LKB) and EUD-based
log-logistic models were adopted (35). The effect of varying alpha-beta ratios for acute and late
complications on EUD and NTCP were discussed accordingly for individual OAR. The
alpha-beta ratios and required biological parameters to calculated NTCP were summarized
in Table 2 (35-40).
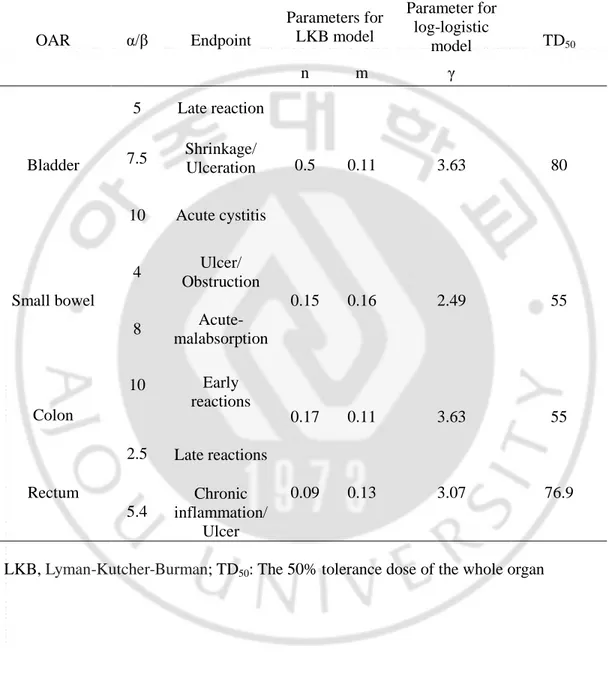
Table 2. Radiobiological parameters to calculate the equivalent uniform dose and
normal tissue complication probabilities for organs at risk in whole pelvic radiation
therapy
OAR
α/β
Endpoint
Parameters for
LKB model
Parameter for
log-logistic
model
TD
50n
m
γ
Bladder
5
Late reaction
0.5
0.11
3.63
80
7.5
Shrinkage/
Ulceration
10
Acute cystitis
Small bowel
4
Ulcer/
Obstruction
0.15
0.16
2.49
55
8
Acute-
malabsorption
Colon
10
Early
reactions
0.17
0.11
3.63
55
Rectum
2.5
Late reactions
0.09
0.13
3.07
76.9
5.4
Chronic
inflammation/
Ulcer
E. Beam complexity of volumetric modulated arc therapy plans
The traveling distances between control points and segment shapes of the VMAT arcs
can affect complexity of the beam intensity modulation. The modulation complexity score
(MCS) using variabilities of leaf sequences (LSV) and segment area (AAV) was adopted to
comprehensively present the plan complexity across all segments (34). It is formulated
using equation [1] by reflecting each segment weight to the corresponding relative arc
weight,
𝑴𝑪𝑺
𝑽𝑴𝑨𝑻=
∑
∑
[(
𝑨𝑨𝑽𝒄𝒑𝒂𝒓𝒄+𝑨𝑨𝑽𝒄𝒑+𝟏𝒂𝒓𝒄 𝟐) × (
𝑳𝑺𝑽𝒄𝒑𝒂𝒓𝒄+𝑳𝑺𝑽𝒄𝒑+𝟏𝒂𝒓𝒄 𝟐) × (
(𝑴𝑼𝒄𝒑+𝟏𝒂𝒓𝒄 −𝑴𝑼𝒄𝒑𝒂𝒓𝒄) 𝑴𝑼𝒂𝒓𝒄)]
(𝒏−𝟏) 𝒄𝒑=𝟏 𝑵 𝒂𝒓𝒄=𝟏eq. [1]
The parameters of n and N mean the total number of control points per each arc and the
total number of arcs used in each VMAT plan. The LSV
cpand the AAV
cpfor each control
point are calculated using Eq. [2] and [3], respectively, where m is the number of MLC
leaves which move underneath the unblocking portion of the field defined by X and Y jaws
for each control point.
𝐿𝑆𝑉
𝑐𝑝=
∑
(𝑝𝑜𝑠
𝐿− |(𝑝𝑜𝑠
𝑖+1𝐿− 𝑝𝑜𝑠
𝑖𝐿)|)
𝑚 𝑖=1(𝑚 − 1) × 𝑝𝑜𝑠
𝐿𝑐𝑝×
∑
(𝑝𝑜𝑠
𝑅− |(𝑝𝑜𝑠
𝑖+1 𝑅− 𝑝𝑜𝑠
𝑖𝑅)|)
𝑚 𝑖=1(𝑚 − 1) × 𝑝𝑜𝑠
𝑅𝑐𝑝eq. [2]
𝐴𝐴𝑉
𝑐𝑝=
∑
(𝑝𝑜𝑠
𝑖 𝐿− 𝑝𝑜𝑠
𝑖𝑅)
𝑚 𝑖=1𝑚 × (𝑝𝑜𝑠
𝐿𝑎𝑟𝑐− 𝑝𝑜𝑠
𝑅𝑎𝑟𝑐)
eq. [3]
The
𝑝𝑜𝑠
𝑖𝐿presents the i-th leaf position of the MLC at the left bank. The
𝑝𝑜𝑠
𝐿𝑐𝑝and
𝑝𝑜𝑠
𝐿𝑎𝑟𝑐mean the furthest position of the MLC leaf from the isocenter among the all MLC
leaves constituting a shape of the individual segment and across the all the control points
of an individual arc. The R denotes the MLC leaves on the right bank. Thus, while the LSV
presents variability of traveling distances of the MLC leaf sweeping the each set of control
points relative to the maximum lateral separation from the isocenter for the each side, the
AAV presents the complexity of the separation of each pair of the MLC leaves relative to
the maximum separation created among all of MLC leaves across all segments consisting
of the arc.
F. Dose homogeneity evaluation
The conformity number (CN), and homogeneity index (HI) were evaluated to know the
dose homogeneity.
The CN was defined as: CN = (V
T,RI/V
T)/(V
T,RI/V
RI). V
T,RIis the target volume covered
by the reference isodose. V
Tis the total target volume, and V
RIis the corresponding volume
to the reference isodose (41).
The HI was defined as:
HI = √∑ (𝐷
𝑖− 𝐷
𝑝)
2×
𝑉𝜈𝑖𝑇
𝑖
D
iis the delivered dose to the i-th
voxel, and D
pis the prescribed dose. V
Tis the total target volume, and v
iis the
corresponding volume of i-th voxel (42).
G. Statistical analysis
To distinguish statistically significant dosimetric effects by the different planning
techniques for WPRT using VMAT,
Wilcoxon signed rank test was performed using
statistical analysis software (SPSS, version 20, SPSS Inc., Chicago, IL). The p-value less
than 0.05 were considered statistically significant.
III. Results
A. Normal organ dosimetry
When the PTV has met the same goal for the dose coverage in VMAT plans using three
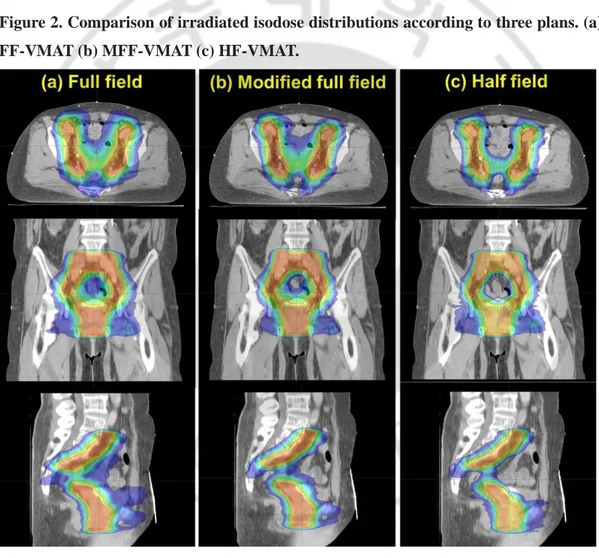
different techniques, dose sparing for the bladder and the small bowel was noticeable in an
axial and a coronal view (figure 2).
Figure 2. Comparison of irradiated isodose distributions according to three plans. (a)
FF-VMAT (b) MFF-VMAT (c) HF-VMAT.
1. Small bowel
Mean small bowel dose of HF-VMAT plan was significantly lower than FF-VMAT plan
(29.57 vs 32.89, p<0.05). V30 and V40 to small bowel were also significantly lower (V30:
46.43 vs 62.38, V40: 21.44 vs 28.72, p<0.05). Although it was no statistically significant
between HF-VMAT and MFF-VMAT plan, HF-VMAT plan showed the improved
dosimetric values of small bowel. V10 to small bowel was 91.74, 91.61, and 91.76 in
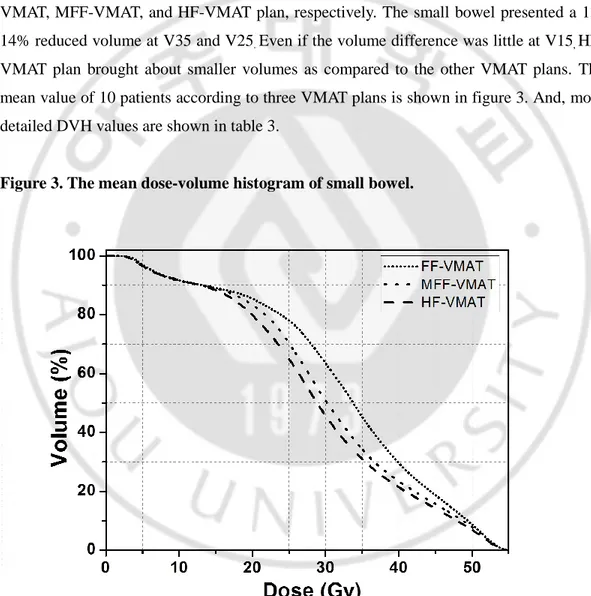
FF-VMAT, MFF-FF-VMAT, and HF-VMAT plan, respectively. The small bowel presented a
13-14% reduced volume at V35 and V25
.Even if the volume difference was little at V15
,HF-VMAT plan brought about smaller volumes as compared to the other HF-VMAT plans. The
mean value of 10 patients according to three VMAT plans is shown in figure 3. And, more
detailed DVH values are shown in table 3.
Table 3. Comparison of dose-volume parameters for small bowel in VMAT plans
using three different techniques. The mean dose (D
mean), maximum dose (D
max), dose
delivered to 2cc of the most irradiated organ volume (D
2cc) were evaluated for small bowel.
Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec)Dmean (Gy) 32.89±3.69 30.68±3.57 29.57±2.28 0.005 0.005 0.074 Dmax (Gy) 55.29±0.56 55.12±34.44 55.39±4.52 0.508 0.114 0.074 D2cc (Gy) 53.91±0.84 53.89±0.29 53.74±17.01 0.169 0.760 0.445 V5 (%) 97.39±30.86 96.56±2.56 96.59±2.41 0.066 0.008 0.953 V10 (%) 91.74±5.09 91.61±5.24 91.76±5.07 0.646 0.173 0.683 V15 (%) 89.16±6.10 89.03±6.29 88.37±5.56 0.017 0.074 0.074 V20 (%) 85.50±8.44 83.35±8.90 79.95±5.62 0.005 0.013 0.037 V25 (%) 78.10±12.60 70.28±12.48 65.01±7.13 0.005 0.005 0.173 V30 (%) 62.38±14.16 50.77±13.88 46.43±9.17 0.009 0.005 0.285 V35 (%) 45.29±11.50 34.36±10.17 31.10±6.65 0.005 0.005 0.074 V40 (%) 28.72±7.51 24.27±8.68 21.44±4.94 0.005 0.047 0.059 V45 (%) 18.72±5.89 15.70±5.18 14.18±3.44 0.009 0.005 0.047
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
2. Bladder
Mean bladder dose of HF-VMAT plan was significantly lower than FF-VMAT and
MFF-VMAT plan (33.64 vs 38.63 vs 37.21, p<0.05). V30 and V40 to bladder were also
significantly lower (V30: 62.57 vs 89.15 vs 86.20, p<0.05, V40: 25.69 vs 37.74 vs 31.27).
V10 to bladder was 100 in all plans. The bladder showed highest volume reduction of 12%
at V40 and 27% at V30. Although
MVMAT plan showed better results than the
FF-VMAT plan at > 35Gy, it was not as good as HF-FF-VMAT plan.
The mean value of 10
patients according to three VMAT plans is shown in figure 4. More detailed DVH values
are shown in table 4.
Table 4. Comparison of dose-volume parameters for bladder in VMAT plans using
three different techniques. The mean dose (D
mean), maximum dose (D
max), dose delivered
to 2cc of the most irradiated organ volume (D
2cc) were evaluated for bladder.
Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec) Dmean (Gy) 38.63±2.25 37.21±2.21 33.64±3.86 0.005 0.017 0.005 Dmax (Gy) 53.85±1.18 53.41±1.42 53.53±1.52 0.721 0.203 0.646 D2cc (Gy) 51.35±1.87 50.88±1.87 50.15±2.14 0.005 0.019 0.013 V5 (%) 100 100 100 - - - V10 (%) 100 100 99.44±1.76 0.317 - 0.317 V15 (%) 100 100 97.86±5.12 0.068 - 0.068 V20 (%) 100 100 91.69±9.99 0.018 - 0.018 V25 (%) 99.87±0.35 99.53±0.97 82.54±17.25 0.018 0.273 0.018 V30 (%) 89.15±12.24 86.20±9.29 62.57±23.62 0.011 0.260 0.017 V35 (%) 66.61±16.61 55.98±14.48 42.13±13.20 0.005 0.022 0.005 V40 (%) 37.74±12.40 31.27±11.10 25.69±10.68 0.005 0.013 0.005 V45 (%) 20.11±10.04 17.41±8.86 14.28±7.81 0.005 0.013 0.005
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
3. Colon
Mean colon dose of HF-VMAT plan was significantly lower than FF-VMAT plan (23.45
vs 25.27, p<0.05). V30 and V40 to small bowel were also significantly lower (V30: 34.85
vs 40.01, V40: 20.22 vs 26.34, p<0.05). Colon showed highest volume reduction at V35
up
to 8%
.The HF-VMAT plan achieved a 4-5% volume decrease for colon at V45
andV35.
Dose sparing for the volumes at the same percentage led to 2 Gy less D
meanfor colon.
MFF-VMAT plan showed better results than the FF-VMAT plan at > 25Gy and it was
statistically significant. However the MFF-VMAT plan was not as good as HF-VMAT plan.
Table 5. Comparison of dose-volume parameters for colon in VMAT plans using three
different techniques. The mean dose (D
mean), maximum dose (D
max), dose delivered to 2cc
of the most irradiated organ volume (D
2cc) were evaluated for colon.
Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec)
Dmean (Gy) 25.27±6.81 23.98±6.34 23.45±6.29 0.005 0.005 0.037 Dmax (Gy) 55.11±0.97 55.12±0.34 54.95±0.46 0.959 0.508 0.959 D2cc (Gy) 52.85±16.73 53.89±0.29 52.31±1.21 0.285 0.114 0.799 V5 (%) 85.98±15.11 85.87±15.18 84.95±15.17 0.074 0.799 0.074 V10 (%) 74.20±15.81 72.49±16.40 71.51±16.10 0.047 0.721 0.074 V15 (%) 65.37±16.89 64.71±17.19 63.12±18.32 0.114 0.333 0.059 V20 (%) 57.91±18.21 55.71±18.56 53.90±2011 0.028 0.093 0.285 V25 (%) 48.45±19.39 44.35±19.99 43.74±18.81 0.005 0.005 0.386 V30 (%) 40.01±19.54 36.60±17.87 34.85±16.17 0.005 0.013 0.093 V35 (%) 35.32±19.65 34.36±10.17 27.68±12.48 0.005 0.005 0.037 V40 (%) 26.34±12.33 20.89±9.27 20.22±8.79 0.005 0.005 0.093 V45 (%) 16.80±8.83 15.70±5.18 13.16±5.98 0.005 0.005 0.333
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
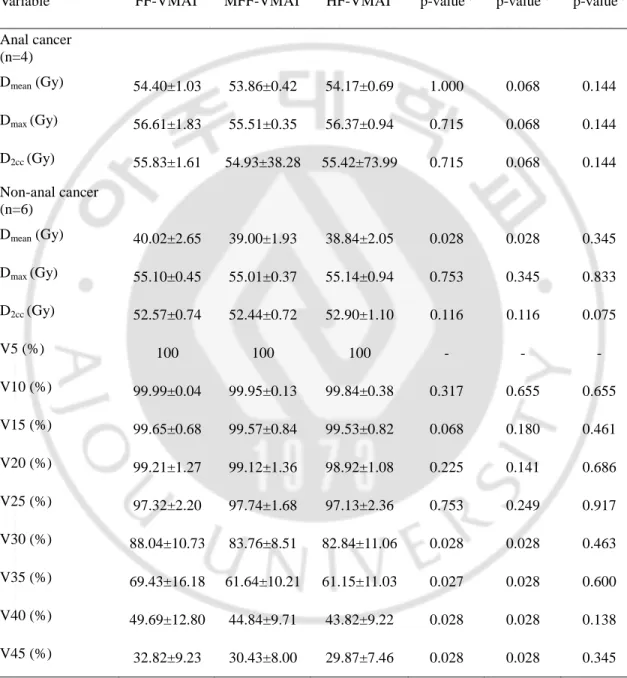
4. Rectum
Because rectum is included in the treatment volume of anal cancer, six patients except
anal cancer were analyzed.
Mean rectal dose of HF-VMAT plan was significantly lower
than FF-VMAT plan (38.84 vs 40.02, p<0.05). V30 and V40 to small bowel were also
significantly lower (V30: 82.84 vs 88.04, V40: 43.82 vs 49.69, p<0.05). HF-VMAT and
MFF-VMAT plans showed improved OAR compared to FF-VMAT plan, and it was
statistically significant differences at > 30Gy. However the difference was small. More
detailed DVH values are shown in table 6.
Table 6. Comparison of dose-volume parameters for rectum in VMAT plans using
three different techniques. The mean dose (D
mean), maximum dose (D
max), dose delivered
to 2cc of the most irradiated organ volume (D
2cc) were evaluated for rectum.
Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec) Anal cancer (n=4) Dmean (Gy) 54.40±1.03 53.86±0.42 54.17±0.69 1.000 0.068 0.144 Dmax (Gy) 56.61±1.83 55.51±0.35 56.37±0.94 0.715 0.068 0.144 D2cc (Gy) 55.83±1.61 54.93±38.28 55.42±73.99 0.715 0.068 0.144 Non-anal cancer (n=6) Dmean (Gy) 40.02±2.65 39.00±1.93 38.84±2.05 0.028 0.028 0.345 Dmax (Gy) 55.10±0.45 55.01±0.37 55.14±0.94 0.753 0.345 0.833 D2cc (Gy) 52.57±0.74 52.44±0.72 52.90±1.10 0.116 0.116 0.075 V5 (%) 100 100 100 - - - V10 (%) 99.99±0.04 99.95±0.13 99.84±0.38 0.317 0.655 0.655 V15 (%) 99.65±0.68 99.57±0.84 99.53±0.82 0.068 0.180 0.461 V20 (%) 99.21±1.27 99.12±1.36 98.92±1.08 0.225 0.141 0.686 V25 (%) 97.32±2.20 97.74±1.68 97.13±2.36 0.753 0.249 0.917 V30 (%) 88.04±10.73 83.76±8.51 82.84±11.06 0.028 0.028 0.463 V35 (%) 69.43±16.18 61.64±10.21 61.15±11.03 0.027 0.028 0.600 V40 (%) 49.69±12.80 44.84±9.71 43.82±9.22 0.028 0.028 0.138 V45 (%) 32.82±9.23 30.43±8.00 29.87±7.46 0.028 0.028 0.345
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
B.
Normal tissue complication probability
The NTCP values of small bowel, colon, bladder and rectum were compared according
to three VMAT plans. In small bowel and colon, HF-VMAT and MFF-VMAT plans
showed improved NTCP compared to FF-VMAT plan, and it was statistically significant.
HF-VMAT plan reduced the NTCP for small bowel toxicity from 12 - 13% to 8 - 9% than
FF-VMAT plan. And, colon toxicity was reduced from 3.2% to 1.8%. Although HF-VMAT
plan showed improved NTCP compared to MFF-VMAT plan, the difference was small.
More detailed NTCP values are shown in table 7.
Table 7. Evaluation of normal tissue complication probabilities and
equivalent-uniform dose (EUD) with statistical significance. The NTCP was evaluated using two
analytic models including Lyman-Kutcher-Burman (LKB) and log-logistic model.
Variable Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec)
Small bowel LKB (%) 12.81±4.24 10.25±3.69 8.98±2.42 0.007 0.005 0.047 Logistic(%) 11.99±4.27 9.41±3.65 8.12±2.38 0.007 0.005 0.047 EUD (Gy) 40.68±1.96 39.57±1.88 39.06±1.32 0.007 0.005 0.093 Colon LKB (%) 3.14±2.63 1.93±1.82 1.75±1.70 0.005 0.005 0.153 Logistic (%) 3.20±2.58 2.02±1.79 1.84±1.68 0.005 0.005 0.139 EUD (Gy) 38.37±3.20 37.07±3.08 36.87±3.02 0.005 0.005 0.203 Bladder LKB (%) 0.00 0.00 0.00 - - - Logistic(%) 0.00 0.00 0.00 - - - EUD (Gy) 39.15±2.20 37.81±2.24 34.77±3.49 0.005 0.013 0.005 Rectum LKB (%) 1.67±0.45 1.43±0.14 1.56±0.24 0.715 0.068 0.144 (anal cancer, n=4) Logistic(%) 2.52±0.55 2.24±0.18 2.40±0.31 0.715 0.068 0.144 EUD (Gy) 54.48±1.06 53.91±0.41 54.25±0.69 0.715 0.068 0.144 Rectum LKB (%) 0.16±0.06 0.15±0.04 0.15±0.05 0.116 0.028 0.345 (non-anal cancer, n=6) Logistic(%) 0.39±0.11 0.36±0.08 0.37±0.10 0.116 0.028 0.345 EUD (Gy) 45.55±1.29 45.20±1.12 45.55±1.29 0.116 0.028 0.345
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
C. Target coverage
HF-VMAT plan showed superiority compared with FF-VMAT plan in CN (0.90 vs 0.86,
p<0.05). The CN of MFF-VMAT plan was also increased than FF-VMAT (0.89 vs 0.86,
p<0.05). And, it was similar between HF-VMAT and MFF-VMAT (0.90 vs 0.89). There
was no statistically significant in the HI. The mean values and comparison are provided in
Table 8. And, the DVH of PTV to three VMAT plans is shown in figure 5.
Table 8. Mean conformity number (CN) and homogeneity index (HI) according to
three plans.
Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec) CN 0.86±0.01 0.89±0.02 0.90±0.03 0.007 0.160 0.005 HI 35.90±2.20 34.87±2.64 36.07±4.31 0.575 0.052 0.139
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
D. Modulation index
HF-VMAT and MFF-VMAT plans showed improved MI compared to FF-VMAT plan, it
showed a statistically significant differences. The MI values of FF-VMAT and HF-VMAT
plans were respectively 366.95 and 306.31, which was about 20% lower. The MI value of
MFF-VMAT plan was similar with HF-VMAT plan. The mean values and comparison are
provided in Table 9.
Table 9. Mean modulation index (MI) according to three plans.
Variable FF-VMAT MFF-VMAT HF-VMAT p-valuea) p-valueb) p-valuec)
MI (MCS) 366.95±92.28 307.88±74.11 306.31±62.95 0.017 0.005 0.959
Values are presented as mean ± standard deviation,
a)Comparison of FF-VMAT and
HF-VMAT,
b)Comparison of FF-VMAT and MFF-VMAT,
c)Comparison of MFF-VMAT and
HF-VMAT
IV. Discussion
The elective WPRT including pelvic lymph node irradiation is regarded as a standard
treatment regimen for intermediate or high-risk rectal and gynecological cancers. Although
the benefits of WPRT has been controversial for the locally advanced prostate cancer
patients (43), its role and gain have been reexamined with consideration of interaction
between RT and adjuvant androgen deprivation therapy (ADT) according to the duration
and timing of the hormone therapy and the field sizes of RT. Long-term outcomes were
suggested in terms of improved free from progression and biochemical failure in ADT plus
RT. In addition, recent results as per a new protocol were reported treatment gains in
short-term ADT plus pelvic lymph node irradiation using IMRT (44, 45).
WPRT can bring out clinical merits with other treatment modalities, or clever radiation
dose delivery techniques such as simultaneously integrated boost using a different dose
scheme can be useful (46-48). However, acute and late radiation-induced complications
have been significant concerns due to the use of a large size of the field (16-18). When
large and irregular irradiated field is required to cover target volumes and regional lymph
nodes, the radiation exposure of normal organ is inevitable. To achieve the target dose
coverage with normal organ sparing in large and irregular anatomical geometries, VMAT is
one of the useful dose delivery methods (21-23). Optimal fluence, which is created by
MLC segments at individual numerous beam angles going through the arc trajectories in
VMAT, can effectively reduce the radiation doses of normal organs.
The present study was conducted to overcome the limitation of MLC motion in wide
field VMAT plan. In order to compensate for MLC leakage and non-blocking phenomena,
HF-VMAT and MVMAT plans were designed and compared with conventional
FF-VMAT. Anal cancer and vaginal cancer patients were enrolled due the benefit of
HF-VMAT plan was expected to be more pronounced in WPRT patients with inguinal field.
Studies about HF-VMAT plan were already published, however they were very rare. Lai et
al. reported that HF-VMAT plan was more effective for normal organ saving and dose
distribution than FF-VMAT plan in breast cancer irradiation. HF-VMAT plan showed
improved dosimetry in ipsilateral lung and heart than FF-VMAT plan (27). There is also a
study that the HF-VMAT plan showed an improved dose distribution in radiotherapy of
scalp and lower neck node (49, 50). Previous studies have shown that RT field is large and
irregular, like breast cancer or head and neck cancer. In present study, we analyzed the
effect of VMAT plan in patients undergoing WPRT with inguinal node, and the
HF-VMAT plan showed some dosimetric benefit compared to FF-HF-VMAT plan. We also
compared MFF-VMAT plan with HF-VMAT and FF-VMAT plan in present study. The
MFF-VMAT plan is a plan that X field size is fixed at the maximum equipment size (14
cm), in order to optimize the MLC modulation. Most of the parameters were not as good as
HF-VMAT plan, but showed better results than FF-VMAT plan. This means that the
adjustment of irradiation field size is possible to improve the dose distribution by the
increasing of MLC modulation.
The small bowel requires careful treatment planning, because it is an organ that can
cause fatal side effects, such as perforation, even at low radiation dose (51, 52). Especially,
for gynecologic malignancies, the possibility of side effects may be increased because the
small bowel can come down to the empty space after hysterectomy. Even in older patients,
there is a high possibility that the small bowel may become deformed due to the elasticity
of the abdominal wall or muscle weakness. Ahamad et al. analyzed the effect of IMRT in
cervical cancer patient undergoing postoperative radiotherapy, and
significantly
less small bowel was irradiated by IMRT than conformal radiotherapy for doses greater
than 25 Gy (53).
For this reason, we focused on small bowel and compared various
dosimetric factors according to various plans. As a result, we were able to confirm the
advantages of HF-VMAT plan in several parameters. The bladder and colon also showed
improved dosimetry and was statistically significant. The dosimetry of rectum did not
show any difference. It is probably due to the inclusion of anal cancer patients in enrolled
patients. Rectum is included in PTV of anal cancer. Except anal cancer patients, the
analysis showed improved dosimetry in HF-VMAT plan.
HF-VMAT plan is effective in reducing the radiation dose of normal organ. However it
is possible to impair the dose homogeneity of PTV. We evaluated this through CN and HI,
and there was no difference between the three plans. On the contrary, CN showed
improved PTV homogeneity in HF-VMAT plan than FF-VMAT plan and it was
statistically significant. Finally, HF-VMAT plan successfully reduced the normal organ
dose without compromising the PTV dose homogeneity compared to FF-VMAT plan. We
anticipated that the optimization of MLC movement enabled it, and MI was analyzed to
access it. The concept of MI has already been applied to evaluate mechanical uncertainty
in several previous studies on the VMAT plan (54, 55). In present study, we tried to
analyze the values of MI using MCS model, which reflects MLC motion and PTV shape.
HF-VMAT plan showed improved MI than FF-VMAT plan, and MFF-VMAT plan also
showed about 20% improved MI value than FF-VMAT plan. This implies that the
optimization of MLC modulation affects the dose distribution of VMAT plan.
This study was conducted to improve the dose distribution when the irradiated field is
large. However, not only the size of the irradiated field but also the shape is important
factors. We conducted a preliminary study on WPRT without inguinal lymph node chain
and obtained unsatisfactory results. In the WPRT without the inguinal lymph node, there
was no effect of HF-VMAT plan. Like the WPRT containing the inguinal lymph node
chain, the more irregular the irradiated field, the more effective HF-VMAT plan is.
Although there are rare studies about HF-VMAT plan, the studies were conducted on head
and neck cancer and breast cancer with supraclavicular lymph node (27, 28). This implies
that the shape of the irradiated field is an important factor.
HF-VMAT plan showed the improved dosimetric impact of normal organ, and the
homogeneity of target volume was comparable with FF-VMAT plan.
HF-VMAT plan can
be more useful option than conventional FF-VMAT plan in patient with large and irregular
irradiation field, such as WPRT with inguinal lymph node chains.
The shape of irradiated
field as well as size is considered as an important factor, therefore the further study about
suitable cases for HF-VMAT plan are required.
References
1. Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(5):872-80.
2. Castaneda SA, Romak LB. Radiotherapy for Anal Cancer: Intensity-Modulated Radiotherapy and Future Directions. Surgical oncology clinics of North America. 2017;26(3):467-75.
3. Chang JH, Jang WI, Kim YB, Kim JH, Kim YS, Kim YS, et al. Definitive treatment of primary vaginal cancer with radiotherapy: multi-institutional retrospective study of the Korean Radiation Oncology Group (KROG 12-09). Journal of gynecologic oncology. 2016;27(2):e17. 4. Nicholas S, Chen L, Choflet A, Fader A, Guss Z, Hazell S, et al. Pelvic Radiation and Normal Tissue Toxicity. Semin Radiat Oncol. 2017;27(4):358-69.
5. Ray A, Sarkar B. Small bowel toxicity in pelvic radiotherapy for postoperative gynecological cancer: comparison between conformal radiotherapy and intensity modulated radiotherapy. Asia Pac J Clin Oncol. 2013;9(3):280-4.
6. Cheung CP, Chiu HS, Chung CH. Small bowel perforation after radiotherapy for cervical carcinoma. Hong Kong Med J. 2003;9(6):461-3.
7. Matsuura S, Royba E, Akutsu SN, Yanagihara H, Ochiai H, Kudo Y, et al. Analysis of individual differences in radiosensitivity using genome editing. Annals of the ICRP. 2016;45(1 Suppl):290-6.
8. Schuster B, Ellmann A, Mayo T, Auer J, Haas M, Hecht M, et al. Rate of individuals with clearly increased radiosensitivity rise with age both in healthy individuals and in cancer patients. BMC geriatrics. 2018;18(1):105.
9. Hong TS, Moughan J, Garofalo MC, Bendell J, Berger AC, Oldenburg NBE, et al. NRG Oncology Radiation Therapy Oncology Group 0822: A Phase 2 Study of Preoperative Chemoradiation Therapy Using Intensity Modulated Radiation Therapy in Combination With Capecitabine and Oxaliplatin for Patients With Locally Advanced Rectal Cancer. Int J Radiat Oncol. 2015;93(1):29-36.
Endometrial Carcinoma: Radiation Therapy Oncology Group Trial 0418. Int J Radiat Oncol. 2012;84(1):E23-E8.
11. Mabuchi S, Isohashi F, Yokoi T, Takemura M, Yoshino K, Shiki Y, et al. A phase II study of postoperative concurrent carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy followed by consolidation chemotherapy in surgically treated cervical cancer patients with positive pelvic lymph nodes. Gynecol Oncol. 2016;141(2):240-6.
12. Holliday EB, Lester SC, Harmsen WS, Eng C, Haddock MG, Krishnan S, et al. Extended-Field Chemoradiation Therapy for Definitive Treatment of Anal Canal Squamous Cell Carcinoma Involving the Para-Aortic Lymph Nodes. Int J Radiat Oncol. 2018;102(1):102-8.
13. Lestrade L, Zilli T, Kountouri M, Jumeau R, Matzinger O, Bourhis J, et al. Early-stage Favourable Anal Cancer: A Retrospective Analysis of Clinical Outcomes of a Moderately Low Dose Elective Nodal Intensity-modulated Radiotherapy Schedule. Clin Oncol-Uk. 2017;29(7):E105-E9. 14. Magli A, Moretti E, Tullio A, Giannarini G, Tonetto F, Urpis M, et al. Hypofractionated simultaneous integrated boost (IMRT-SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer P D. 2018;21(2):269-76.
15. Lin YW, Lin LC, Lin KL. The early result of whole pelvic radiotherapy and stereotactic body radiotherapy boost for high-risk localized prostate cancer. Frontiers in oncology. 2014;4:278. 16. Roach M, 3rd, DeSilvio M, Valicenti R, Grignon D, Asbell SO, Lawton C, et al. Whole-pelvis, "mini-Whole-pelvis," or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. International journal of radiation oncology, biology, physics. 2006;66(3):647-53.
17. Roeske JC, Bonta D, Mell LK, Lujan AE, Mundt AJ. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2003;69(2):201-7.
18. Yang TJ, Oh JH, Son CH, Apte A, Deasy JO, Wu A, et al. Predictors of acute gastrointestinal toxicity during pelvic chemoradiotherapy in patients with rectal cancer. Gastrointestinal cancer research : GCR. 2013;6(5-6):129-36.
19. Kang HJ, Kay CS, Son SH, Kim M, Jo IY, Lee SJ, et al. Hypofractionated intensity-modulated radiotherapy in patients with localized prostate cancer: a preliminary study. Radiat Oncol J. 2016;34(1):45-51.
20. Yang R, Wang J, Xu F, Li H, Zhang X. Feasibility study of volumetric modulated arc therapy with constant dose rate for endometrial cancer. Med Dosim. 2013;38(3):351-5.
21. Ishii K, Ogino R, Hosokawa Y, Fujioka C, Okada W, Nakahara R, et al. Whole-pelvic volumetric-modulated arc therapy for high-risk prostate cancer: treatment planning and acute toxicity. Journal of radiation research. 2015;56(1):141-50.
22. Wong E, D'Souza DP, Chen JZ, Lock M, Rodrigues G, Coad T, et al. Intensity-modulated arc therapy for treatment of high-risk endometrial malignancies. International journal of radiation oncology, biology, physics. 2005;61(3):830-41.
23. Ishii K, Ogino R, Hosokawa Y, Fujioka C, Okada W, Nakahara R, et al. Comparison of dosimetric parameters and acute toxicity after whole-pelvic vs prostate-only volumetric-modulated arc therapy with daily image guidance for prostate cancer. The British journal of radiology. 2016;89(1062):20150930.
24. Huang B, Fang Z, Huang Y, Lin P, Chen Z. A dosimetric analysis of volumetric-modulated arc radiotherapy with jaw width restriction vs 7 field intensity-modulated radiotherapy for definitive treatment of cervical cancer. The British journal of radiology. 2014;87(1039):20140183.
25. Rossi M, Boman E, Skytta T, Kapanen M. A novel arc geometry setting for pelvic radiotherapy with extensive nodal involvement. Journal of applied clinical medical physics. 2016;17(4):73-85.
26. Zhang WZ, Lu JY, Chen JZ, Zhai TT, Huang BT, Li DR, et al. A Dosimetric Study of Using Fixed-Jaw Volumetric Modulated Arc Therapy for the Treatment of Nasopharyngeal Carcinoma with Cervical Lymph Node Metastasis. Plos One. 2016;11(5).
27. Lai Y, Chen Y, Wu S, Shi L, Fu L, Ha H, et al. Modified Volumetric Modulated Arc Therapy in Left Sided Breast Cancer After Radical Mastectomy With Flattening Filter Free Versus Flattened Beams. Medicine. 2016;95(14):e3295.
28. Casey KE, Wong PF, Tung SS. Effect of interfractional shoulder motion on low neck nodal targets for patients treated using volumetric-modulated arc therapy (VMAT). Journal of applied clinical medical physics. 2015;16(4):40-51.
29. Benson AB, 3rd, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Anal Carcinoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(7):852-71.
30. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(1):64-84.
31. Small W, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic
2008;71(2):428-34.
32. Gay HA, Barthold HJ, O'Meara E, Bosch WR, El Naqa I, Al-Lozi R, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. International journal of radiation oncology, biology, physics. 2012;83(3):e353-62. 33. Myerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. International journal of radiation oncology, biology, physics. 2009;74(3):824-30.
34. McNiven AL, Sharpe MB, Purdie TG. A new metric for assessing IMRT modulation complexity and plan deliverability. Medical physics. 2010;37(2):505-15.
35. Moiseenko V, Song WY, Mell LK, Bhandare N. A comparison of dose-response characteristics of four NTCP models using outcomes of radiation-induced optic neuropathy and retinopathy. Radiat Oncol. 2011;6.
36. Dasu A, Toma-Dasu I, Olofsson J, Karlsson M. The use of risk estimation models for the induction of secondary cancers following radiotherapy. Acta Oncol. 2005;44(4):339-47.
37. Fowler JF. The radiobiology of prostate cancer including new aspects of fractionatedradiotherapy. Acta Oncol. 2005;44(3):265-76.
38. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol. 2004;60(4):1013-5. 39. Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation Dose-Volume Effects in Radiation-Induced Rectal Injury. Int J Radiat Oncol. 2010;76(3):S123-S9.
40. Luxton G, Keall PJ, King CR. A new formula for normal tissue complication probability (NTCP) as a function of equivalent uniform dose (EUD). Phys Med Biol. 2008;53(1):23-36. 41. Feuvret L, Noel G, Mazeron JJ, Bey P. Conformity index: a review. International journal of radiation oncology, biology, physics. 2006;64(2):333-42.
42. Yoon M, Park SY, Shin D, Lee SB, Pyo HR, Kim DY, et al. A new homogeneity index based on statistical analysis of the dose-volume histogram. Journal of applied clinical medical physics. 2007;8(2):9-17.
43. Dirix P, Haustermans K, Junius S, Withers R, Oyen R, Van Poppel H. The role of whole pelvic radiotherapy in locally advanced prostate cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2006;79(1):1-14.
44. Lawton CA, DeSilvio M, Roach M, 3rd, Uhl V, Kirsch R, Seider M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. International journal of radiation oncology, biology, physics.
2007;69(3):646-55.
45. Roach M, Moughan J, Lawton CAF, Dicker AP, Zeitzer KL, Gore EM, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. The Lancet Oncology. 2018;19(11):1504-15.
46. Mabuchi S, Isohashi F, Yokoi T, Takemura M, Yoshino K, Shiki Y, et al. A phase II study of postoperative concurrent carboplatin and paclitaxel combined with intensity-modulated pelvic radiotherapy followed by consolidation chemotherapy in surgically treated cervical cancer patients with positive pelvic lymph nodes. Gynecologic oncology. 2016;141(2):240-6.
47. Holliday EB, Lester SC, Harmsen WS, Eng C, Haddock MG, Krishnan S, et al. Extended-Field Chemoradiation Therapy for Definitive Treatment of Anal Canal Squamous Cell Carcinoma Involving the Para-Aortic Lymph Nodes. International journal of radiation oncology, biology, physics. 2018;102(1):102-8.
48. Lestrade L, Zilli T, Kountouri M, Jumeau R, Matzinger O, Bourhis J, et al. Early-stage Favourable Anal Cancer: A Retrospective Analysis of Clinical Outcomes of a Moderately Low Dose Elective Nodal Intensity-modulated Radiotherapy Schedule. Clinical oncology. 2017;29(7):e105-e9. 49. Lai Y, Shi L, Lin Q, Fu L, Ha H. Planning study of flattening filter free beams for volumetric modulated arc therapy in squamous cell carcinoma of the scalp. Plos One. 2014;9(12):e114953.
50. Casey KE, Wong PF, Tung SS. Effect of interfractional shoulder motion on low neck nodal targets for patients treated using volumetric-modulated arc therapy (VMAT). Journal of applied clinical medical physics. 2015;16(4):40-51.
51. Yamashita H, Nakagawa K, Tago M, Igaki H, Shiraishi K, Nakamura N, et al. Small bowel perforation without tumor recurrence after radiotherapy for cervical carcinoma: report of seven cases. J Obstet Gynaecol Res. 2006;32(2):235-42.
52. Pietrzak L, Sopylo R, Bujko K. Acute small bowel perforation following preoperative radiotherapy and abdominoperineal resection: a case report. Acta Oncol. 2006;45(3):344-5.
53. Ahamad A, D'Souza W, Salehpour M, Iyer R, Tucker SL, Jhingran A, et al. Intensity-modulated radiation therapy after hysterectomy: comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. International journal of radiation oncology, biology, physics. 2005;62(4):1117-24.
54. Park JM, Park SY, Kim H. Modulation index for VMAT considering both mechanical and dose calculation uncertainties. Phys Med Biol. 2015;60(18):7101-25.
evaluate the degree of modulation for volumetric modulated arc therapy. Medical physics. 2014;41(11).