저작자표시-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이 저작물을 영리 목적으로 이용할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
The Effect of Lycii Radicis Cortex Extract
on Bone Formation in vitro and in vivo
by
Yilan Jin
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
The Effect of Lycii Radicis Cortex Extract
on Bone Formation in vitro and in vivo
by
Yilan Jin
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Master of Medicine
Supervised by
Seon-Yong Jeong, Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
-ABSTRACT -
The Effect of Lycii Radicis Cortex Extract on Bone Formation
in vitro and in vivo
Osteoporosis is a common skeletal disease. It is caused by decreased bone mass and enhances the risk of bone fracture. In this study, I aimed to discover novel herbal extract(s) for the treatment of osteoporosis.
I screened 64 ethanol extracts of edible plants native to Korea for their ability to increase the cellular proliferation and differentiation of two osteoblastic cell lines: C3H10T1/2 and MC3T3-E1. I selected a Lycii Radicis Cortex (LRC), Lycium Chinense root bark, as the primary candidate. Treatment with LRC extract showed enhanced alkaline phosphatase activity and an increased expression of bone metabolic markers Alpl, Runx2, and Bglap genes in both cell lines. There was no effect on the osteoclastic differentiation of primary-cultured monocytes from the mouse bone marrows.
Furthermore, the effect of LRC extract in vivo was examined in ovariectomized (OVX) mice. The OVX mice administered LRC extract for 8 weeks and 16 weeks, respectively, exhibited significantly increased bone mineral density (BMD) compared to the non-treated control OVX mice, and no toxic effects were observed. My results indicated that LRC extract reduced the OVX-induced BMD loss in mice via promoting the differentiation of osteoblast linage cells. It also suggested that LRC extract may be a good candidate for the
ii
treatment of osteoporosis with few side effects. This is the first report demonstrating the effect of LRC extract on bone formation in vitro and in vivo.
__________________________________________________________________________
Keywords: herbal extract, Lycii Radicis Cortex, osteoporosis, osteoblast, ovariectomized
TABLE OF CONTENTS
ABSTRACT ··· i
TABLE OF CONTANTS ··· iii
LIST OF FIGURES ··· v
LIST OF TABLES ··· vi
I. INTRODUCTION ··· 1
II. MATERIALS AND METHODS ··· 5
1. Herbal extracts and materials ··· 5
2. Cell culture ··· 5
3. Water-soluble tetrazolium salt (WST) assay and alkaline phosphatase (ALP) assay ··· 6
4. In vitro generation of osteoclasts and tartrate-resistant acid phosphatase (TRAP) assay ··· 6
5. Quantitative reverse-transcription PCR (qRT-PCR) ··· 7
6. In vivo experiment ··· 8
7. Measurement of whole body fat (%) and right femur BMD and BMC ··· 9
8. Statistical analysis ··· 9
III. RESULTS ··· 10
1. Sixty-four plants native to Korea were screened for cellular proliferation and the differentiation of osteoblastic C3H10T1/2 and MC3T3-E1 cell lines ··· 10
iv
2. LRC extract increased cellular proliferation and differentiation of osteoblast cell
lines ··· 12
3. LRC extract increased mRNA expression of osteoblastic markers ··· 15
4. LRC extract inhibited BMD loss in ovariectomized (OVX) mice ··· 17
IV. DISCUSSION ··· 21
V. CONCLUSION ··· 24
REFERENCES ··· 25
LIST OF FIGURES
Figure 1. Bone remodeling process ··· 4
Figure 2. Lycii Radicis Cortex (LRC) extract increased cellular proliferation and differentiation in C3H10T1/2 and MC3T3-E1 osteoblastic cell lines ··· 13
Figure 3. No changes in osteoclastic differentiation after treatment of
Lycii Radicis Cortex (LRC) extract in primary-cultured monocytes ··· 14
Figure 4. Lycii Radicis Cortex (LRC) extract increased the mRNA expression of
Alpl, Runx2, and Bglap in C3H10T1/2 and MC3T3-E1 cells ··· 16
Figure 5. Lycii Radicis Cortex (LRC) extract inhibited the loss of bone mineral density in ovariectomized (OVX) mice ··· 19
Figure 6. The long-term effects of Lycii Radicis Cortex (LRC) extract in ovariectomized (OVX) mice ··· 20
vi
LIST OF TABLES
Table 1. Results of water-soluble tetrazolium salt (WST) assay and alkaline phosphatase (ALP) assay of the ethanol extracts of the 64 plants native to Korea in
I. INTRODUCTION
Bone is a complex rigid organ composed of several types of cells undergoing a continuous remodeling process of bone formation and bone resorption followed by the replacement of bone to regulate calcium homeostasis and repair damaged bones (Feng and McDonald, 2011).
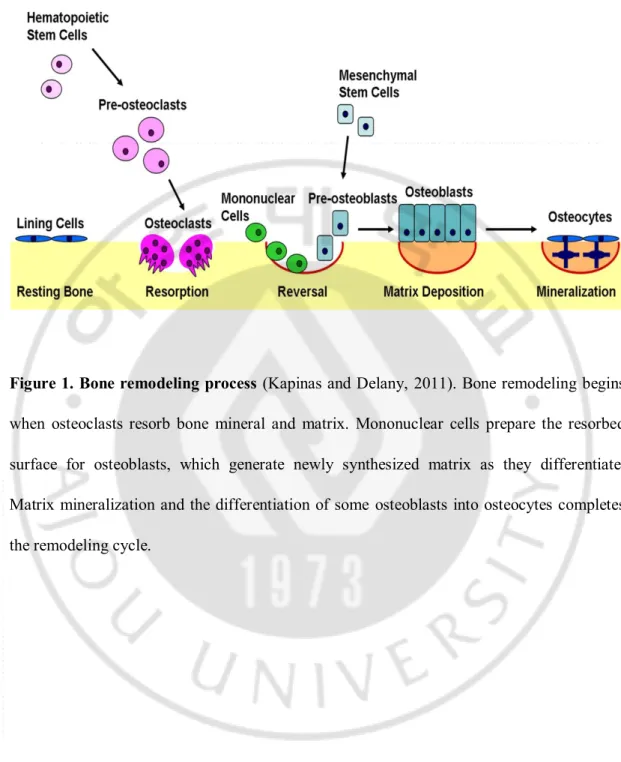
There are two different types of bone cells: osteoblasts, which form new bone, and osteoclasts, which break bone down, and they both play an important role in the regulation of bone metabolism (Feng and McDonald, 2011). The action of osteoblasts and osteoclasts, coupled via paracrine signaling, is controlled by a number of factors that either enhance or inhibit bone remodeling cells. The subtle balances between osteoblast and osteoclast numbers and activities mediated bone remodeling process (Kapinas and Delany, 2011) (Figure 1). Some cytokines at the remodeling site recruits osteoclasts to the bone surface (Rachner et al., 2011). A boarder formed by osteoclasts allows their tight adherence to the bone surface. The bone remodeling phase begins with mononuclear cells preparing the bone surface for new osteoblasts and providing signals to recruit them. When the osteoblasts differentiate, the matrix matures and is mineralized (Kapinas and Delany, 2011). However, the imbalanced regulation of bone remodeling between two processes—bone resorption and bone formation—leads many metabolic bone diseases, including osteoporosis (Rachner et al., 2011).
Osteoporosis is a common skeletal disease that can lead to an increased risk of fracture (caused by decreased bone mass) and an enhanced risk of bone fragility and susceptibility to
2
-fracture (Sambrook and Cooper, 2006; Rachner et al., 2011). In osteoporosis, the bone mineral density (BMD) is reduced, bone microarchitecture deteriorates, and the amount and variety of proteins in bone are altered. Osteoporosis is defined as a bone mineral density of 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults) as measured by dual-energy X-ray absorptiometry; the term "established osteoporosis" includes the presence of a fragility fracture (Kanis, 1994).
For the pharmacological therapy of postmenopausal osteoporosis, supplements and medications usually include certain ingredients to help maintain bone health: calcium, magnesium, vitamin D, vitamin K, phytoestrogen, and prebiotic fiber (Hanley et al., 2010; Jose Ramon et al., 2012; Rizzoli et al., 2013). Novel interventions that target regulators of bone remodeling have been suggested to be promising agents for the treatment of osteoporosis. An inhibitor of cathepsin K, odanacatib, is in clinical trials for the treatment of postmenopausal osteoporosis and monoclonal antibodies to sclerostin, such as AMG 785, have osteoanabolic properties with the potential to improve clinical outcomes in patients with osteoporosis (Lewiecki, 2011). However, some of these medications have side effects, including long-lasting ingestion and an increased risk of endometrial and breast cancers (Bonura, 2009). Therefore, the number of ingredients should be minimized in order to decrease the side effects of medications used to treat osteoporosis.
Recently, as an alternative long‐term therapeutic option against osteoporosis, herbal medicine has come to our attention. This is because the advantages of natural plants have shown the potential of having fewer side effects, thereby making them more suitable for
chemotherapeutic effects of several natural products derived from plants, including Carthamus tinctorius, Drynaria fortunei, Gardenia jasminoides, Schizandra chinensis, and Ulmus davidiana, have been reported (Ha et al., 2003; Suh et al., 2007; Kim et al., 2008; Caichompoo et al., 2009; Jeong et al., 2012). Several natural products, such as Curculigoside (Shen et al., 2013), Withania somnifera (Nagareddy and Lakshmana, 2006), Pueraria mirifica, and miroestrol (Chatuphonprasert et al., 2013), have also been reported to prevent bone loss in osteoporosis animal models with an improvement in total body bone mineral density and bone mineral content.
In this study, my aim was to discover alternative herbal therapeutic drugs for effective osteoporosis treatment in vitro and in vivo. Sixty-four ethanol extracts of edible plants native to Korea were screened for their ability to increase the proliferation and differentiation of two osteoblast cell lines. Based on the screening data of both cell lines, Lycii Radicis Cortex
(LRC) extract was selected as a potential natural‐source candidate. I carried out further in
vitro experiments in cell lines and in vivo experiments in the osteoporosis model mice to
4
-Figure 1. Bone remodeling process (Kapinas and Delany, 2011). Bone remodeling begins
when osteoclasts resorb bone mineral and matrix. Mononuclear cells prepare the resorbed surface for osteoblasts, which generate newly synthesized matrix as they differentiate. Matrix mineralization and the differentiation of some osteoblasts into osteocytes completes the remodeling cycle.
II. MATIRIALS AND METHODS
1. Herbal extracts and materials.
The 70% ethanol extracts of 64 plants native to Korea were provided from the Korea Promotion Institute for Traditional Medicine Industry (Gyeongsan, Korea) (http://www.kotmin.kr). The plant materials were added 5-fold volume of 70% ethanol, extracted at 100 °C for 3 h, filtered, and then concentrated. Cell culture media (DMEM and α-MEM), antibiotics (penicillin and streptomycin), and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, USA). An EZ-Cytox Cell Viability Assay Kit was purchased from Daeil (Seoul, Korea), and ascorbic acid and β-glycerophosphate were purchased from Sigma-Aldrich (St. Louis, USA).
2. Cell culture.
The murine mesenchymal stem cell line (C3H10T1/2 cells) was purchased from the Korean Cell Line Bank (Seoul, Korea) and grown in a DMEM medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Mouse MC3T3-E1 pre-osteoblast cells were purchased from the RIKEN Cell Bank (Tsukuba, Japan) and grown in a α-MEM medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Osteoblast differentiation was induced by adding an osteogenic medium containing ascorbic acid (50 μg/ml) and β-glycerophosphate (10 mM) after allowing 24 h for cell
6
-adherence (day 0), and the medium was changed every three days. All cultured cells were incubated in a humidified atmosphere at 37 °C and at 5% CO2.
3. Water-soluble tetrazolium salt (WST) assay and alkaline phosphatase (ALP) assay.
To test cell viability, cells (3×103 cells/well) were incubated in a 96-well plate overnight and treated with different concentrations of the plant extracts (10, 50 and 100 μg/ml) in the medium for 48 h. An EZ-Cytox Cell Viability Assay Kit was used for cell viability assay. WST (20 μl, 5 mg/mL in PBS) was added to each well, the cells were incubated for another 4 h, and the media were carefully removed. Absorbance was measured at 450 nm and 655 nm using a microplate reader (BioTek; Winooski, USA). For ALP activity assay, cells (1.5×10⁴cells/well) were incubated in a 48-well plate for 24 h and then treated with different concentrations of the plant extracts (10, 50 and 100 μg/ml) in the medium for 48 h. After washing twice with 1×PBS, the cells were added 200 μl of extraction solution and incubated overnight at 4 °C. ALP activity was measured in total cell lysates after homogenization in a buffer containing 1 mmol/l Tris-HCl (pH 8.8), 0.5% Triton X-100, 10 mmol/l Mg2+, and 5 mmol/L p-nitrophenylphosphate as substrates. The absorbance was read at 405 nm (BioTek).
4. In vitro generation of osteoclasts and tartrate-resistant acid phosphatase (TRAP) assay.
For primary-cultured monocytes, bone marrow cells were flushed from the femoral bones of 6-week-old mice in the presence of ascorbate-2-phosphate (1 mM). Monocyte cells
were identified by immunophenotypic analysis with a CD11b antibody using the FACS Aria III cell sorter (BD Biosciences, San Jose, USA) and FACS Diva software (BD Biosciences). Monocyte cells were cultured in the presence of 30 ng/ml M-CSF (PeproTech, Rocky Hill, USA) and 50 ng/ml RANKL (PeproTech) to induce differentiation to osteoclasts (Sun et al., 2006). The differentiated osteoclast cells from monocytes were measured by a TRAP activity assay and staining using the Acid-Phosphatase Kit (Sigma-Aldrich).
5. Quantitative reverse-transcription PCR (qRT-PCR).
To determine the mRNA expression levels of the bone metabolic genetic markers, Alp,
Runx2, Ocn (Osteocalcin, Bglap), quantitative real-time PCR was performed. Total RNA
was extracted from culture cells using a TRIzol reagent (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions and quantified by a spectrophotometer (Beckman Coulter, Brea, USA). The extracted RNA were subsequently reverse transcribed using the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas Inc., Hanover, USA) with the oligo(dT)15–18 at a random primer. All real-time PCR measurements were performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems; Foster City, USA). All PCR amplifications (40 cycles) were performed in a total volume of 25 μl containing 150 ng cDNA using the SYBR Green I qPCR kit (TaKaRa, Shiga, Japan) according to the manufacturer’s recommendations. The specific primers for osteoblast markers were as follows: 5′-TCCCACGTTTTCACATTCGG-3′ and 5′-GGCCATCCTATA TGGTAACGGG-3′ for mouse Alpl, 5′-TAAAGTGACAGTGGACGGTCCC-3′ and 5′-CCT CAGTGATTTAGGGCGCA-3′ for mouse Runx2, and 5′-TAGTGAACAGACTCCGGCG
8
-CTA-3′ and 5′-AT GGCTTGAAGACCGCCTACA-3′ for mouse Bglap. To normalize the efficiency of real-time RT-PCR reactions, the mouse Gapdh gene was used as a relative quantification standard with the following primers: 5′-TGACCACAGTCCATGCCATC-3′ and 5′-GACGGACACATTGGGGGTAG-3′. The following amplification parameters were used: 10 min preincubation for hot start polymerase activation at 95°C, followed by 45 amplification cycles at 95 °C for 20 s, 62 °C for 20 s, 72 °C for 40 s. After the end of the last cycle, the melting curve was generated by starting fluorescence acquisition at 60 °C, and taking measurements every 0.2 °C until 95 °C. By normalizing to Gapdh, a relative quantification of gene expression was performed using the comparative threshold (Ct) method as described by the manufacturer (Applied Biosystems). The values were expressed as fold change over control. Relative gene expression was displayed as 2-ΔCt (ΔCt = Ct target gene − Ct Gapdh). Fold change was calculated as 2-ΔΔCt (ΔΔCt = ΔCt control − Ct treatment).
6. In vivo experiment.
The ovariectomized (OVX, n = 30) and sham-operated (Sham, n = 12) 8-week-old female ddY mice were purchased from Shizuoka Laboratory Center Inc. (Hamamatsu, Japan). The mice were acclimated for 10 days prior to experimentation. They were maintained on a diet of Formula-M07 (5.0 g/day) (Feedlab Co. Ltd., Hanam, Korea) and tap water (15 ml/day). All mice were housed individually in clear plastic cages under controlled temperature (23 ± 2 °C), humidity (55 ± 5%), and illumination (12-hmy light/dark cycle). The mice were administered different concentrations of LRC extract: either 50, 150 and 300
mg/kg/day for 8 weeks or 150 mg/kg/day for 16 weeks. Each calculated concentration of LRC extract was added to tap water. The LRC extract-containing water was changed for fresh water every three days, and the volume of LRC extract-containing water was measured every three days for the administered LRC amount. The right femur bone mineral density (BMD) and bone mineral content (BMC), as well as the whole body percentage of body fat (% fat) and body weight, were measured before and after the administration of LRC extract. The animal research protocol was approved by the Animal Care and Use Committee of the Ajou University School of Medicine, and all experiments were conducted in accordance with the institutional guidelines established by the Committee.
7. Measurement of whole body fat (%) and right femur BMD and BMC.
Whole body fat (% fat) and right femur BMD and BMC were measured using a PIXImus bone densitometer (GE Lunar, Madison, USA) and calculated by on-board PIXImus software for small animals, adjusted in relation to body weight. After anesthetization using tiletamine/zolazepam (Zoletil; Virbac Laboratories, Carros, France), the mice were placed on the specimen tray for measurement.
8. Statistical analysis.
A statistical software package (SPSS 11.0 for Windows, SPSS Inc., Chicago, USA) was used to perform the statistical tests. The statistical significance of differences was assessed by Student’s t-test. P < 0.05 values were considered significant. Results were expressed as mean ± SEM.
- 10 -
III. RESULTS
1. Sixty-four plants native to Korea were screened for cellular proliferation and the differentiation of osteoblastic C3H10T1/2 and MC3T3-E1 cell lines.
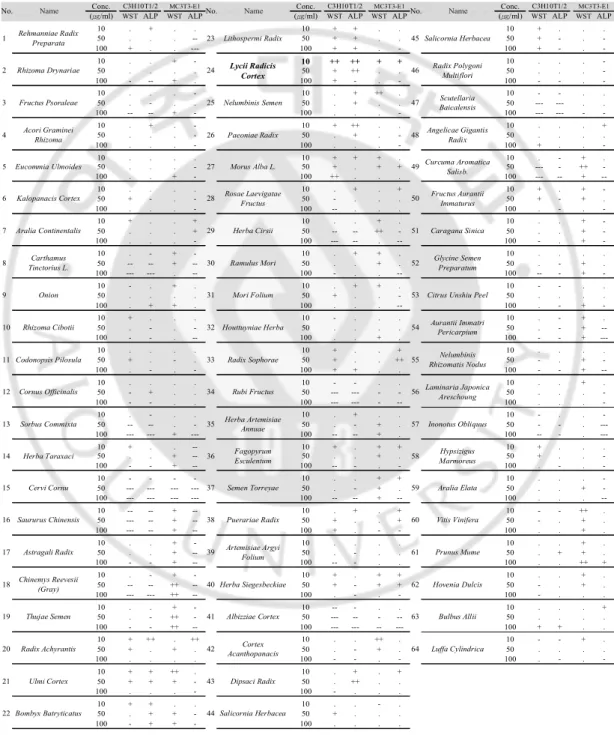
To discover the potential natural source(s) having an influence on bone formation augmentation, I screened the ethanol extracts of 64 different plants native to Korea. ALP is a glycoprotein found on the surface of osteoblasts and is a sensitive and reliable indicator of bone metabolism. Hence, I used an ALP assay as the main method for screening the extracts’ effects on bone formation. Three different concentrations (10, 50 and 100 μg/ml) of each plant extract were treated in the osteoblastic cell lines C3H10T1/2 and MC3T3-E1. After three days of incubation with the 64 extracts, cell viability was examined by a WST assay, and osteoblastic differentiation levels were determined using the ALP assay (Table 1).
Based on the ALP activity and cell viability of the two cell lines, Lycii Radicis Cortex (LRC), Lycium Chinense root bark, was selected as a primary candidate plant. LRC is widely used in eastern Asia as a traditional medicine. However, the effect of LRC extract on osteoporosis has not yet been explored.
Table 1. Results of water-soluble tetrazolium salt (WST) assay and alkaline phosphatase (ALP) assay of the ethanol extracts of the 64 Korean native plants in C3H10T1/2 and MC3T3-E1 cells
Conc. Conc. Conc.
(㎍/ml) WSTALP WST ALP (㎍/ml) WSTALP WSTALP (㎍/ml) WSTALP WSTALP
10 . + . . 10 + + . . 10 + . . . 50 . . . -- 50 + + . . 50 + - . -100 + . . --- 100 + + . - 100 + - . -10 . . + - 10 ++ ++ + + 10 . . . -50 . . . . 50 + ++ . . 50 . . . -100 - -- + . 100 + - . - 100 - - . -10 . . . - 10 . + ++ . 10 - - . . 50 . - . . 50 . + . . 50 --- --- . . 100 -- -- + - 100 . . . - 100 --- --- - -10 . + . - 10 + ++ . . 10 . . . + 50 . . . - 50 . + . - 50 . . . . 100 . . . - 100 . . . - 100 + . . -10 . . . . 10 + + + . 10 . - + . 50 . . . - 50 + . + + 50 --- - ++ -100 . . + - 100 ++ . . . 100 --- -- + --10 . . . . 10 . + . + 10 + . + -50 + - . - 50 - . . . 50 + - + . 100 . . . - 100 -- . . . 100 . - . -10 + . . + 10 . . + . 10 . . + -50 . . . + 50 -- -- ++ - 50 . . + -100 . . . - 100 --- -- . -- 100 - . + -10 . - + - 10 . + + . 10 . . . . 50 -- -- + -- 50 . . + . 50 . - + . 100 --- --- . -- 100 - . . -- 100 -- - + -10 - . + . 10 . + + . 10 - - . . 50 . . . . 50 + . . - 50 . . . . 100 . + + . 100 . . . -- 100 . - + -. 10 + . . . 10 - . . . 10 . - + . 50 . - . - 50 . . . . 50 . - + --100 - - . -- 100 . . + . 100 - - + ---10 . . . . 10 + . . + 10 . . . . 50 + . - . 50 + . . ++ 50 - . + -100 . - . - 100 + + . . 100 - - + --10 . . . . 10 - - . . 10 . . + . 50 - + . . 50 --- --- - - 50 . . . -100 - - . . 100 --- --- - -- 100 . . . -10 . - . . 10 . + . . 10 - . . -50 -- -- . - 50 . - + . 50 - - . ---100 --- --- + --- 100 -- -- + . 100 -- - . ---10 + . . -- 10 + . + + 10 + . . . 50 . . + -- 50 . . + . 50 + . . -100 - - + -- 100 -- - . - 100 . - . -10 - - - - 10 . . + + 10 . . . -50 --- --- --- --- 50 . - + . 50 . . + -100 --- --- --- --- 100 -- -- + -- 100 . . . --10 -- -- + -- 10 . + . + 10 - - ++ . 50 --- -- + -- 50 + . . + 50 - . + . 100 --- -- + -- 100 + . - - 100 . . + . 10 . . + - 10 . . . . 10 . . + . 50 . . + -- 50 . - . . 50 . + + . 100 - - + -- 100 -- - . . 100 . . ++ + 10 . - + - 10 + . + + 10 . . + . 50 -- -- ++ -- 50 + - + + 50 - . + . 100 --- --- ++ -- 100 . - . - 100 - . . . 10 . . + - 10 -- - . . 10 . . . + 50 . - ++ - 50 --- -- - -- 50 . . . . 100 - - ++ -- 100 --- --- -- --- 100 + + . . 10 + ++ . ++ 10 . . ++ . 10 - - + . 50 + . + . 50 . - + . 50 . . . . 100 . . . . 100 - - . - 100 . - . -10 + + ++ . 10 . + . + 50 + + + - 50 . ++ . . 100 . . . - 100 - . . . 10 + + . . 10 . . - . 50 . + + - 50 + . . . 100 - + + - 100 . . . .
Symbols: ---, 0~50%; --, 50~70%; -, 70~90%; ., 90~110%; +, 110~130%; ++, 130~150%; +++, 150~ vs. non-treated control.
22 Bombyx Batryticatus 44 Salicornia Herbacea 21 Ulmi Cortex 43 Dipsaci Radix
20 Radix Achyrantis 42 Cortex
Acanthopanacis 64 Luffa Cylindrica
19 Thujae Semen 41 Albizziae Cortex 63 Bulbus Allii
18 Chinemys Reevesii(Gray) 40 Herba Siegesbeckiae 62 Hovenia Dulcis
17 Astragali Radix 39 Artemisiae Argyi
Folium 61 Prunus Mume
16 Saururus Chinensis 38 Puerariae Radix 60 Vitis Vinifera
15 Cervi Cornu 37 Semen Torreyae 59 Aralia Elata
14 Herba Taraxaci 36 Fagopyrum
Esculentum 58
Hypsizigus Marmoreus
13 Sorbus Commixta 35 Herba Artemisiae
Annuae 57 Inonotus Obliquus
12 Cornus Officinalis 34 Rubi Fructus 56 Laminaria Japonica
Areschoung
11 Codonopsis Pilosula 33 Radix Sophorae 55 Rhizomatis NodusNelumbinis 10 Rhizoma Cibotii 32 Houttuyniae Herba 54 Aurantii Immatri
Pericarpium
9 Onion 31 Mori Folium 53 Citrus Unshiu Peel 8 Carthamus
Tinctorius L. 30 Ramulus Mori 52
Glycine Semen Preparatum
7 Aralia Continentalis 29 Herba Cirsii 51 Caragana Sinica
6 Kalopanacis Cortex 28 Rosae Laevigatae
Fructus 50
Fructus Aurantii Immaturus
5 Eucommia Ulmoides 27 Morus Alba L. 49Curcuma Aromatica
Salisb.
No.
4 Acori GramineiRhizoma 26 Paeoniae Radix 48 Angelicae GigantisRadix 3 Fructus Psoraleae 25 Nelumbinis Semen 47 Scutellaria
Baicalensis
2 Rhizoma Drynariae 24 Lycii Radicis
Cortex 46
Radix Polygoni Multiflori
Name C3H10T1/2 MC3T3-E1
1 Rehmanniae Radix
Preparata 23 Lithospermi Radix 45 Salicornia Herbacea
- 12 -
2. LRC extract increased cellular proliferation and differentiation of osteoblast cell lines.
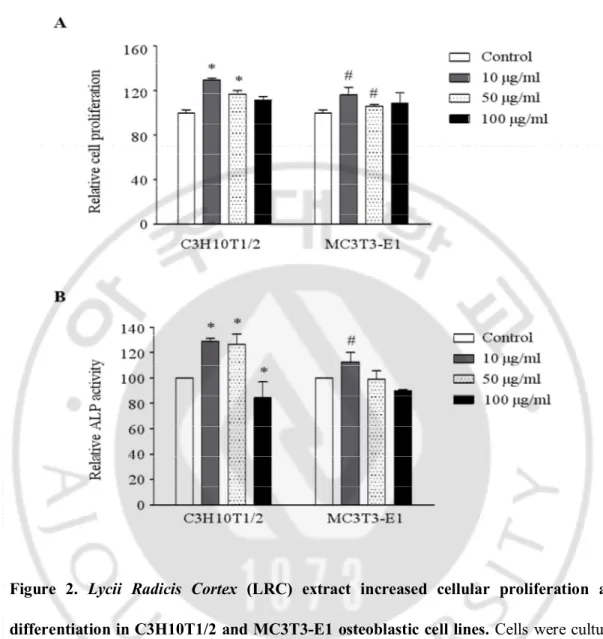
I further investigated the anti-osteoporotic effects of LRC extract. Treatment using 10 μg/mL and 50 μg/mL of LRC extract, respectively, showed a significantly increased proliferation in both cell lines compared to the control group, indicating that LRC extract has no toxic effects on cell growth (Figure 2A). The highest ALP activity in both C3H10T1/2 and MC3T3-E1 cell lines was observed in 10 μg/ml extracts of LRC (Figure 2B).
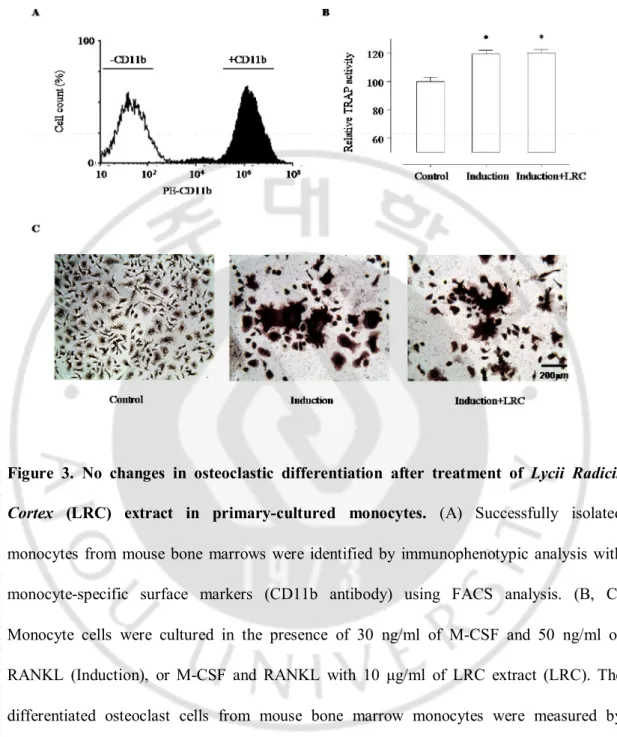
Imbalanced bone remodeling is one of the key factors in inducing osteoporosis and many other metabolic bone diseases. Naturally, the daily removal of bone mineral (bone resorption) must be balanced by equal amounts of new mineral deposition, which leads to gradual restructuring of the bone. I further examined whether LRC extract has any effect on the differentiation of osteoclast cell lines. For primary-cultured monocytes, bone marrow cells were flushed from the femoral bones of 6-week-old mice in the presence of ascorbate-2-phosphate. Monocyte cells were identified by immunophenotypic analysis with monocyte-specific surface markers (CD11b antibody) using fluorescence activated cell sorter (FACS) analysis (Figure 3A). The TRAP assay results revealed that osteoclastic differentiation of the primary-cultured monocytes was significantly increased by induction with 30 ng/ml of M-CSF and 50 ng/ml of RANKL, but there was no changes by additional treatment of 10 μg/ml of LRC extract in the M-CSF and RANKL-treated monocytes (Figure 3B, 3C). These results suggested that LRC extract may promote the proliferation and differentiation of osteoblast cells rather than the inhibition of osteoclastic differentiation.
Figure 2. Lycii Radicis Cortex (LRC) extract increased cellular proliferation and differentiation in C3H10T1/2 and MC3T3-E1 osteoblastic cell lines. Cells were cultured
with three different concentrations of LRC extract (10, 50 and 100 μg/ml) for three days, and cell viability (A) and ALP activity (B) were analyzed. Control: LRC non-treated cells. *, #: p < 0.05 vs. Control.
- 14 -
Figure 3. No changes in osteoclastic differentiation after treatment of Lycii Radicis
Cortex (LRC) extract in primary-cultured monocytes. (A) Successfully isolated monocytes from mouse bone marrows were identified by immunophenotypic analysis with monocyte-specific surface markers (CD11b antibody) using FACS analysis. (B, C) Monocyte cells were cultured in the presence of 30 ng/ml of M-CSF and 50 ng/ml of RANKL (Induction), or M-CSF and RANKL with 10 μg/ml of LRC extract (LRC). The differentiated osteoclast cells from mouse bone marrow monocytes were measured by tartrate-resistant acid phosphatase (TRAP) activity assay (B) and TRAP staining (C). Control: Monocyte cells cultured without M-CSF and RANKL. *: p < 0.05 vs. Control.
3. LRC extract increased mRNA expression of osteoblastic markers.
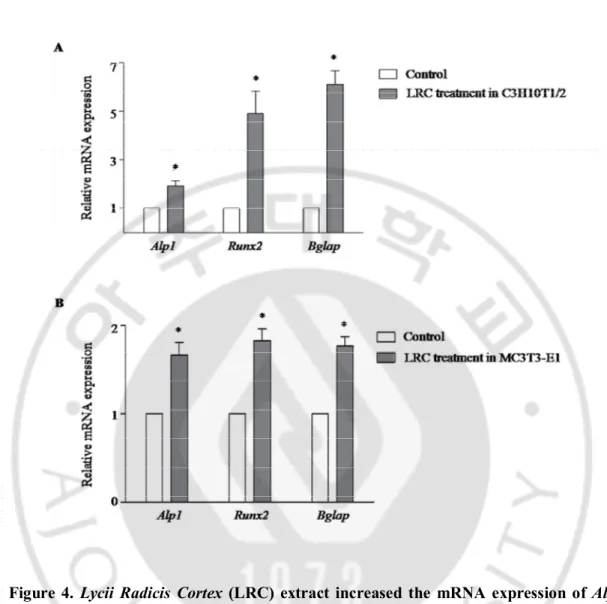
To further confirm the effect of LRC on the cellular differentiation of osteoblasts, I investigated any changes in the expression of representative osteoblastic marker genes Alpl (alkaline phosphatase, ALP), Runx2 (runt-related transcription factor 2, Runx2), and Bglap (bone gamma carboxyglutamate protein, Osteocalcin). After treatment with 10 μg/mL of LRC extract for three days in C3H10T1/2 and MC3T3-E1 cell lines, total RNAs were prepared and used as a template for quantitative RT-PCR.
There was significant increased expression of Alpl, Runx2, and Bglap genes in both LRC-treated cell lines compared to the non-treated control cell lines (Figure 4). This result suggested that LRC extract may stimulate osteoblast differentiation by up-regulating osteoblastic-inducing genes such as Alpl, Runx2, and Bglap.
- 16 -
Figure 4. Lycii Radicis Cortex (LRC) extract increased the mRNA expression of Alpl,
Runx2, and Bglap in C3H10T1/2 and E1 cells. C3H10T1/2 cells (A) and MC3T3-E1 cells (B) were treated with 10 μg/mL of LRC extract for three days. The expression level of mRNA was calculated quantitatively by RT-PCR using targeted gene-specific primers and then normalized to Gapdh mRNA expression. Control: LRC non-treated cells. *: p < 0.05 vs. Control.
4. LRC extract inhibited BMD loss in ovariectomized (OVX) mice.
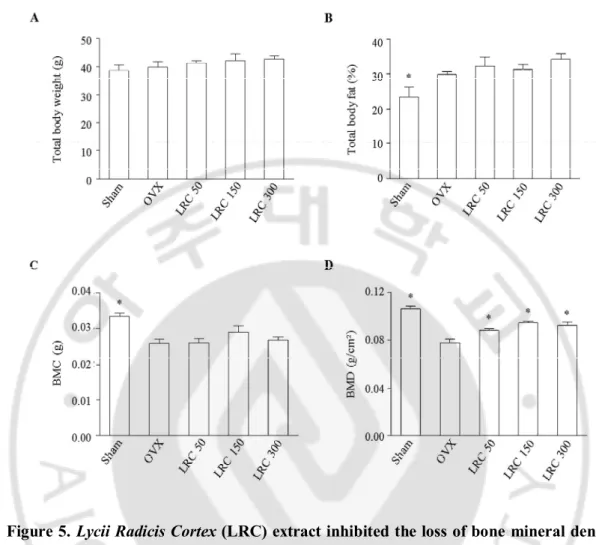
Based on my in vitro results, I further investigated the effect of LRC extract in the osteoporosis model animals. It is known that OVX mice present reduced bone mass and quality. In addition, ovariectomy leads to an increase in body weight in mice with significant decreased right femur BMD and BMC. I first investigated the effects of LRC extract on OVX mice for 8 weeks. Thirty of the 8-week-old female ddY mice underwent either ovariectomy or sham surgery (Sham). The mice were then divided into five groups of six mice each: 1) Sham, 2) OVX control, 3) OVX administrated with 50 mg/kg/day of LRC extract, 4) OVX administrated with 150 mg/kg/day of LRC extract, and 5) OVX administrated with 300 mg/kg/day of LRC extract. The mice were housed for 8 weeks and their total body weight, total body fat (%fat), and right femur BMD and BMC were compared.
First, I compared the two non-LRC-administered mice groups: the Sham group and the OVX control group. The OVX group showed increased % body fat and decreased BMC and BMD compared to the Sham group, indicating that the OVX mice were an appropriate menopause-induced osteoporosis model (Figure 5). Next, I compared the OVX control and the LRC-administered groups. While body weight, body fat (% fat), and BMC were not significantly different among OVX control and LRC-administered groups, the BMD of the right femur bone was significantly increased in all of the LRC-administered groups compared to the OVX control group (Figure 5). The highest BMD was observed in the 150 mg/kg/day LRC extract-administered group.
- 18 -
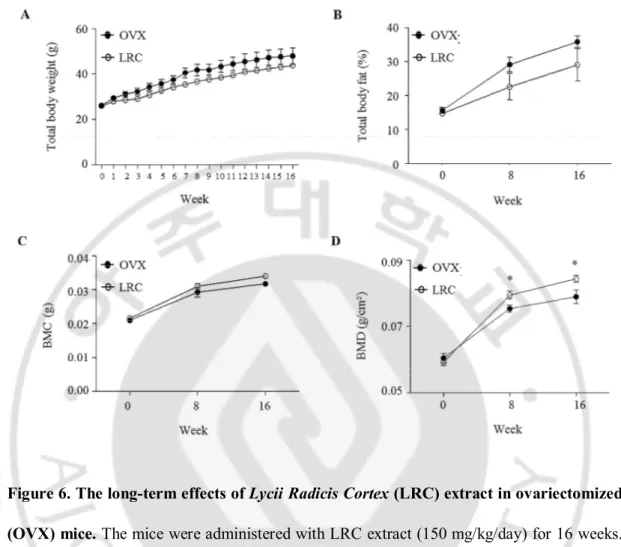
To investigate the effects of LRC extract in vivo for a longer period of time, six mice from the OVX control group and six mice from the LRC extract-administered group (150 mg/kg/day) were housed for 16 weeks. Total body weights were measured every week, and % body fat, BMC, and BMD of the right femur were measured at 0, 8 and 16 weeks, respectively. Similarly, there were no significant differences between the OVX control and the LRC-administered groups in terms of body weight, % fat, and BMC, although the LRC group showed a slight decrease in body weight and % fat, and a slight increase in BMC (Figure 6). As expected, BMD was significantly higher in the LRC-administered group than the OVX control group (Figure 6).
These results indicated that LRC extract inhibited BMD loss through ovariectomy in mice, and its effect continued for the duration of the test period (16 weeks). No toxic effects were observed in either mouse group.
Figure 5. Lycii Radicis Cortex (LRC) extract inhibited the loss of bone mineral density in ovariectomized (OVX) mice. The OVX mice were administered with different
concentrations of LRC extract (50, 150, 300 mg/kg/day) for 8 weeks. OVX control: non-treated mice. Their total body weight (A), percentage of total body fat (% fat) (B), right femur bone mineral content (BMC) (C), and right femur bone mineral density (BMD) (D) were measured before and after treatment of LRC extract. *: p < 0.05 vs. OVX control.
- 20 -
Figure 6. The long-term effects of Lycii Radicis Cortex (LRC) extract in ovariectomized (OVX) mice. The mice were administered with LRC extract (150 mg/kg/day) for 16 weeks.
OVX control: non-treated mice. Total body weights (A) were measured every week and percentage of total body fat (% fat) (B), right femur bone mineral content (BMC) (C), and
right femur bone mineral density (BMD) (D) were measured at 0, 8 and 16 weeks. *: p <
IV. DISCUSSION
Many studies have suggested pharmacological therapy (with several ingredients) for osteoporosis, but some medications have shown negative effects, including endometrial and breast cancers, when used long term (Bonura, 2009). Recently, natural herbal medicines have been used for the alternative long-term therapeutic treatment of several human diseases. Screening of herbal extracts for increasing osteoblast differentiation may be an attractive approach to finding new therapeutic osteoporosis drugs that have fewer effects. It has been reported that several natural products derived from plants can be beneficial for both preventing and treating osteoporosis through the induction of osteoblast differentiation (Li et al., 2012).
To discover novel herbal extracts for the treatment of osteoporosis, I screened 64 ethanol extracts of edible plants native to Korea for their ability to increase cellular proliferation and differentiation using the osteoblastic cell lines C3H10T1/2 and MC3T3-E1. I selected LRC as a primary candidate because the treatment of LRC extract showed the greatest increase in cellular proliferation and differentiation of osteoblastic cells among the herbal extracts tested. Until this point, the effect of LRC extract on osteoporosis has not been
explored.
Since new bone formation is mediated by osteoblasts, either increasing the
proliferation or inducing osteoblastic differentiation of the osteoblastic lineage cells
promotes bone formation
(Ducy et al., 2000).My results indicate that LRC extract
promotes both proliferation and differentiation of osteoblastic lineage cells, which
may leads to enhancement of new bone formation.
- 22 -
To further confirm the effect of LRC on the cellular differentiation of osteoblasts, I investigated any changes in the expression of representative osteoblastic marker genes Alpl, and Bglap. Previous studies have suggested the clinical utility of bone remodeling biochemical markers (Watts, 1999). ALP increases during active bone formation with the induction of osteoblast activity, suggesting that ALP plays a crucial role in the mineralization of newly formed bone (Watts, 1999). Runx2 is a key transcription factor related to osteoblast differentiation (Gilbert et al., 2002). Bglap (Osteocalcin), which is secreted from osteoblasts, is an important factor in the regulation of bone metabolism and in the implication of bone mineralization and calcium ion homeostasis (Lee et al., 2007). The RT-PCR results suggested that LRC extract may stimulate osteoblast differentiation by up-regulating osteoblastic-inducing genes such as Alpl, Runx2, and Bglap.
After confirming the effect of LRC extract in vitro experiments, I further explored the effect of LRC extract in vivo using ovariectomized (OVX) mice as an osteoporosis model animal. The OVX mice administered LRC extract for either 8 or 16 weeks exhibited significantly increased bone mineral density compared to non-treated control OVX mice, and no toxic effects were observed. Based on the in vitro and in vivo data, my results suggest that LRC extract may be a good candidate for the treatment of osteoporosis with few side effects.
This is the first study demonstrating the effect of LRC extract on bone formation in
vitro and in vivo. Many studies have demonstrated that LRC extract contains a variety of
physiologically active compounds such as apigenin, luteolin, kaempferol, quercetin, oleanolic acid, and urosolic acid (Potterat, 2010). In addition, LRC extract promotes certain biological activities, including insulin resistance, inhibition of tumor growth, and lipid
metabolism in obese diabetic rats (Ye et al., 2008). Earlier pharmacology studies have suggested new anti-hepatotoxic cerebroside from Lycium chinense fruits (Kim et al., 1997) and its mechanisms related to free radical scavenger activity and anti-inflammatory activity (Lin et al., 1997). In addition, LRC extract has been reported to have many beneficial effects, including hepato-protective action, an inhibitory effect on the rennin/angiotensin system (Potterat, 2010), improvements in insulin resistance and lipid metabolism (Shen et al., 2013), and antioxidant enzyme activity (Zhang et al., 2010).
In summary, my results demonstrated that LRC extract increased the proliferation and differentiation of osteoblast cells and prevented OVX-induced BMD loss in mice. Notably, in the 16-week in vivo experiment, the LRC-administered mice maintained better BMD than the non-treated OVX control mice for the full test period and showed no deleterious effects on body weight, physical size, morphological characteristics, and diet. This suggests that LRC extract may be a good candidate for the treatment of osteoporosis, but with fewer side effects than traditional medications.
Further studies of the components isolated from the LRC may lead to the identification of the active substance(s) that inhibit bone loss and activate bone formation, and may provide invaluable information toward elucidating the therapeutic properties of LRC for the treatment of osteoporosis. I believe that this study will have a significant impact on the field of phytotherapy research and will be helpful for investigators studying the ethnopharmacological treatment of osteoporosis.
- 24 -
V. CONCLUSION
I screened 64 ethanol extracts of edible plants native to Korea were screened for their ability to increase cellular proliferation and differentiation using the osteoblastic cell lines C3H10T1/2 and MC3T3-E1 and selected Lycii Radicis Cortex (LRC), Lycium Chinense root bark, as a primary candidate because the treatment of LRC extract showed the greatest increase in cellular proliferation and differentiation of osteoblastic cells. The OVX mice administered LRC extract for either 8 or 16 weeks exhibited significantly increased bone mineral density compared to non-treated control OVX mice, and no toxic effects were observed.
This is the first report demonstrating the effect of LRC extract on bone formation. Based on the in vitro and in vivo data, my results suggest that LRC extract may be a good
REFERENCES
1. Assessment of fracture risk and its application to screening for postmenopausal
osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843: 1-129, 1994
2. Bonura F: Prevention, screening, and management of osteoporosis: an overview of
the current strategies. Postgrad Med 121: 5-17, 2009
3. Caichompoo W, Zhang QY, Hou TT, Gao HJ, Qin LP, Zhou XJ: Optimization of
extraction and purification of active fractions from Schisandra chinensis (Turcz.) and its osteoblastic proliferation stimulating activity. Phytother Res 23: 289-292, 2009
4. Chatuphonprasert W, Udomsuk L, Monthakantirat O, Churikhit Y, Putalun W,
Jarukamjorn K: Effects of Pueraria mirifica and miroestrol on the antioxidation-related enzymes in ovariectomized mice. J Pharm Pharmacol 65: 447-456, 2013
5. Ducy P, Schinke T, Karsenty G: The osteoblast: a sophisticated fibroblast under
central surveillance. Science 289: 1501-1504, 2000
6. Feng X, McDonald JM: Disorders of bone remodeling. Annu Rev Pathol 6:
121-145, 2011
7. Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS,
Nanes MS: Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol
- 26 -
8. Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH, Kim C: Effects of Eucommiae Cortex
on osteoblast-like cell proliferation and osteoclast inhibition. Arch Pharm Res 26: 929-936, 2003
9. Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD, Guidelines Committee of
the Scientific Advisory Council of Osteoporosis C: Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada (summary).
CMAJ 182: 1315-1319, 2010
10. Jeong JC, Kim SJ, Kim YK, Kwon CH, Kim KH: Lycii cortex radicis extract
inhibits glioma tumor growth in vitro and in vivo through downregulation of the Akt/ERK pathway. Oncol Rep 27: 1467-1474, 2012
11. Jose Ramon V, Mora Mora M, Marino Alarcon O, Hernandez G, Josefina Linares L,
Urdaneta Romero H, Arevalo Gonzalez E: [Comparative study of the urinary excretion of boron, calcium, magnesium and phosphorus in postmenopausal women with and without osteoporosis]. Invest Clin 53: 3-15, 2012
12. Kanis JA: Assessment of fracture risk and its application to screening for
postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group.
Osteoporos Int 4: 368-381, 1994
13. Kapinas K, Delany AM: MicroRNA biogenesis and regulation of bone remodeling.
Arthritis Res Ther 13: 220, 2011
14. Kim KW, Suh SJ, Lee TK, Ha KT, Kim JK, Kim KH, Kim DI, Jeon JH, Moon TC,
Kim CH: Effect of safflower seeds supplementation on stimulation of the proliferation, differentiation and mineralization of osteoblastic MC3T3-E1 cells. J
Ethnopharmacol 115: 42-49, 2008
15. Kim SY, Choi YH, Huh H, Kim J, Kim YC, Lee HS: New antihepatotoxic
cerebroside from Lycium chinense fruits. J Nat Prod 60: 274-276, 1997
16. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ,
McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G: Endocrine regulation of energy metabolism by the skeleton. Cell 130: 456-469, 2007
17. Lewiecki EM: New targets for intervention in the treatment of postmenopausal
osteoporosis. Nat Rev Rheumatol 7: 631-638, 2011
18. Li TM, Huang HC, Su CM, Ho TY, Wu CM, Chen WC, Fong YC, Tang CH:
Cistanche deserticola extract increases bone formation in osteoblasts. J Pharm
Pharmacol 64: 897-907, 2012
19. Lin CC, Chuang SC, Lin JM, Yang JJ: Evaluation of the antiinflammatory
hepatoprotective and antioxidant activities of Lycium chinense from Taiwan.
Phytomedicine 4: 213-220, 1997
20. Nagareddy PR, Lakshmana M: Withania somnifera improves bone calcification in
calcium-deficient ovariectomized rats. J Pharm Pharmacol 58: 513-519, 2006
21. Potterat O: Goji (Lycium barbarum and L. chinense): Phytochemistry,
pharmacology and safety in the perspective of traditional uses and recent popularity.
Planta Med 76: 7-19, 2010
22. Rachner TD, Khosla S, Hofbauer LC: Osteoporosis: now and the future. Lancet
- 28 -
23. Rizzoli R, Boonen S, Brandi ML, Bruyere O, Cooper C, Kanis JA, Kaufman JM,
Ringe JD, Weryha G, Reginster JY: Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin 29: 305-313, 2013
24. Sambrook P, Cooper C: Osteoporosis. Lancet 367: 2010-2018, 2006
25. Shen Q, Zeng D, Zhou Y, Xia L, Zhao Y, Qiao G, Xu L, Liu Y, Zhu Z, Jiang X:
Curculigoside promotes osteogenic differentiation of bone marrow stromal cells from ovariectomized rats. J Pharm Pharmacol 65: 1005-1013, 2013
26. Suh SJ, Yun WS, Kim KS, Jin UH, Kim JK, Kim MS, Kwon DY, Kim CH:
Stimulative effects of Ulmus davidiana Planch (Ulmaceae) on osteoblastic MC3T3-E1 cells. J Ethnopharmacol 109: 480-485, 2007
27. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL,
Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M: FSH directly regulates bone mass. Cell 125: 247-260, 2006
28. Watts NB: Clinical utility of biochemical markers of bone remodeling. Clin Chem
45: 1359-1368, 1999
29. Ye Z, Huang Q, Ni HX, Wang D: Cortex Lycii Radicis extracts improve insulin
resistance and lipid metabolism in obese-diabetic rats. Phytother Res 22: 1665-1670, 2008
30. Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD, Chae S, Park JS, Youn UJ, Hyun JW: Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J Ethnopharmacol 130: 299-306, 2010
- 30 - - 국문요약 –
지골피
추출물의 조골세포 및 난소절제 마우스
골형성
효과에 대한 연구
아주대학교 대학원 의학과 김 예 란 (지도교수: 정 선 용) 골다공증은 뼈 미세구조의 질적인 변화로 인하여 뼈의 강도가 약화되어 척추와 대퇴부, 요골 등 부위의 골절 위험도가 증가되는 질환이다. 생활 수준이 향상되고 의학기술이 발전하여 사람들의 평균 수명이 길어지면서, 최근 급속한 고령화에 따른 골다공증이 크게 증가되고 있다. 골다공증 환자의 골밀도 개선을 위해서는 장기간의 약물 치료가 필요하기 때문에 부작용이 적은 천연물 유래의 치료약물의 개발이 필요하다. 본 연구에서는 64 종의 천연물 유래 추출물을 대상으로 2 종류의 조골세포주(C3H10T1/2 와 MC3T3-E1)의 분화 및 활성 증진 효과를탐색하여, 그 중 가장 효과가 있는 천연물로서 지골피(Lycii Radicis Cortex)
추출물을 발굴하였다. 조골세포주를 이용한 in vitro 연구에서 지골피 추출물의
처리에 의해 조골세포의 분화 유도와 세포 활성은 크게 증진되었다. 하지만 마우스 골수 유래의 단핵세포에서 파골세포로의 분화에는 영향이 없었다.
다음으로, 골다공증 모델 동물인 난소절제 마우스 실험을 통해 지골피 추출물의 in vivo 효과를 관찰하였다. 8 주간의 1 차 실험을 통해 지골피 추출물의 최적농도(150 mg/kg/day)를 결정한 후 16 주 동안 2 차 실험을 진행한 결과, 지골피 추출물이 골다공증이 유발된 마우스의 골밀도(bone mineral density) 개선에 유의한 효과가 있음을 증명하였다. 결론적으로, 본 연구에서는 지골피 추출물이 부작용이 적고 효과적인 골다공증 예방 또는 치료제로서의 활용 가능성이 있음을 처음으로 밝혔다. __________________________________________________________________________ 핵심어 : 천연물 추출물, 지골피, 골다공증, 조골세포, 난소절제 마우스, 골밀도