800
Copyright © 2016 The Korean Society of Fisheries and Aquatic Science pISSN:0374-8111, eISSN:2287-8815
서 론
대장균(Escherichia coli)은사람이나동물의장관또는자연 계에널리분포하고있는세균으로대부분병원성이없는것으 로알려져있다. 그러나설사및급성위장염증상을일으키는 일부병원성대장균은발병기작및독성인자에의해장관병원 성대장균(enteropathogenic E. coli, EPEC), 장관독소성대장균 (enterotoxigenic E. coli, ETEC), 장관출혈성대장균(enetrohe- morrhagic E. coli, EHEC), 장관침입성대장균(enetroinvasive E. coli, EIEC), 및 장관응집성대장균(enetroaggregative E.
coli, EAEC) 등 5개범주로나뉜다(Turner et al., 2006). 병원 성세균에의한감염질환의예방및치료를위해다양한항균제 의사용으로내성균의출현은점점증가하고있으며, 특히그람 음성간균은다양한항균제내성유전자를지속적으로획득하여 현재널리사용되는있는항균제에내성을나타내는내성균은 다양한시료에서쉽게분리되고있는실정이다(Schwartz et al., 2003; Kümmerer, 2009; Cabello et al., 2013; Xu et al., 2015;
Kim, 2016). 항균제에대한내성은 plasmid, transposon, DNA insertion elements 및 genomic islands에매개된내성유전자의 획득에의해생기며, 내성유전자는염색체또는 plasmid에존
곰소만 해역의 바지락( Ruditapes philippinarum)에서 분리한 대장균 ( Escherichia coli)의 항균제 내성 및 병원성 유전자의 보유성
김태옥·엄인선·박광호·박권삼*
군산대학교 식품생명공학과
Antimicrobial Resistance and the Presence of Virulence Genes in Escherichia coli Strains Isolated from Ruditapes philippinarum in
Gomso Bay, Korea
Tae-Ok Kim, In-Seon Eom, Kwang-Ho Park and Kwon-Sam Park*
Department of Food Science and Biotechnology, Kunsan National University, Gunsan 54150, Korea
In total, 151 Escherichia coli isolates from Ruditapes philippinarum in Gomso Bay were analyzed for their suscep- tibility to 18 different antimicrobial agents and for genes associated with virulence. For virulence genes, each strain of the isolates was positive for the enterotoxigenic E. coli (ETEC)-specific heat-stable toxin (estA), enteroinvasive E. coli (EIEC)-specific invasion-associated locus (iaa) gene and enteropathogenic E. coli (EPEC)-specific attaching and effacing (eae) gene. According to a disk diffusion susceptibility test, resistance to ampicillin was most preva- lent (23.2%), followed by resistance to amoxicillin (22.5%), ticarcillin (20.5%), tetracycline (18.5%), nalidixic acid (12.6%), ciprofloxacin (10.6%), streptomycin (9.9%), and chloramphenicol (6.6%). More than 35.8% of the isolates were resistant to at least one antimicrobial agent, and 19.9% were resistant to four or more classes of antimicrobi- als; these were consequently defined as multidrug resistant. Minimum inhibitory concentration (MIC) ranges for the antimicrobial resistance of the 15 different antimicrobial agents of 54 E. coli strains were confirmed by varying the concentrations from 32-2,048 µg/mL. Overall, these results not only provide novel insights into the necessity for seawater and R. philippinarum sanitation in Gomso Bay but they also help to reduce the risk of contamination by antimicrobial-resistant bacteria.
Key words: Escherichia coli, Antimicrobial resistance, Virulence genes, Minimum inhibitory concentration, Gomso Bay
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial Licens (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
http://dx.doi.org/10.5657/KFAS.2016.0800 Korean J Fish Aquat Sci 49(6) 800-866, December 2016
Received 29 September 2016; Revised 19 October 2016; Accepted 19 October 2016
*Corresponding author: Tel: +82. 63. 469. 1822 Fax: +82. 63. 469. 7448 E-mail address: parkks@kunsan.ac.kr
재한다(Rowe-Magnus et al., 2002). 그람음성세균에서항균
제다제내성유전자가삽입되어있는 integron은세균염색체
에서이동성을가진 DNA 단편인 transposon을통하여유전자 의한복제단위에서다른복제단위로이동되는데일반적으로 접합을통하여동종및이종세균으로확산된다고보고되어있 다(Martinez, 2009). 국내에서대장균의항균제내성에관한연 구는주로사람과가축에서분리한대장균에대한연구가대부 분을차지하고있다(Kang et al., 2005; Lee et al., 2005; Cho et al., 2008; Byun et al., 2012; Cho et al., 2012; Han et al., 2015;
Kim et al., 2015; Kim et al., 2016). 수산물유래대장균의경우 는해수, 어류및패류양식장에서분리한대장균을대상으로항 균제내성및감수성중심으로연구가이루어져있으며연구내 용의다양성은많이빈약한실정이다(Lee et al., 2003; Son et al., 2009; Park, 2013; Park et al., 2013; Jo et al., 2016). 따라서 본연구에서는 2014년 3월부터 2016년 2월까지전라북도곰소 만해역의바지락에서분리한대장균에대한병원성유전자의 존재유무, 항균제내성및감수성양상과내성항균제에대한 최소발육억제농도를조사하였다.
재료 및 방법
균주 및 시약
실험에사용한균주는전북곰소만해역의바지락에서분리한 대장균 151균주및항균제감수성결과의정도관리를위하여 E. coli ATCC 25922와 Staphylococcus aureus ATCC 25923을 사용하였다. 유전자증폭을위한효소는 Takara (Otsu, Japan) 사제품, 항균제디스크는 Becton Dickinson (BBL Sensi-Disk,
Sparks, MD, USA)사제품및각종항균제는 Sigma (St. Louis, MO, USA)사제품을사용하였다.
대장균의 분리 및 동정
갯벌에서채취한바지락은근처해수로뻘등의부착물을제
거한후멸균된용기에담아 10℃ 이하로유지하여실험실로
운반한다음흐르는수돗물로 표면에묻어있는부착물과물 기를제거한후탈각하여실험에사용하였다. 대장균의분리는 Recommended Procedures for the Examination of Seawater and Shellfish (A.P.H.A., 1970)에준하여실시하였다. 추정시험 에는 Lauryl Tryptose broth (Difco, USA)를확정시험에는 EC broth (Merck, Germany)를사용하였다. EC broth 양성시험관 의균은 TBX medium (Oxoid, UK)에접종하여 44±1.0℃에 서 24시간배양한후푸른색을나타내는균주는 API 20E Kit (bioMerieux, Marcy-l’Etoile, France)를사용하여동정하였다. 병원성 유전자의 확인
대장균의병원성유전자는 polymerase chain reaction (PCR) assay로확인하였다. PCR assay에사용한병원성유전자증폭 용 primers의염기서열및예상 DNA 크기등은 Table 1에나 타내었다. Primers는 Bioneer (Daejon, Korea)에의뢰하여합 성하였다. PCR assay 조건은 95℃에서 3분 1회변성후, 95℃
에서 30초, 57℃ 또는 58℃에서 30초, 72℃에서 30초또는 1 분을한 단위로하여 30회반복하여 DNA를증폭하였다. 증 폭된 DNA 산물은 1.5% agarose gel을사용하여전기영동후 ethidium bromide로염색하여 Vilber Lourmat (Bio-Paint ST4, Marne-la-Vallee, France)사 Gel-Doc system으로확인하였다. Table 1. Oligonucleotide primers used in this study
Gene Primer Nucleotide sequence (5' to 3') Annealing temp.
(℃) Product size
(bp) Reference
estA ST-F GCTAAACCAGTAGGGTCTTCAAAA
57 147 Talukdar et al., 2013
ST-R CCCGGTACAGGCAGGATTACAACA
eltB LT-F CACACGGAGCTCCTCAGTC
57 508 Talukdar et al., 2013
LT-R CCCCCAGCCTAGCTTAGTTT
eae eae-F CCCGAATTCGGCACAAGCATAAGC
57 881 Talukdar et al., 2013
eae-R CCCGGATCCGTCTCGCCAGTATTCG
aat pCVD432-F CTGGCGAAAGACTGTATCAT
57 650 Talukdar et al., 2013 pCVD432-R CAATGTATAGAAATCCGCTGTT
stx1 stx1F CACAATCAGGCGTCGCCAGCGCACTTGCT
58 606 Talukdar et al., 2013
stx1R TGTTGCAGGGATCAGTGGTACGGGGATGC
stx2 stx2F CCACATCGGTGTCTGTTATTAACCACACC
58 372 Talukdar et al., 2013 stx2R GCAGAACTGCTCTGGATGCATCTCTGGTC
iaa ial upper CTGGATGGTATGGTGAGG
57 320 Talukdar et al., 2013 ial lower GGAGGCCAACAATTATTTCC
항균제 감수성 시험
각종 항균제에대한 내성및감수성은 Acar and Goldstein (1991)의디스크확산법과미국 NCCLS (National Committee for Clinical Laboratory Standards, 2002)법에준하여시험하 였다. 시험균주는 Luria-Bertani (tryptone 1%, yeast-extract 0.5%, NaCl 0.5%) broth에접종하여하룻밤배양한균을생 리식염수로 2회세정후 농도를 McFarland 0.5로 조정하고, 두께 4 mm의 Mueller Hinton agar (Difco, USA) 평판에도 말하였다. 여기에항균제디스크를고착하여 35℃에서 16-18 시간배양한다음균의증식저해대크기는 calipers로측정하 였으며, 항균제는 amikacin (30 μg; AK), amoxicillin (10 μg;
AML), ampicillin (10 μg; AMP), cefepime (30 μg; FEP), ce- fotaxime (30 μg; CTX), cefoxitin (30 μg; FOX), ceftriaxone (30 μg; CRO), cephalothin (30 μg; KF), cephazolin (30 μg;
KZ), chloramphenicol (30 μg; C), ciprofloxacin (5 μg; CIP), gentamicin (10 μg; GN), imipenem (10 μg; IPM), kanamycin (30 μg; K), nalidixic acid (30 μg; NA), streptomycin (10 μg;
S), tetracycline (30 μg; TE), ticarcillin (75 μg; TIC) 등 18종 을사용하였다.
최소발육억제농도(minimum inhibitory concentra- tion, MIC)의 측정
최소발육억제농도는미국 NCCLS (National Committee for Clinical Laboratory Standards, 2002)법에기초하여변법으로 측정하였다. 멸균된 Muller-Hinton broth에 4,096 μg/mL에서
1 μg/mL까지절반씩농도를달리한항균제를첨가한후멸균
된소형시험관에배지를 2 mL씩분주하였다. 여기에 LB broth 에서전배양한균액 2 μL 접종하여 35℃에서 16시간정치배 양한후균증식여부를육안으로확인하여 MIC를측정하였다.
결과 및 고찰
대장균의 분리 및 병원성 유전자의 확인
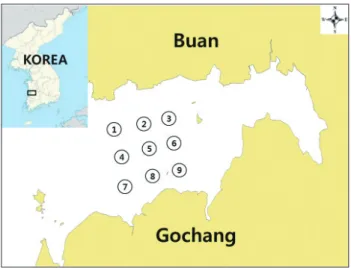
바지락은 2014년 3월부터 2016년 2월까지전라북도곰소만 해역의 9개지점에서총 16회합계 144시료를채취하여대장 균을분리하였으며채취지점은 Fig. 1과같다. 하나의시료에 서동일대장균의중복분리를배제하기위하여원칙적으로하 나의시료에서 1균주의대장균분리를시도하여총 151균주의 대장균을분리하였다. 분리된대장균에대해 7종의대장균관 련병원성유전자(estA, eltB, eae, aat, stx1, stx2, 및 iaa)의존 재유무를 PCR assay로확인하였으며그결과는 Table 2에나 타내었다. 시험결과장관독소성대장균(enterotoxigenic E. coli, ETEC)의 estA, 장관병원성대장균(enteropathogenic E. coli, EPEC)의 eae 및 장관침입성대장균(enteroinvasive E. coli, EIEC)의 iaa 병원성유전자를가지고있는각 1균주씩총 3균주
(2.0%)가병원성유전자보유대장균으로확인되었으며나머지
4종의병원성유전자는모든균주에서확인되지않았다. 결과적 으로곰소만해역의바지락에서분리한대장균은병원성유전 자보유율은매우낮았으며대부분이비병원성대장균으로확 인되었다. Park (2013)은곰소만해수에서분리한 131균주대 장균에대한병원성유전자의보유성을 PCR assay로검토한결 과, 장관응집성대장균(enteroaggregative E. coli, EAEC)의병 원성유전자(pCVD432)를보유하고있는 1개균주및 EPEC의 병원성유전자(eae)을보유하고있는 3개균주로파악되어전체
대장균의 3.1%가병원성대장균이며나머지는비병원성대장
균인것으로보고하였는데본연구결과와유사한결과이다. 또 한 Van et al. (2008)은베트남시장에서판매되는패류에서분 리한대장균의병원성유전자보유율은 3.5%였다는결과와크 게다르지않았다.
Fig. 1. Location of sample collection stations in Gomso Bay, Re- public of Korea, from March 2014 to February 2016.
Table 2. Virulence genes presence of Escherichia coli strains iso- lated from Ruditapes philippinarum in Gomso Bay
Classes of
pathogenic E. coli Target gene No. of virulence strains
ETEC estA 1
eltB 0
EHEC stx1 0
stx2 0
EPEC eae 1
EAEC aat 0
EIEC iaa 1
ETEC, Enterotoxigenic E. coli; EHEC, Enterohemorrhagic E.
coli; EPEC, Enteropathogenic E. coli; EAEC, Enteroaggregative E. coli; EIEC, Enteroinvasive E. coli.
대장균의 항균제 내성 및 감수성
분리된 151균주 대장균의 18종 항균제에 대한 내성및 감 수성 결과는 Table 3과 같다. 18종의 항균제 중 ampicillin (23.2%)에가장높은내성율을보이며, 다음으로 amoxicillin (22.5%), ticarcillin (20.5%), tetracycline (18.5%), nalidixic acid (12.6%), ciprofloxacin (10.6%), streptomycin (9.9%), 및 chloramphenicol (6.6%) 순서로내성을보였다. Cefotaxime, cefoxitin, ceftriaxone, cephalothin, cephazolin, gentamicin, 및 kanamycin은 5% 이하의낮은내성율을보이며, amikacin, cefepime, 및 imipenem 등 3종의항균제에대해서는모든균 주가감수성인것으로확인되었다. 23.2%로내성율이가장높 은 ampicillin의경우, Son et al. (2009)이남해안어류양식장에 서분리한대장균의 ampicillin에대한내성율이 46.2%이였다 는결과및 Jo et al. (2016)이서해안패류양식장에서분리한대 장균의 ampicillin에대한내성율이 37.2%이였다는결과보다 는다소낮은수준이나, Park (2013)이곰소만해수에서분리 한대장균의 ampicillin에대한내성율이 22.1%이였다는결과 와는매우비슷한데이는대장균의분리장소가동일하기때문 인것으로판단된다.
22.5%의내성율을나타내는 amoxicillin의경우, Park et al.
(2013)이남해안패류양식장에서분리한대장균은모두 amox- icillin에감수성이었다는결과및 Park (2013)의곰소만해수에 서분리한대장균의 amoxicillin 내성율은 0%이였다는결과와
는많은차이가있었으며, Jo et al. (2016)는서해안패류양식장 에서분리한대장균의 amoxicillin에대한내성율은 7.9%이였 다는결과및 Son et al. (2009)의남해안어류양식장에서분리
한대장균의 경우는내성율이 14.9%이였다는결과보다는다
소높았다. 본연구결과를기존의국내수산물유래대장균의 연구결과와비교해보면대장균의분리장소, 시기, 분리원에 따라항균제에 대한감수성및내성율은차이가있었는데특 히 amoxicillin, tetracycline, cephalothin, 및 streptomycin 등 의항균제에서차이가큰것으로확인되었다(Lee et al., 2003;
Son et al., 2009; Park, 2013; Park et al., 2013; Jo et al., 2016).
해수또는수산물에서분리된대장균의각종항균제에대한내 성차이는항균제및항균제내성균을포함하는가축분변의해 역으로유입및해역주변의양식장에서질병예방과치료를목 적으로사용되고있는항균제에의한내성균의발생이주원인 으로추측된다.
대장균의 항균제 내성 양상
전체 151균주중실험에사용한 18종의항균제중어느하나 라도 내성을나타내는 대장균은 54균주(35.8%)이였으며, 나 머지 97균주(64.2%)는모든항균제에감수성을보였다(Table 4). 4종이상의항균제에내성을나타내는다제내성균(multiple antimicrobial resistance bacteria, MARB)은 30균주(19.9%) 로 확인되었으며, 나머지 24균주(15.9%)는 4종이하의항균 Table 3. Antimicrobial resistance of Escherichia coli strains isolated from Ruditapes philippinarum in Gomso Bay
Antimicrobials Disc content
(μg) No. of isolates
Resistant Intermediate Susceptible
Amikacin (AK) 30 0 85 66
Amoxicillin (AML) 10 34 51 66
Ampicillin (AMP) 10 35 28 88
Cefepime (FEP) 30 0 0 151
Cefotaxime (CTX) 30 3 48 100
Cefoxitin (FOX) 30 1 27 123
Ceftriaxone (CRO) 30 3 38 110
Cephalothin (KF) 30 3 72 76
Cephazolin (KZ) 30 5 37 109
Chloramphenicol (C) 30 10 30 111
Ciprofloxacin (CIP) 5 16 61 74
Gentamicin (GN) 10 1 44 106
Imipenem (IPM) 10 0 14 137
Kanamycin (K) 30 4 82 65
Nalidixic acid (NA) 30 19 16 116
Streptomycin (S) 10 15 106 30
Tetracycline (TE) 30 28 43 80
Ticarcillin (TIC) 75 31 13 107
제에내성을보였다. 1종의항균제에서내성을나타내는균주 는 12균주(8.0%)이였는데 특히 tetracycline에내성을나타내 는균주는 8균주로다른항균제에비해내성균주가많았다. 3
종의항균제에내성을나타내는균주는 7균주(4.6%)이였는데
amoxicillin-ampicillin-ticarcillin의내성조합이 5균주로가장 많은것으로파악되었다. 5종의항균제에내성을나타내는균주 는 13균주(8.6%)로다른조합보다도가장높은빈도를보였는
데 amoxicillin-ampicillin-cephazolin-nalidixic acid-ticarcillin 의 조합이 8균주로가장많았다. 가장다양한항균제에내성 을나타내는조합은 amoxicillin-ampicillin-cefotaxime-ceftri- axone-cephalothin-cephazolin-nalidixic acid-streptomycin- tetracycline-ticarcillin의 10종의항균제에내성을나타내는 1
균주(0.7%)이였다. 결과적으로곰소만해역의바지락에서분
리한대장균은다제내성균의비율이비다제내성균의비율보다 Table 4. Antimicrobial resistance profiles of Escherichia coli strains isolated from Ruditapes philippinarum in Gomso Bay
No. of antimicrobials Antimicrobial resistance profiles No. of resistance isolates Total (%)
0 97 64.2
1
AMP 1
C 1 8.0
S 2
TE 8
2
AML-AMP 1
C-CIP 1 3.3
K-TE 1
S-TE 2
3
AML-AMP-TIC 5
4.6
C-S-TE 1
K-NA-TE 1
4
AML-AMP-C-TE 1
5.3
AML-AMP-CIP-NA 1
AML-AMP-CIP-TIC 1
AML-AMP-NA-TIC 2
AML-AMP-S-TIC 1
AML-AMP-TE-TIC 1
C-CIP-NA-TE 1
5
AML-AMP-C-TE-TIC 1
8.6
AML-AMP-FOX-KZ-TIC 1
AML-AMP-KZ-NA-TE 1
AML-AMP-KZ-NA-TIC 8
AML-AMP-S-TE-TIC 1
KZ-NA-S-TE-TIC 1
6 AML-AMP-C-S-TE-TIC 2 1.3
7
AML-AMP-C-K-S-TE-TIC 1
AML-AMP-C-NA-S-TE-TIC 1 2.7
AML-AMP-CTX-CIP-NA-S-TIC 1
AML-AMP-CTX-CRO-KF-KZ-TIC 1
9 AML-AMP-CIP-GN-K-NA-S-TE-TIC 1
AML-AMP-CRO-KF-KZ-CIP-NA-TE-TIC 1 1.3
10 AML-AMP-CTX-CRO-KF-KZ-NA-S-TE-TIC 1 0.7
AML, amoxicillin; AMP, ampicillin; CTX, cefotaxime; FOX, cefoxitin; CRO, ceftriaxone; KF, cephalothin; KZ, cephazolin; C, chloram- phenicol; CIP, ciprofloxacin; GN, gentamicin; K, kanamycin; NA, nalidixic acid; S, streptomycin; TE, tetracycline; TIC, ticarcillin.
높다는점에서심각성이대두된다.
내성 항균제에 대한 대장균의 최소발육억제농도 측정 대장균에내성을나타내는 15종항균제의 MIC를측정한결 과는 Table 5과같다. 내성을갖는모든균주의 MIC가 512 µg/
mL 이상인항균제는 amoxicillin, ampicillin, cefoxitin, chlor- amphenicol, ciprofloxacin, nalidixic acid, 및 ticarcillin 등으 로이들항균제의 MIC는다른항균제에비해매우높은편이 였다. Amoxicillin에내성을나타내는 34균주중 MIC가 2,048 µg/mL인균주는 27균주(79.4%), MIC가 1,024 µg/mL인균 주는 7균주(20.6%)로평균 MIC는 1,837 µg/mL이였다. Am- picillin에내성을나타내는 35균주중 MIC가 2,048 µg/mL인 균주는 15균주(42.9%), 1,024 µg/mL의 MIC를나타내는균주 는 11균주(31.4%), 512 µg/mL의 MIC를나타내는균주는 9 균주(25.7%)로 평균 MIC는 1,331 µg/mL이였다. Cefoxitin, chloramphenicol, 및 ciprofloxacin에 대한모든 내성균주의 MIC는 512 µg/mL으로파악되었다. Nalidixic acid에내성을 나타내는 19균주중 17균주(89.5%)의 MIC는 2,048 µg/mL이 였고나머지 2균주(10.5%)는 512 µg/mL로평균 MIC는 1,886 µg/mL이였다. Ticarcillin에내성을나타내는 31균주중 MIC 가 2,048 µg/mL인균주는 24균주(77.4%), MIC가 1,024 µg/
mL인균주는 7균주(22.6%)로평균 MIC는 1,817 µg/mL이였 다. 또한 streptomycin 및 tetracycline의평균 MIC는 324 및 158 µg/mL으로다른항균제에비해 MIC는낮은편이었다. 기 존수산물유래대장균의항균제 MIC에관한연구결과가없 어본연구결과와직접비교할수는없지만 Kuo et al. (2009)가
건강한닭및돼지에서분리한대장균의대부분은 ampicillin, chloramphenicol, 및 nalidixic acid 등의항균제에대한 MIC가 512 및 1,024 µg/mL 이상으로높게나타났다고보고한결과및 Cho et al. (2008)가수생조류에서분리한대장균의 tetracycline 에대한 MIC 범위가 16-512 µg/mL으로나타났다는결과와대 체로유사하였다. 결과적으로곰소만해역의바지락에서분리 한대장균의 15종항균제에대한 MIC는 32-2,048 µg/mL으로 MIC의범위가넓고높다는것이특징인것으로확인되었다. 또 한다제내성균의양상이보편화되어있다는점에서곰소만해 역의해수및바지락유래대장균의항균제내성에관한지속적 인모니터링이절실히필요하다고판단된다.
References
Acar JF and Goldstein FW. 1991. Disk susceptibility test. In:
Antibiotics in Laboratory Medicine, Lorian V, ed. Williams
& Wilkins, Baltimore, U.S.A., 17-52.
A.P.H.A. 1970. Recommended procedures for the examination of seawater and shellfish. 4th Ed. American public Health Association, Washington D. C., U.S.A., 1-47.
Byun JW, Kim HY, Jung BY, Bae YC and Lee WK. 2012. An- timicrobial resistance and frequency of BlaTEM in Esche
richia coli isolated from non-diarrheic and diarrheic piglets.
Korean J Vet Res 52, 133-139.
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Mil- lanao A and Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial re- sistance and to animal and human health. Environ Microbiol Table 5. Minimum inhibitory concentrations of Escherichia coli strains isolated from Ruditapes philippinarum in Gomso Bay
Antimicrobials μg/mL
32 64 128 256 512 1024 2048
Amoxicillin 7 (20.6%) 27 (79.4%)
Ampicillin 9 (25.7%) 11 (31.4%) 15 (42.9%)
Cefotaxime 1 (33.3%) 2 (66.7%)
Cefoxitin 1 (100.0%)
Ceftriaxone 1 (33.3%) 2 (66.7%)
Cephalothin 3 (100%)
Cefazolin 1 (25.0%) 4 (75.0%)
Chloramphenicol 10 (100%)
Ciprofloxacin 16 (100%)
Gentamicin 1 (100%)
Kanamycin 1 (25.0%) 3 (75.0%)
Nalidixic acid 2 (10.5%) 17 (89.5%)
Streptomycin 4 (26.7%) 4 (26.7%) 2 (13.3%) 3 (20.0%) 2 (13.3%)
Tetracycline 2 (7.1%) 2 (7.1%) 15 (53.6%) 9 (32.2%)
Ticarcillin 7 (22.6%) 24 (77.4%)
15, 1917-1942. http://dx.doi.org/10.1111/1462-2920.12134.
Cho JK, Lee SM and Kim KS. 2008. Antimicrobial resistance and distribution of tetracycline resistance determinants in Escherichia coli isolated from aquatic birds. Korean J Vet Res 43, 295-303.
Cho SH, Lim YS and Kang YH. 2012. Comparison of antimi- crobial resistance in Escherichia coli strains isolated from healthy poultry and swine farm workers using antibiotics in Korea. Osong Public Health Res Perspect 3, 151-155. http://
dx.doi.org/10.1016/j.phrp.2012.07.002.
Han SB, Lee SC, Lee SY, Jeong DC and Kang JH. 2015. Ami- noglycoside therapy for childhood urinary tract infection due to extended-spectrum β-lactamase-producing Esch
erichia coli or Klebsiella pneumoniae. BMC Infect Dis 15, 414. http://dx.doi.org/10.1186/s12879-015-1153-z.
Jo MR, Park YS, Park K, Kwon JY, Yu HS, Song KC, Lee HJ, Oh EG, Kim JH, Lee TS and Kim PH. 2016. Antimicrobial resistance in Escherichia coli isolated from shellfish farms on the west coast of Korea. Korean J Fish Aquat Sci 49, 13- 19. http://dx.doi.org/10.5657/KFAS.2016.0013.
Kang HY, Jeong YS, Oh JY, Tae SH, Choi CH, Moon DC, Lee WK, Lee YC, Seol SY, Cho DT and Lee JC. 2005. Charac- terization of antimicrobial resistance and class 1 integrons found in Escherichia coli isolates from humans and animals in Korea. J Antimicrob Chemother 55, 639-644.
Kim HB, Jang JY, Chang BJ, Kim AR and Choe BH. 2016.
Prevalence and antimicrobial resistance of Salmonella spp. and Escherichia coli isolated from ducks in Korea.
Korean J Vet Res 56, 91-95. http://dx.doi.org/10.14405/
kjvr.2016.56.2.91.
Kim S, Sung JY and Choi SG. 2015. Molecular characteriza- tion of Escherichia coli isolates from humans and chickens in the Chungcheong area using MLST analysis. Korean J Clin Lab Sci 47, 71-77. http://dx.doi.org/10.15324/kj- cls.2015.47.2.71.
Kim YH. 2016. Conditional probability analysis of multidrug resistance in Gram-negative bacilli isolated from tertiary medical institutions in South Korea during 1999-2009. J Microbiol 54, 50-56. http://dx.doi.org/10.1007/s12275-016- 5579-9.
Kümmerer K. 2009. Antibiotics in the aquatic environment -A review- Park I. Chemosphere 75, 417-434. http://dx.doi.
org/10.1016/j.chemosphere.2008.11.086.
Kuo HC, Chou CC, Tu C, Gong SR, Han CL and Liao JW.
2009. Characterization of plasmid-mediated quinolone re- sistance by the qnrS gene in Escherichia coli isolated from healthy chickens and pigs. Vet Med 54, 473-482.
Lee JI, Han GY and Park HH. 2003. Characteristics and antibi- otics susceptibility of Escherichia coli isolated from fishery products. Korean J Food Nutr 16, 111-115.
Lee YJ, Kim AR, Jung SC, Song SW and Kim JH. 2005. Antibi- otic resistance pattern of E. coli and Salmonella spp. isolated
from chicken feces. Korean J Vet Serv 45, 75-83.
Martinez JL. 2009. The role of natural environments in the evo- lution of resistance traits in pathogenic bacteria. Proc Biol Sci 276, 2521-2530.
National Committee for Clinical Laboratory Standards (NC- CLS). 2002. Performance standards for antimicrobial sus- ceptibility testing. Twelfth informational supplement M100- S12. Wayne, Pennsylvania, U.S.A., 19087-19098.
Park K, Jo MR, Yu HS, Lee HJ, Kim JH, Oh EG, Shin SB, Kim YK and Lee TS. 2013. Antimicrobial resistance Escherichia coli isolated from shellfish farms in the southern coast of Korea. Korean J Fish Aquat Sci 46, 528-533. http://dx.doi.
org/10.5657/KFAS.2013.0528.
Park KS. 2013. Antimicrobial resistance and virulence genes presence in Escherichia coli strains isolated from Gomso Bay, Korea. Fish Aquat Sci 16, 221-227. http://dx.doi.
org/10.5657/FAS.2013.0221.
Rowe-Magnus DA, Guerout AM and Mazel D. 2002. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol Microbiol 43, 1657-1669.
Schwartz T, Kohnen W, Jansen B and Obst U. 2003. Detec- tion of antibiotic resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol Ecol 43, 325-335. http://dx.doi.
org/10.1111/j.1574-6941.2003.tb01073.x.
Son KT, Oh EG, Park K, Kwon JY, Lee HJ, Lee TS and Kim JH.
2009. Antimicrobial susceptibility of Escherichia coli iso- lated from fish farms on the southern coast of Korea. Kore- an J Fish Aquat Sci 42, 322-328. http://dx.doi.org/10.5657/
KFAS.2009.0322.
Turner SM, Scott-Tucker A, Cooper LM and Henderson IR.
2006. Weapons of mass destruction: virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol Lett 263, 10-20. http://dx.doi.org/10.1111/j.1574- 6968.2006.00401.x.
Van TTH, Chin J, Chapman T, Tran LT and Coloe PJ. 2008.
Safety of raw meat and shellfish in Vietnam; an analysis of Escherichia coli isolations for antibiotic resistance and viru- lence genes. Int J Food Microbiol 124, 217-223.
Xu G, An W, Wang H and Zhang X. 2015. Prevalence and char- acteristics of extended-spectrum β-lactamase genes in Esch
erichia coli isolated from piglets with post-weaning diarrhea in Heilongjiang province, China. Front Microbiol 6, 1103.
http://dx.doi.org/10.3389/fmicb.2015.01103.