저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
醫學 碩士學位 論文
Bone Marrow-Derived Buffy Coat Can
Supplement the Bone Marrow Stimulation
Technique for Articular Cartilage Repair
By
Long Hao Jin
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Bone Marrow-Derived Buffy Coat Can
Supplement the Bone Marrow Stimulation
Technique for Articular Cartilage Repair
by
Long Hao jin
A Dissertation Submitted to The Graduate School of Ajou
University in Partial Fulfillment of the Requirements for the Degree
of
MASTER IN MEDICINE
Supervised by
Byoung-Hyun Min, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
B
one Marrow-Derived Buffy Coat Can Supplement the Bone
Marrow Stimulation Technique for Articular Cartilage Repair
This certifies that the dissertation of
Long Hao Jin
is approved.
SUPERVISORY COMMITTEE
Approved by
: (Sign)
Byoung-Hyun Min, Thesis Adviser, Professor
Department of Orthopedic Surgery, School of Medicine, Ajou University
(Sign)
Ye-Yeon Won, Professor
Department of Orthopedic Surgery, School of Medicine, Ajou University
(Sign)
Kyeong-Jin Han, Professor
Department of Orthopedic Surgery, School of Medicine, Ajou University
The Graduate School, Ajou University
December, 22th, 2008
i
-
ABSTRACT
Bone Marrow-Derived Buffy Coat Can Supplement the Bone
Marrow Stimulation Technique for Articular Cartilage Repair
Bone marrow stimulation (BMS) has been used as a popular operating technique to repair defect of knee articular cartilage. However, the regenerated tissue from BMS is known as a fibrocartilage rather than a hyaline cartilage and thinner than native cartilage. The objective of this study was to investigate the feasibility of autologous bone marrow-derived buffy coat transplantation in the regeneration of large full-thickness cartilage defect.Rabbits
were divided into four groups:the defect was remained untreated as a negative control
(Group 1), performed with BMS only using a drill (diameter: 5 mm) and a 17 gauge needle (Group 2), injected with concentrated autologous buffy coat isolated from the iliac crest after BMS (Group 3) and implanted with autologous osteochondral graft (AOG) as a positive control (Group 4). The cartilage defect was finally covered with a thin membrane made of cartilage matrix material using cross suture method in groups 1, 2 and 3. Bone marrow aspirated from iliac crest was diluted with phosphate-buffered saline. Buffy coat was isolated by centrifugation through a Ficoll density gradient at 2000 rpm for 30 min. The repaired cartilages in each experimental group were evaluated by macroscopic observation, histology with Safranin-O staining, immunohistochemistry for collagen type IIand ICRS scoring at 4 and 8 weeks post-operation. In the results of gross observation and Safanin-O staining, the fibrous/hyaline cartilage was regenerated in groups 1 and 2, while the hyaline cartilage tissues with mature matrix and columnar organization of chondrocytes were observed in groups 3 and 4. In the immunohistochemical staining, expression of type II collagen was gradually increased along with time at the pericellular region in the repaired tissues of groups 2, 3 and 4. In the total ICRS, histological score increased significantly along with time in all groups. The ICRS score was higher in groups 3 and 4 than in other groups. This
ii
study demonstrated that injection of autologous bone marrow buffy coat after BMS effectively regenerated cartilage defect in rabbit model, being more effective than the BMS alone and similar to AOG. It is speculated that the buffy coat could probably provide more mesenchymal stem cells to regenerate the articular cartilage defect. In addition the extracellular matrix membrane used to cover the defect efficiently prevented leakage of injected cells and blood clots from cartilage defect into the knee joint cavity. In conclusion, the injection of autologous bone marrow-derived buffy coat in case of BMS arthroplasty could be a useful clinical protocol for cartilage repair.
Key Words: Cartilage, Regeneration, Bone marrow stimulation, Autologous buffy coat
iii
TABLE OF CONTENTS
ABSTRACT...
ⅰ TABLE OF CONTENTS...
ⅲ LIST OF FIGURES...
ⅳ LIST OF TABLES...
ⅴ LIST OF ABBREVIATIONS...
ⅵ Ⅰ. INTRODUCTION...
1Ⅱ. MATERIALS AND METHODS
...
4A. Experimental design and surgery
...
4B. Macroscopic and histological evaluation
...
7C. Histological scoring (ICRS score)
...
7D. Chemical assay of repaired tissue
...
9Ⅲ. RESULTS
...
101. Colony-forming unit-fibroblasts (CFU-Fs) assay
...
102. Gross finding of cartilage defects
...
133. Expression of type II collagens
...
174. Sirius red staining
···
195. Chemical assay of repaired tissue
···
21Ⅳ. DISCUSSION
···
24Ⅴ. CONCLUSION
···
28REFERENCES
···
28iv
LIST OF FIGURES
Figure. 1. Procedure for the implantation of autologous buffy coat ··· 6
Figure. 2. Number of MSCs colony ··· 11
Figure. 3. Gross finding of cartilage defects ··· 14
Figure. 4. Histological Safanin-O staining ··· 15
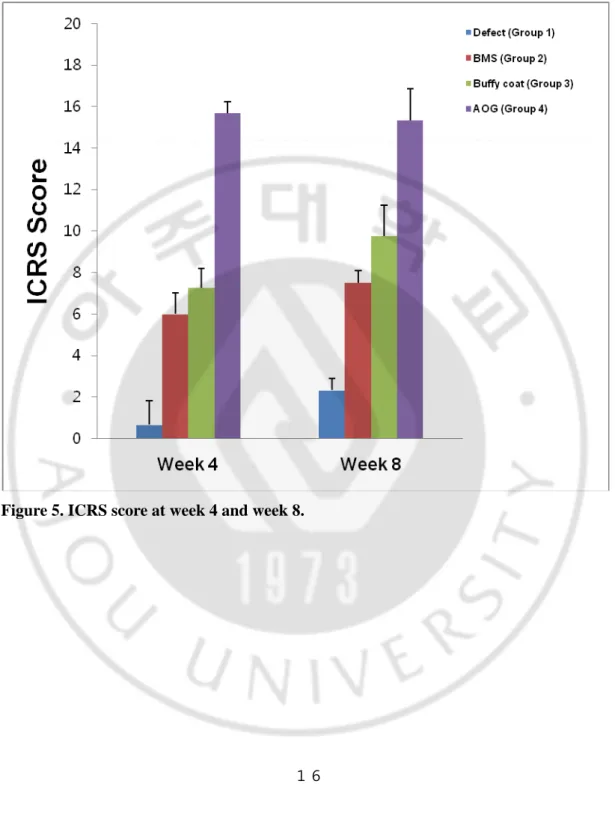
Figure. 5. ICRS score at week 4 and week 8 ··· 16
Figure. 6. The immunohistochemistry for collagen type II expression ··· 18
Figure. 7. Sirius red staining ··· 20
Figure. 8. Changes and comparison of GAG Contents among the experimental groups along with time ··· 22
v
LIST OF TABLES
Table 1. Histological scoring system ··· 8
vi
LIST OF ABBREVIATIONS
ACT: Autologous chondrocyte transplantation
AOG: Autologous osteochondral graft
BMS: Bone marrow stimulation
CFU-F: Colony-forming unit-fibroblasts
CPM: Continuous passive motion
ECM: Extracellular matrix
HSC: Haematopoietic stem cell
MSC: Mesenchymal stem cell
1
Ⅰ. INTRODUCTION
Articular cartilage is a resilient load-bearing tissue that forms the articulating surfaces of diarthrodial joints and provides the low friction, lubrication, and wears characteristics required for repetitive gliding motion. It also absorbs mechanical shock and spreads the applied load onto subchondral bone. Human articular cartilage provides these essential biomechanical functions mostly for 8 decades or more. However, it has restricted self-healing potential. The reason for this low repair potential has been unknown, but articular cartilage has neither vascular supply nor easy access to stem cells (Buckwalter and Mankin 1997; Hunziker 2002; Prakash et al. 2002), and low cell mobility due to surrounding matrix, and a limited number of progenitor cells could be contributing factors (Tallheden et al. 2003). Until now much effort has been made to repair cartilage defects. For example, autologous chondrocyte transplantation, marrow-stimulation procedures like subchondral bone microfracture and AOG have been developed to restore normal structure and function of cartilage (Chang et al. 2008). But, the reconstruction of hyaline cartilage and subchondral bone has not been fully realized (Kuo et al. 2006). Autologous chondrocytes have significant limitations as a cell source for cartilage regeneration, such as the small number of cells available and their restricted proliferation capacity (Heng et al. 2004), Moreover they tend to dedifferentiate and gradually lose their specific phenotypes during monolayer in vitro culture (Tallheden et al. 2005). In the case of AOG, multiple osteochondral grafts are removed from non-weight bearing area of the joint surface and implanted into the defect area. However, the osteochondral grafts may exhibit fibrillation and degradation with time (Horas et al. 2003). Furthermore, the size of the defect that can be repaired is still limited. In the case of large articular cartilage defects by osteoarthritis (OA) or rheumatoid arthritis (RA), for example, AOG cannot be an effective repair method. Total joint replacement, osteotomy, or arthrodesis can be performed for elderly patients with severely damaged joints but, there is no available treatment for mild injuries in young patients. Among these surgery techniques, BMS technique has been widely used for the regeneration of articular cartilage defect, because it could provide a suitable environment for new tissue formation and it might utilize the natural
2
healing power of the living body. BMS can facilitate the intrinsic healing response of MSCs or progenitor cells recruited by subchondral penetration to the bone marrow and can stimulate cartilage regeneration. But it was shown to regenerate fibrocartilage having poor mechanical properties when compared with the desired hyaline cartilage. And also the thickness of repaired tissue after BMS was usually thinner than that of the native articular cartilage (Kang et al. 2008). The reasons of these phenomena are known as excessive loading condition and low density of MSCs caused by loss of bone marrow and blood clots from the BMS region to the joint cavity. Until now, many studies have been conducted to overcome these weaknesses. For example, Howard et al (2000) covered the cartilage defect with collagen film after a microfracture to prevent the induced bone marrow flowing into the joint cavity. As a result, the cartilage regeneration was better in the group that was covered than in the group of uncovered microfracture. Taner et al (2006) covered the defective cartilage area with autologous periosteum membrane after a microfracture, and then, fixed the leg with an assistant device to reduce overloading.
It is well known that bone marrow contains multipotent stem cells that can differentiate not only into haematopoietic cells (including B lymphocytes, T lymphocytes, neutrophils and macrophages) but also into nonhaematopoietic cells (including endothelial cells, pericytes, chondrocytes, osteoblasts, adipocytes, neural component cells, fibroblasts and probably keratinocytes). Azizi et al (1998) reported consequently, many investigators treating various intractable diseases (including ischaemic myocardial diseases, osteogenesis imperfecta, Duchenne’s muscular dystrophy, and central nervous system disorders such as Parkinson’s disease) are focusing on the bone marrow stem cells. Many researchers reported the availability of bone marrow derived mononuclear cells for regeneration of several tissues. Karatoprak et al (2008) showed the treatment of the nontraumatic avascular necrosis of the femoral head with bone marrow derived mononuclear cells, Yip et al (2008) used them to treat infarction of the myocardium, Chang et al (2007) also showed the good effect on cartilage regeneration using them and Viqqeswarapu et al (2001) applied them to a continuous spinal fusion mass. Mesenchymal stem cells (MSCs), also known in the literature as bone marrow stem cells, skeletal stem cells, and multipotent mesenchymal stromal cells, are nonhaematopoietic progenitor cells isolated from adult tissues. Chen et al (2008) reported
3
that MSCs could be characterized in vitro by their extensive proliferation ability and the potential to differentiate along various lineages of mesenchymal origin including chondrocyte, adipocyte, and osteoblast lineages in appropriate environments.
In an attempt to find a safe and effective method to repair articular cartilage defect, this study examined the feasibility of BMS plus transplantation of autologous uncultured bone marrow cells from buffy coat in a full-thickness articular cartilage defect model. In addition, extracellular matrix (ECM) membrane was used to prevent leakage of injected cells and blood clots from cartilage defect into the knee joint cavity. It is expected that transplantation of buffy coat will provide large number of bone marrow stem cells and other biochemical factors and thereby significantly enhance regeneration of cartilage defect.
4
Ⅱ. MATERIALS AND METHODS
A. Experimental design and surgery
The experimental protocol using animals was approved by the Institutional Animal Experiment Committee of Ajou University. New Zealand white rabbits were used in this study with average weight of 3.0~3.5 kg. The surgical procedures were performed under
general anesthesia with a mixture (0.2 ml/kg body weight) of Zoletil (50 mg/ml; Virbac
Laoratoires-06516 Carros, France) and xylazine hydrochloride (Rompun; Bayer, Ansan,
Korea) (Zoletil: Lompun=1:2), including limb preparation and draping. Both knee joints
were operated in the same surgery. An arthrotomy was made through a midline longitudinal incision on a medial parapatellar with the patella dislocated laterally to expose the femoral condyles. A 5 mm drill in diameter was used to create an osteochondral defect of 2 mm in depth at the patella groove (Jin et al. 2007). BMS arthroplasty was performed using 5 mm drill and 17 gauge needles.
To measure the amount of MSCs in isolated bone marrow from iliac crest or blood clots after BMS on the cartilage defect. CFU-F analysis was performed. Briefly, 4 ml and 10~20 ul of bone marrow were harvested from iliac crest and cartilage defect after BMS, respectively. The samples were diluted in PBS and buffy coat was isolated by centrifugation using a Ficoll density gradient (Amersham Biosciences, 17-1440-02, SWEDEN) at 2000 rpm
for 30 min. Isolated buffy coat was suspended in MSCs culture medium consisting α–MEM
supplemented with 10% FBS, and plated in 6 well culture dishes. And also bone marrow harvested from blood clots made by BMS was suspended in the same manner. After 6 days, non-adherent cells were discarded and the adherent cells were replenished with fresh medium after washing. The cells were then cultured for about 12 days with the culture medium replaced every 3 days, and stained with 5% crystal violet solution in 100% methanol for 10 min for CFU-F assay. Colonies greater than 2 mm in diameter were counted.
The defect was remained untreated as negative control (Group 1), performed with BMS only using 5 mm drill and 17 gauge needle (Group 2), injected with concentrated autologous
5
buffy coat isolated from the iliac crest after BMS arthroplasty (Group 3) and implanted with AOG as positive control (Group 4). The cartilage defect was finally covered with a thin membrane made of cartilage matrix material using cross suture method in groups 1, 2 and 3. After surgery, knee joint was fixed with assisting device. The repair of cartilage was evaluated by macroscopic, histological, immunohistochemical and biochemical analysis for repaired tissue at week 4 and 8 post-operation.
6
7
B. Macroscopic and histological evaluations
At 4 and 8 weeks after surgery, the rabbits were euthanized by over-dose injection of pentobarbital to retrieve the femoral condyles. The macroscopic images of the condyles were first observed, and the samples were paraffin-embedded, sectioned, and processed for routine safranin-O, sirius red staining and immunohistochemical analysis. For immunohistochemical analysis, the sections were incubated with mouse monoclonal anti-rabbit collagen type II antibody (Chemicon, Temecula, CA) at 1:200 dilution for 1 hr at room temperature. The sections were then incubated sequentially with biotinylated secondary antibody at 1:200 dilution for 1 hr and peroxidase-conjugated streptavidin solution for 30 min at room temperature (DAKO LSAB System, Carpinteria, CA). Counterstained with Mayer’s hematoxylin (Sigma, St Louis, MO), the sections were mounted with a mount solution before microscopic observation (Nikon E600, Japan) (Cui and Min. 2007; Jin et al. 2007).
C. Histological scoring (ICRS score)
To evaluate the quality of the repaired articular cartilage in the defects, the modified version of the histological grading scale was used as shown in the table 1. The scale consists of seven categories and assigns a score ranging from 0 to 18.
9
D. Chemical assay of repaired tissue
For the chemical assays of the repaired tissue, samples were harvested from the defect by surgical knife and culet. The harvested tissue was dried and digested in papain solution (5
mM L-cysteine, 100 mM Na2HPO4, pH 6.4, 5 mM EDTA and 125 μg/ml papain type III) at
60°C for 24 hrs and then centrifuged at 12,000 g for 10 min. In order to measure the glycosaminoglycan (GAG) contents, the supernatant was subjected to 1,9-Dimethylmethylene Blue (DMB) colorimetric assay. The DNA content was measured using Hoechst 33258 dye (0.2μl/ml) in the dark for 30 min at RT. After incubation, fluorescence was quantified using a 96 well plate reader (Excitation 350 nm, Emission 460 nm, Perkin Elmer LS-55) and concentration was determined against a standard curve made from mouse thymus DNA (Jin et al. 2007). In all assays, the normal cartilage from rabbit knee alone was used as a control. The GAG contents were determined using chondroitin sulfate from the shark cartilage (Sigma; St Louis, MO, USA) as a standard (Cui et al. 2007).
10
Ⅲ. RESULTS
1. Colony-forming unit-fibroblasts (CFU-Fs) assay
The crystal violet staining showed that there is no significant difference in the number of MSCs colony between same volumes of bone marrows isolated from blood clot located in the BMS region and iliac crest (Fig 1). From the CFU-Fs assay, the number of MSCs could be calculated in achieved original bone marrow volumes from each harvest region (Table 2).
11
12
13
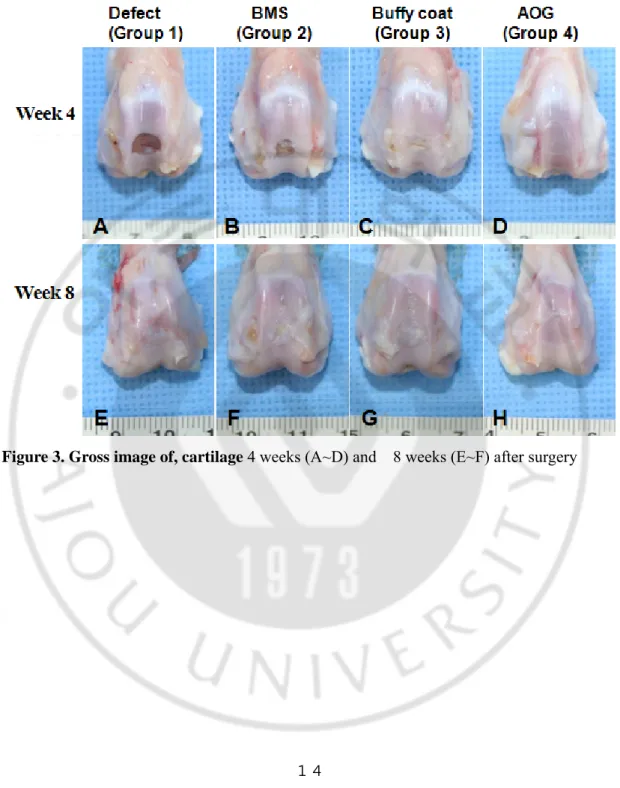
2. Gross finding of cartilage defects
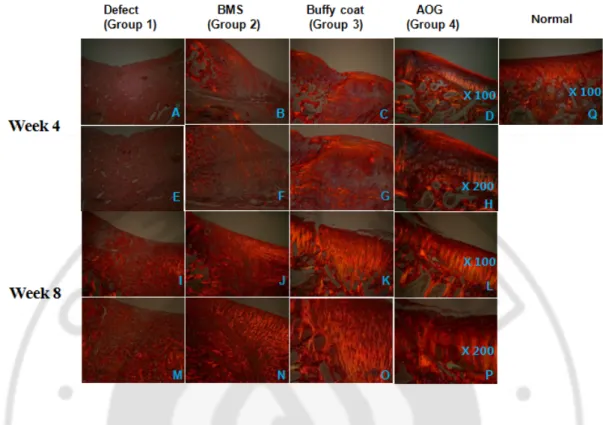
At week 4 after surgery, the repaired defects in groups 3 and 4 showed a smooth and glistening appearance and continuity with the surrounding host cartilage tissue. In contrast, the defect was not repaired well in group 1 and filled partially with fibrous tissues in group 2. At week 8 the white and glistening appearance of repaired tissues was shown on defects in all groups. In the Safranin-O staining, the defect was partially filled with fibrous tissues in groups 1 and 2 at week 4. In contrast, hyaline cartilage-like tissues were observed partially in group 3 and completely in group 4, respectively. At week 8, the fibrous/hyaline cartilage was regenerated in groups 1 and 2. In contrast, the hyaline cartilage tissues with mature matrix and columnar organization of chondrocytes were observed in groups 3 and 4. The total ICRS histological score increased significantly along with time in all groups. Among them, it was higher in groups 3 and 4 at week 4 and 8, respectively.
14
15
Figure 4. Safranin-O staining. 4 weeks (A~H) and 8 weeks (I~P) after surgery. An Safranin-O staining image of normal cartilage was also provided (Q). Magnification was at x20 (A~D, I~L), and x200 (E~H, M~P), respectively.
16
17
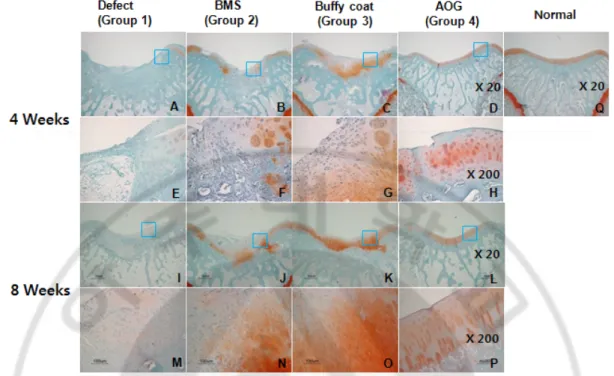
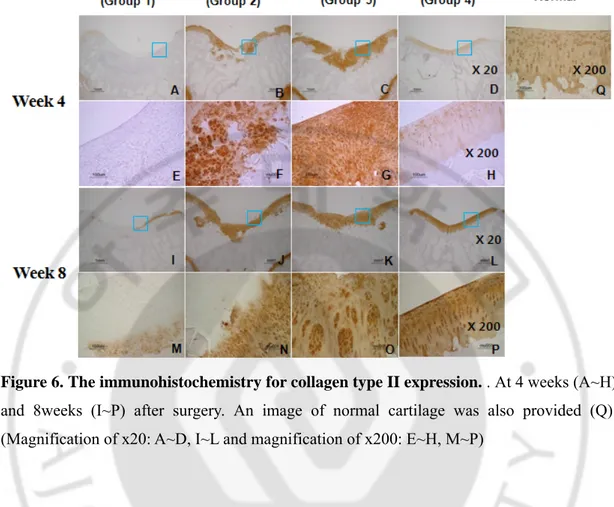
3. Expression of type II collagens
Expression of type II collagen was gradually increased along with time at the pericellular region in the repaired tissues of group 2, 3 and 4. It was not much detected in group 1 both at week 4 and 8. The most significant expression of type II collagen with dark brown color was observed in zonal-structure in groups 3 and 4 at 8 weeks.
18
Figure 6. The immunohistochemistry for collagen type II expression. . At 4 weeks (A~H) and 8weeks (I~P) after surgery. An image of normal cartilage was also provided (Q). (Magnification of x20: A~D, I~L and magnification of x200: E~H, M~P)
19
4. Sirius red staining
In the Sirius red staining, collagen fiber was found only in small amount in the samples of groups 1 and 2 at week 4 and no oriented pattern was observed. Collagen fibers were found in large amount in group 3. In the Group 4 (AOG group), oriented collagen fibers perpendicular to cartilage surface were found, but no integration was observed between implant cartilage tissue and host cartilage. At week 8 after surgery, the amount of collagen fibers increased in all groups, but oriented arrangement of collagen fibers not observed in groups 1 and 2. In the groups 3 and 4, well oriented arrangement of collagen fibers similar to normal cartilage were found at 8 weeks, but integration between implant tissue and host tissue was also not observed.
20
Figure 7. The sirius red staining image at 4 weeks (A~H) and 8 weeks (I~P) after surgery. An image of normal cartilage was also provided (Q). Magnification was at x100 (A~D, I~L) and x200 (E~H, M~P), respectively.
21
5. Chemical assay of repaired tissue
The results showed that contents of GAG were gradually increased along with time in the repaired tissues of all groups as shown in figure 5. Gag contents of groups 3 and 4 were higher than those of groups 1 and 2 but lower than those of normal cartilage at both of measuring time, week 4 and 8 after surgery (Fig 7). In all groups, DNA contents were increased along with time. The highest level of DNA contents were detected in the Group 3 (buffy coat group) (Fig 8).
22
Figure 8. Changes and comparison of GAG Contents among the experimental groups along with time.
23
24
Ⅳ. DISCUSSION
In the present study, the injection of autologous bone marrow buffy coat after BMS arthroplasty effectively regenerated cartilage defect in a rabbit model, being more effective than the BMS arthroplasty alone. It is speculated that the injected buffy coat could provide more MSCs to regenerate the defect and the ECM membrane covering the defect prevented injected cells and blood clots from leakage into the joint fluid.
Yip et al (2008) reported that bone marrow-derived MSCs have a lot of advantages for cell therapy, however, when bone marrow derived MSCs were injected into infarcted rat myocardium, single MSC could not differentiated completely into myocardia like cells. Also it was shown to have low viability, when injected after expansion in vitro , so could not find any clinical effect. In many studies, MSC and HSC are not used alone, but either mononuclear cells that include MSC and HSC, or a buffy coat is used as a cell source (Karatoprak et al 2008; Yip et al 2008; Chang et al 2007 and Viqqeswarapu et al 2001). Usually in vitro expended MSCs and HSCs are used for the regeneration of various tissues defects, however, mononuclear cells or buffy coat can be used immediately without culturing
in vitro nor with any risk. And, it is considered that T cell, B cell, macrophages and cytokines
secreted by platelets in buffy coat may have influence on the defect healing (Chang et al 2008).
The BMS is a most commonly used method for cartilage regeneration. But it was also shown to regenerate fibrocartilage with poor mechanical properties when compared with the desired hyaline cartilage. Besides, the thickness of repaired tissue after BMS was usually thinner than that of the native articular cartilage (Kang et al. 2008). It is believed that the reasons of these phenomena are excessive loading condition and low density of MSCs caused by loss of bone marrow and blood clots from the BMS region to the joint cavity. It is also considered as a reason of fibro-cartilage repair that bone marrow and blood clots could be washed away by the fluid of knee joint cavity from microfractured defect region. To overcome this problem, ECM membrane made by cultured chondrocytes was used in the previous study. It is known that ECM membrane not only could cover the cartilage defect for
25
preventing the loss of bone marrow, but also has high biocompatibility, biodegradable, and cartilage affinity (Jin et al. 2008). So it is considered as good biomaterials for cartilage regeneration.
Even though the group using ECM membranes showed better results than the other groups in the ICRS Score, it also showed big difference compared to the normal cartilage. We thought that the reason of this result was the low MSC concentration of the bone marrow that was flew out through the BMS, and therefore, the number of MSCs after the BMS was counted through the CFU-F assay. As a result, only approximately 20 MSC flowed out through the BMS (Table 2). In order to solve this problem, the autologous bone marrow derived buffy coat isolated from the iliac crest, is additionally injected into the damaged cartilage area after BMS. The buffy coat was separated from 4 ml of bone marrow, which is the maximum amount that can be harvested from the iliac crest safely at one time in our experience. There were approximately 2000 MSCs (Table 2) measured by CFU-F assay, additionally HSCs, lymphocytes, platelets (Chang et al 2008), and small amounts of serum were consist in the buffy coat. It is considered that these various cells and other materials will promote the chondrogenesis of MSCs and cartilage regeneration (Chang et al 2008).
In the results, buffy coat injected group showed higher amount of hyaline cartilage, better arrangement of the collagen fiber, and higher ICRS Score compared to the unused control group. Also the group with buffy coat showed higher contents of GAG and DNA compared to the group that had only BMS.
The osteochondral defect in 2~4 mm in depth was commonly used in rabbit cartilage regenerative model. The cartilage thickness of the patellar groove in rabbit is approximately 200 μm, which means that the cartilage defect does not have enough volume to transplant cells or scaffolds (Funayama 2008; Kang 2008). But having thick subchondral bone in patellar groove, it is approximately 4.5~5 mm in thickness, So that, in present study, the defect in 5 mm in diameter and 2 mm in depth was made for transplant the buffy coat. This is conducted by assuming that the defect volume would be approximately 40 μl, and when about 20 μl of buffy coat is injected, the remaining 20 μl of bone marrow will flow out due to BMS, and it will form blood clots and perform a role as scaffold. In the result of observing the re-grown tissue, at 8 weeks, the group that received buffy coat injection showed better
26
regeneration of subchondral bone and the hyaline cartilage at 8 weeks, especially.
Steinwachs et al (2008) reported various BMS techniques rely on the same biological principles of promoting resurfacing with the formation of fibro-cartilaginous tissue with lower biomechanical qualities. And the BMS technique can be applied in small isolated cartilage defects (1−3 cm²) in young and active patients. The osteochondral grafting method includes autografting and allografting. These surgical methods can be applied to focal cartilage defect or osteochondral defect, such as the defect of 1~4 cm2 in size and 1 cm in
deep. Since autologous chondrocyte transplantation (ACT) needs to be performed by isolating the chondrocyte from the healthy area of the cartilage and then culturing it in vitro, it can damage the normal cartilage. Also the surgery needs to be performed twice and cells can be contaminated during in vitro culture procedure.
In this study, we successfully regenerated the cartilage defects of 5 mm in diameter and 2 mm in depth in rabbits defect model. When the knee size ratio of humans to rabbit is
assumed to be 10:1, it can be converted into a diameter of 5 cmand a depth of 2 cm in a
human. Only a few animal studies have worked with large sized defect like our experiment (Howard et al. 2000; Chang et al. 2008). Although it was the large sized defect, it could be regenerated well in this study. Kelly et al. (2005) reported that high stress might harm to cartilage regeneration, particularly at the early stage. Salter et al. (1980) observed that in a model of an osteochondral defect, the high stress at the articular surface-induced fibrous tissue formation and inhibited chondrogenesis. Also Salter et al. (1984) clinically and experimentally found that continuous passive motion (CPM) of the synovial joint is more effective in repairing the cartilage and other tissues of the joint than intermittent motion or immobilization. Chang et al. (2008) reported that CPM increases the nutrition and metabolic activity of the articular cartilage and stimulates differentiation of MSCs to chondrocytes. Recently, the effects of mechanically controlled motion on cartilage regeneration have been investigated using in vivo models which demonstrated that appropriate mechanical stresses and motions contributed to cartilage regeneration. Therefore, in this study, a new assist device was used to fix the rabbit knee joint for 1 week after surgery. During the 1 week, rabbit knee joint assist device was removed for only 10 min/day and rabbits could be exercised under free loading condition on the defect region.
27
V. CONCLUSION
In this study, the feasibility of BMS plus autologous uncultured bone marrow cell transplantation using buffy coat isolation method was examined in case of full-thickness articular cartilage defect model. In the result of observing the re-grown tissue for 8 weeks, the group that received BMS and buffy coat injection showed better regeneration of subchondral bone and the hyaline cartilage at week 8.
From the results, I could come to the conclusion. BMS technique alone is not a suitable and sufficient method for the regeneration of osteochondral defect. The transplantation of buffy coat derived uncultured bone marrow cells can be a good candidate to repair articular cartilage defect. This additional treatment technique was relatively simple and safe as well.
28
REFERENCES
1. Azizi SA, Stokes D, Augelli BJ et al.: Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA 95:3908–13, 1998
2. Buckwalter JA, Mankin HJ: Articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am 79:612–632, 1997
3. Buckwalter JA, Mankin HJ: Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect 47:477-86, 1998
4. Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, Ogawa T, Ochiai N: Repair of Large Full-Thickness Articular Cartilage Defects by Transplantation of Autologous Uncultured Bone-Marrow-Derived Mononuclear Cells. J Orthop Res. 26(1):18-26,2008
5. Chen FH, Tuan RS: Mesenchymal stem cells in arthritic diseases. Arthritis Res
Ther. 10;10(5):223, 2008
6. Cui JH, Park SR, Park K, Choi BH, Min BH: Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 13(2): 351-60, 2007
7. Funayama A, Niki Y, Matsumoto H, Maeno S, Yatabe T, Morioka H, Yanagimoto S, Taguchi T, Tanaka J, Toyama Y: Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci. 13(3);225-32. Epub 2008 Jun 6. 2008
29
8. Heng BC, Cao T, Lee EH: Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cell 22(7):1152-67, 2004
9. Hunziker EB: Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10:432–463, 2002
10. Horas U, Pelinkovic D, Herr G, et al.: Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg 85-A: 185–192, 2003
11. Jin CZ, Park SR, Choi BH, Park k, Min BH; In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold. Artif Organs 31(3); 183-92, 2007 12. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH: Human amniotic
membrane as a delivery matrix for articular cartilage repair. Tissue Eng 13(4):693-702, 2007
13. Jin CZ, Min BH, Jin LH et al.: Cartilage regeneration with bone marrow stimulation technique(BST) using extracellular matrix(ECM) membrane in full thickness defect beagle model. Termis-AP 2008 P1-178/20, 2008
14. Kang SW, Bada LP, Kang CS, Lee JS, Kim CH, Park JH, Kim BS: Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett 30(3):435-9. Epub 2007 Oct 31, 2008
15. Kelly DJ, Prendergast PJ: Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J Biomech 38:1413–1422, 2005
30
16. Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M: Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. OsteoArthritis and Cartilage. 14(11):1126-35. 2006 17. Prakash D, Learmonth D. Natural progression of osteochondral defect in the
femoral condyle. Knee. 9:7–10, 2002
18. Salter RB, Hamilton HW, Wedge JH et al.: Clinical application of basic research on continuous passive motion for disorders and injuries of synovial joints: a preliminary report of a feasibility study. J Orthop Res 1:325–342, 1984
19. Salter RB, Simmonds DF, Malcolm BW, et al.: The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am 62:1232–1251, 1980 20. Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. Injury 39
Suppl 1:S26-31, 2008
21. Tallheden T, Dennis JE, Lennon DP, Sjögren-Jansson E, Caplan AI, Lindahl A: Phenotypic plasticity of human articular chondrocytes J Bone Joint Surg Am. 85-A Suppl 2:93-100, 2003
22. Tallheden T, Bengtsson C, Brantsing C, Sjögren-Jansson E, Carlsson L, Peterson L, Brittberg M, Lindahl A: Proliferation and differentiation potential of chondrocytes from osteoarthritic patients Arthritis Res Ther. 7(3):R560-8. Epub 2005 Mar 3. 2005
31
-
국문요약-
골수자극술의 관절연골 재생 증진을 위한 골수유래 Buffy coat
첨가 효과
아주대학교 대학원 의학과
김 룡 호
(지도교수: 민 병 현)
골수자극술은 무릎연골손상치료에서 가장 많이 이용되는 치료 방법이지만 손상부위가 초자연골 대신 내구성이 약한 섬유연골로 재생된다는 문제점을 가지고 있다. 이 연구의 목적은 골수자극술을 시행한 후 자가골수유래 buffy coat를 연골결손부위에 추가로 주입하여 buffy coat의 연골재생증진효과를 확인하는 것이다. 실험동물은 뉴질랜드 화이트 토끼를 사용했고 실험군은 아래와 같이 4군으로 나누었다. 대퇴골 활차부위에 직경이 5mm, 깊이가 2mm인 연골손상만 제작한 군을 음성대조군으로(그룹 1), 손상부위에 직경 5 mm 인 드릴날과 17G 바늘로 골수자극술을 시행한 군(그룹 2), 골수자극술 후 자가골수유래 buffy coat를 추가 주입한 군(그룹 3), 그리고 자가연골하골 이식군을 양성 대조군으로(그룹 4) 했다. 골수자극술 후 형성된 혈병과 주입한 buffy coat의 유실을 방지하기 위해 그룹 1, 2 와 3에는 얇은 세포외기질막으로 손상부위를 덮어주었다. 장골에서 채취한 자가골수유래 Buffy coat는 Ficoll gradient 분리 법으로 분리했다. 연골손상의 재생여부는 수술 후 4주와 8주 째 육안 및 조직학적 염색, 면역조직화학 염색과 ICRS 조직학적 평점을 이용하여 평가하였다. 결과를 살펴보면 육안과 조직학적 염색상 1, 2 그룹에서는 섬유연골조직이 많이 섞여 있는 반면에 3, 4 그룹에서는 대부분 초자연골로32 재생된 것을 관찰 할 수 있었다. 면역조직화학 염색상 3, 4 그룹에서는 1, 2 그룹보다 제2형 교원질이 현저히 많이 발현된 것을 관찰할 수 있었다. ICRS 조직학적 평점에서도 3, 4 그룹은 1, 2 그룹보다 높았으며 통계학적으로도 유의한 차이를 보였다. 본 실험을 통하여 골수자극술 후 자가골수유래 buffy coat를 추가 주입하고 얇은 세포외기질막을 덮어주므로 연골재생효과를 증진할 수 있음을 확인할 수 있었다. 이러한 연구 결과는 자가골수유래 buffy coat를 주입하여 보다 많은 중간엽줄기세포들 (MSCs)을 제공함과 동시에 그 분화능력을 향상시키고, 세포외기질막을 덮어줌으로서 주입된 자가골수유래 buffy coat와 형성된 혈병이 관절강 내로의 유실되는 것을 방지하여 연골재생 효과를 증진한 것이 원인으로 판단된다. 결론적으로 연골재생에 있어 골수자극술 후 자가골수유래 buffy coat을 주입하는 술법은 임상에서도 유용한 연골재생 치료법이 될 수 있을 것으로 기대된다. 핵심어: 연골재생, 골수자극술, 자가골수유래 buffy coat, 세포외기질막.