For patients who require long-term chronic hemodialysis but with the obstruction of the usual central veins, innovative salvage central venous access techniques provide successful alternatives. These techniques are recanalization of occluded central veins, translumbar, transhepatic or transrenal inferior vena cava access, and catheterization of collateral veins. The inferior vena cava is large and can easily accommodate hemodialysis catheters and these access routes are in general quite durable. Success rates for these access techniques are relatively high and complications are uncommon. Once placed, devices are managed and exchanged like any routinely placed venous access device. The choice of technique and access route will depend on the patient’s anatomy, the device availability, and the operator’s expertise.
Key Words: Hemodialysis, Catheter, Salvage Therapy
Hemodialysis Access Salvage Techniques in Patients with Exhausted Access
Ji Hoon Shin
Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
INTRODUCTION
Long-term chronic hemodialysis in patients with end- stage renal disease (ESRD) requires a durable vascular access.
Prolonged venous access often leads to recurrent infections, vascular stenosis, and vascular thrombosis, which contribute to progressive loss of vascular access and significant morbidity and mortality [1]. ESRD patients typically have complex medical problems and present as a comprehensive challenge to their team of nephrologists, vascular surgeons, and interventional radiologists [2].
Despite our success in increasing the use of reliable, image- guided central venous access procedures, ingenious solutions must be addressed in the management of complicated patients with central venous occlusions. These salvage procedures are considerably more complex than the usual venous access procedures and frequently present a challenge to individual interventional radiologists for maintaining their competency [1].
This article will review the commonly performed techniques used for “salvage” central venous access.
PROCEDURE INDICATIONS AND CLINICAL EVALUATION
The indication for salvage venous access techniques is a lack of or contraindication to a standard central venous access through jugular or subclavian veins. Typical examples include patients with superior vena cava (SVC) obstruction, or patients with unilateral brachiocephalic vein obstruction in whom access using the contralateral side is contraindicated.
A detailed history of a patient's venous anatomy and prior access sites and physical examination are essential in choosing both the best salvage technique to use as well as the device.
The presence of coagulopathy should be addressed and both bleeding and thrombotic disorders must be checked in advance.
Physical examination includes an evaluation for dilated
Corresponding Author : Ji Hoon Shin
Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea
Tel: 82-2-3010-5665, Fax: 82-2476-0090, E-mail: jhshin@amc.seoul.kr
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Copyright © The Korean Society for Dialysis Access | eISSN: 0000-0000
superficial collateral veins, extremity edema, and scars from previous accesses and devices and provides clues regarding sites of stenosis. Before deciding the salvage techniques, all efforts should be made to ensure that any prior access sites cannot be salvaged, including recanalization of occluded central veins.
Current imaging studies should be correlated in order to understand the venous anatomy. Ultrasound evaluation or diagnostic venography can aid in determining if traditional catheter access sites can be salvaged [3]. CT scans of the chest and abdomen are particularly helpful in clearly evaluating venous anatomy as well as the relationship of patent veins to superficial anatomy. If no imaging studies are available, performing an anatomic study before attempting a salvage technique is highly recommended in selected patients.
CHOICE OF VENOUS ACCESS FOR SALVAGE TECHNIQUES
The advantages and disadvantages of salvage techniques are summarized in Table 1. When occlusion of the right internal jugular vein, which is usually the first site for hemodialysis access, is identified, recanalization of the right internal jugular vein is attempted first, after which the right external jugular vein or left side internal or external jugular vein should be considered. In anticipation of a left arm arteriovenous fistula/
graft, the right external jugular vein is preferred (Fig. 1).
There is controversy over which approach is the best next access. The femoral vein is technically easy, however, femoral vein catheters are more susceptible to infection as well as occlusion, and thus requiring frequent catheter care [4].
Placement of femoral catheters has also the definite disadvantage of limiting patient mobility during walking and exercise. Then, supradiaphragmatic collateral vein access and as a next step, infradiaphragmatic access, such as translumbar inferior vena cava (IVC) or transhepatic, can be considered in order to avoid the high complication rates of femoral access sites.
RECANALIZATION OF OCCLUDED CENTRAL VEINS
It should be attempted to preserve traditional access sites
Table 1. Advantages and disadvantages of salvage central venous access techniques
Advantages Disadvantages
Recanalization of occluded central
veins Can preserve future alternative access
routes Technical success depends on the degree of obstruction Venous occlusion can worsen venous blockage (edema) Translumbar IVC access Relatively secure long-term hemodialysis Difficult IVC puncture
Catheter dislodgement due to patient motion
Retroperitoneal hemorrhage, aorta or ureter injury can occur
Transhepatic IVC access Technically easy Catheter kinking / migration due to respiration Relatively short catheter patency due to thrombosis Intraperitoneal hemorrhage or hemothorax can occur Transrenal IVC access Seems more durable than transhepatic
access Risk of renal artery injury
Access through collateral veins Can preserve future alternative access
routes Collateral veins can be too small
Procedure can be complicated
*IVC, inferior vena cava.
A B
Fig. 1. A 64-year-old female patient with an obstructed right internal jugular vein. (A) The right external jugular vein was punctured and its venogram shows good patency into the right atrium. Note the typical, curved course (arrows) of the external jugular vein. (B) A non-tunneled hemodialysis catheter (arrows) was successfully placed.
even if chronically occluded. This traditional crossing technique is to use occluded veins using guidewire recanalization, angioplasty, and stent placement [5,6] (Fig. 2). Negotiation of a guidewire through an occluded vein is a basic technique:
A 0.035-inch guidewire (often a stiff guidewire) is initially used after puncturing the occluded vein or its proximal patent segment using a micropuncture kit. A catheter and guidewire are occasionally advanced through the occluded jugular vein using the femoral approach. From the occluded vein access site, the guidewire could be grasped using a snare and pulled through the occluded vein. In this manner, through-and-through access is obtained which allows vein dilatation and catheter placement.
This is a technically appealing approach, however, technical success depends largely on the degree of venous obstruction.
Venous strictures can also occur at the tip of a catheter because of local thrombosis or vein irritation. Occlusion of the vein may obstruct the catheter and may worsen symptoms of venous blockade, such as edema [1].
TRANSLUMBAR IVC ACCESS
This translumbar IVC catheterization has many advantages.
First of all, the IVC can accommodate any catheter currently available, rarely thromboses completely, and allowing multiple times of replacement. A preoperative CT scan is recommended to confirm normal IVC anatomy and to help plan the best position and angle for needle placement. In adults, the use of
single- and multi-lumen catheters ranging in size from 6 to 18 French has been reported [1].
1. Technique
The two, brief procedure steps are the establishment of venous access and tunneling the catheter (Fig. 3).
1) For venous access, with the patient in prone position, advance a needle (21-gauge needle of appropriate length based on pre-procedure evaluation) 45º medial and cephalad from the skin entry point (approximately 5 cm above the iliac crest and 10 cm right of the midline [3]. Access to the IVC can be obtained under CT guidance or assistance. The guidewire, catheter or balloon catheter into the IVC through patent femoral/popliteal or hepatic venous access, will be a target for percutaneous IVC access (Fig. 3). IVC opacification by intermittent contrast injections from collateral networks is also helpful for making the exact puncture. After confirming needle placement in the IVC by contrast injection, pass a 0.018-inch guidewire into the right atrium and exchange it for a coaxial transitional sheath, i.e. Neff set: Cook, Bloomington, IN, USA. A150-cm long, stiff 0.035-inch guidewire is then inserted into the IVC in the routine fashion using a coaxial transitional sheath.
2) Tunneling of the catheter is performed between the vein entry point and the exit site near the mid-axillary line. Be sure that longer peel-away sheaths of a suitable diameter for the hemodialysis catheter are available before starting the procedure.
Then serially dilate the tract and insert a peel-away sheath. Insert
A B C D
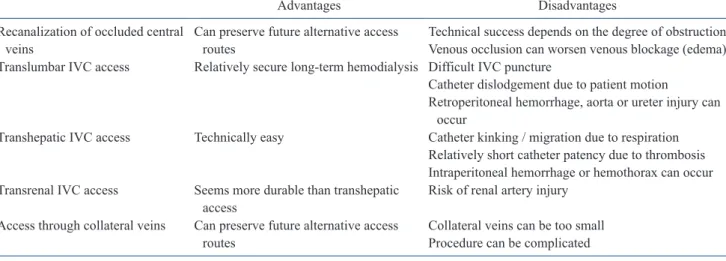
Fig. 2. A 74-year-old female patient with an obstructed left internal jugular vein. The right internal jugular vein was also obstructed (not shown). (A) A left internal jugular venogram taken after using a micropuncture kit, shows nearly complete obstruction (arrows) of the left brachiocephalic vein. (B) After successful negotiation of the tight stricture with a stiff 0.035-inch guidewire, the nearly obstructed, left brachiocephalic vein was dilated using a 6-mm-diameter balloon catheter (arrows). (C, D) The hemodialysis catheter could be inserted after an additional 8-mm balloon dilatation (arrows) of the stenotic superior vena cava over the stiff guidewire.
the catheter confirming it is in the mid- to low right atrium. Peel away the sheath and close access site in the routine manner.
2. Outcomes and Complications
Translumbar hemodialysis catheters seem to have a higher rate of dysfunction compared to those with traditional access.
In the same large series, the 1- and 3-year cumulative assisted patency rates of translumbar hemodialysis catheters were 73.2% and 27.9%, respectively [7]. At the same medical center, conventional jugular hemodialysis catheters had assisted patency rates of 77.8% and 44.0% at one and three years, respectively [8].
In one large series based on 84 translumbar catheter insertions in 28 patients [9], the initial, revised, and total catheter placement interval was 65, 84, and 244 days respectively, and the initial catheter patency rate at three, six, and 12 months was 43%, 25%, and 7%, respectively.
Retroperitoneal hemorrhage, aortic puncture, ureteral injury, and puncture of an abdominal viscus, such as the duodenum or colon can occur during the procedure. Catheter tip migration can occur due to the combination of patient and respiratory motion.
It is recommended to leave the catheter tip approximately 2 cm above its final desired position. A translumbar catheter can be removed using standard techniques if it becomes nonfunctional, infected or unnecessary, however, coagulation and platelet studies with correction are warranted before removal.
TRANSHEPATIC IVC ACCESS
Transhepatic venous access to the IVC and right atrium is obtained via the right or middle hepatic vein (Fig. 4). Review of available CT scans or ultrasound is desirable to evaluate patency of hepatic veins, variant anatomy, and estimate catheter lengths.
1. Technique
Advance a 21-gauge needle into the liver aiming through the intercostal space toward the T12 vertebral body with a similar needle trajectory as that of the percutaneous transhepatic biliary drainage. A subcostal approach should be avoided due to the tendency to kink and dislodge with respiration. Slowly inject contrast as the needle is withdrawn until the hepatic vein is visualized. After confirming needle placement in the IVC by contrast injection, pass a 0.018-inch guidewire into the right atrium and exchange it for a coaxial transitional sheath followed by insertion of a 150-cm long, stiff 0.035-inch guidewire into the SVC using a coaxial transitional sheath in the routine fashion.
Create a subcutaneous tunnel for the hemodialysis catheter and insert it in the normal fashion through a peel-away sheath following tract dilatation.
2. Outcomes and Complications
Immediate catheter failures are most often due to migration and can be minimized by placing the catheter tip in the mid-
A B C D
Fig. 3. Translumbar inferior vena cava (IVC) access in a 54-year-old male patient with exhausted venous access in the upper thorax.
(A) A venogram via the left internal jugular vein shows complete obstruction of the superior vena cava. (B) The inflated 20-mm- diameter balloon catheter which was inserted through the right femoral vein, was punctured using a 21-gauge Chiba needle, and an inserted, 0.018-inch guidewire (arrows) could be confirmed to be in the lumen of the balloon catheter, and which indicates successful IVC cannulation. (C) After exchanging with a stiff 0.035-inch guidewire, serial dilation (up to 14Fr, arrows) was performed. Note the inserted guidewire and deflated balloon catheter (arrowheads) via the right femoral vein along the IVC course. (D) The final spot radiograph with the patient in the prone position shows the successfully inserted hemodialysis catheter with the tip at the entrance of the right atrium.
or even upper right atrium so as to avoid caudal migration into the hepatic veins caused by respiratory motion [3]. The mean primary and secondary patency rates were 27 days and 70 days, respectively, with an average of 7.5 exchanges in order to maintain catheter functionality [10,11].
A perihepatic hematoma or hepatic arterial injury can occur during the procedure. Early complications include high rates of dislodgement and migration (37%), catheter-related sepsis (21.7%), and catheter thrombosis (17.4%) [11].
TRANSRENAL IVC ACCESS
Transrenal venous access to the IVC can be considered when conventional and translumbar access options are exhausted (Fig.
5).
1. Technique
Identify the mid- or inferior renal parenchyma and advance a 21-gauge Chiba needle into the parenchyma under ultrasound guidance with a similar needle trajectory of the placement of a percutaneous nephrostomy tube. Segmental interpolar veins are the ideal target. Slowly inject contrast as the needle is withdrawn until a renal vein tributary is visualized.
Once contrast injection confirms the intraluminal location in a renal vein, pass a 0.018-inch guidewire, i.e. V-18 200-cm, Boston Scientific, Watertown, MA, USA, into the right atrium and exchange for a coaxial transitional sheath (Fig. 5). Then a 150-cm long, stiff 0.035-inch guidewire is inserted into the right
A B
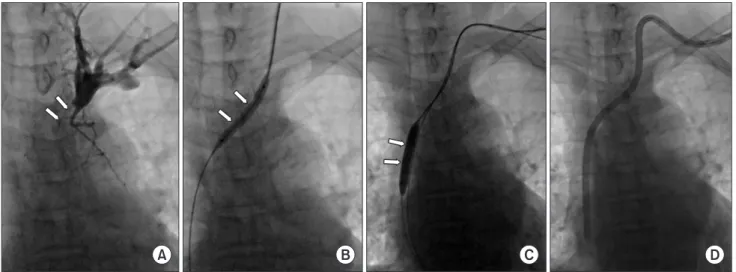
Fig. 4. Transhepatic venous access in a 47-year-old female patient with exhausted venous access in the upper thorax. (A) The right hepatic vein was successfully punctured with a 21-gauge needle (arrows) with the opacification of the right hepatic vein by contrast injection (arrowheads). (B) After serial dilation of the tract, a hemodialysis catheter could be in serted with the tip in the right atri um.
A B
Fig. 5. Transrenal inferior vena cava (IVC) access in a 64-year- old female patient with exhausted venous access in the upper thorax.
(A) After several attempts, the left renal vein was successfully punctured with a 21-gauge needle (arrows) and a 0.018-inch guidewire (arrowheads) was inserted into the IVC. (B) After serial dilation of the tract, a hemodialysis catheter could be inserted with the tip in the upper IVC.
atrium in the routine fashion. Create a subcutaneous tunnel for the hemodialysis catheter and insert the hemodialysis catheter in the normal fashion through a peel-away sheath following tract dilatation.
2. Outcomes and Complications
Transrenal hemodialysis catheters seem more durable than transhepatic catheters. In one case report, the catheter functioned without further intervention for six months, at which point the patient was lost to follow-up [12]. In another case report, the catheter functioned for two years, at which time it became thrombosed and was successfully exchanged over a wire after fibrin sheath disruption [13].
The risk of arterial injury can occur, however, embolization can be performed for arterial injury.
ALTERNATIVE ACCESS THROUGH COLLATERAL VEINS
Even in patients with extensive central vein occlusion, including the IVC, alternative routes of percutaneous catheterization can frequently be found. Collateral veins can often be hypertrophied from high venous flow secondary to central vein obstruction. The modern angiography suite with digital angiography and imaging tools, such as rotational angiography, cone-beam CT, and road mapping, can assist in the localization and puncture of small collateral veins [1]. Small- vessel catheterization systems, including 21-gauge needles, 0.018-inch (0.46 mm) platinum or gold-tipped guidewires, and small sheaths, are used for cannulation and passage of catheters through collateral networks to the central veins. However,
collateral veins may be too small to allow for the insertion of large-caliber devices, such as hemodialysis catheters.
Azygos and hemiazygos veins could be used by percutaneous puncture of these veins under fluoroscopic control during contrast injection using a catheter in the left renal vein. A guidewire, catheter, and sheath are advanced through the hemiazygos and azygos veins to the SVC and right atrium [14,15]. The accessory hemiazygos vein can also be utilized for venous access (Fig. 6). As a consequence of brachiocephalic vein occlusion or stenosis, collateral circulation via the superior right intercostal and azygos veins (right side of the body) or left superior intercostal and accessory hemiazygos vein (left side of the body) will develop [16]. Through the intercostal route, in addition to 6 Fr or 6.5 Fr tunneling catheter, a hemodialysis catheter could be inserted with the catheter tip into azygos or hemiazygos/accessory hemiazygos veins, depending on the anatomy.
CONCLUSION
In patients with obstructed routine access, there are multiple choices available for salvage of central venous access. Choice of the technique and access route will depend on the device availability, the patent’s anatomy, and the operator’s own skill.
REFERENCES
1. Denny DF. Venous access salvage techniques. Tech Vasc Interv Radiol, 2011; 14(4): 225-32.
2. Lew SQ, Nguyen BN, Ing TS. Unusual sites for hemodialysis vascular access construction and catheter placement: A
A B
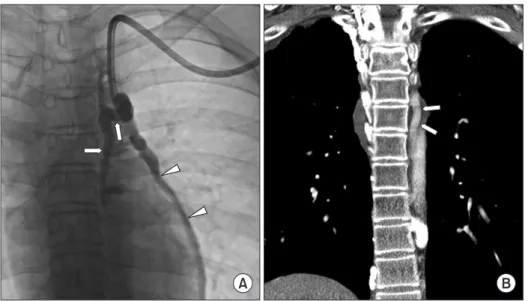
Fig. 6. Hemodialysis catheter into the accessory hemiazygos vein in a 17-year-old male patient with bilateral brachiocephalic veins and superior vena cava obstruction.
(A) A hemodialysis catheter was inserted into the entrance into the accessory hemiazygos vein (arrows) through the left internal jugular vein. Note the left pericardiophrenic vein (arrowheads). (B) On a coronal reconstructed CT scan, a co r res- ponding, prominent accessory he- mi azygos vein is noted (arrows).
review. Int J Artif Organs, 2015; 38(6): 293-303.
3. Rahman S, Kuban JD. Dialysis catheter placement in patients with exhausted access. Tech Vasc Interv Radiol, 2017; 20(1):
65-74.
4. Zaleski GX, Funaki B, Lorenz JM, et al. Experience with tunneled femoral hemodialysis catheters. AJR Am J Roentgenol, 1999; 172(2): 493-6.
5. Ferral H, Bjarnason H, Wholey M, et al. Recanalization of occluded veins to provide access for central catheter placement. J Vasc Interv Radiol, 1996; 7(5): 681-5.
6. Pereira K, Osiason A, Salsamendi J. Vascular access for placement of tunneled dialysis catheters for hemodialysis: A systematic approach and clinical practice algorithm. J Clin Imaging Sci, 2015; 5: 31.
7. Power A, Singh S, Ashby D, et al. Translumbar central venous catheters for long-term haemodialysis. Nephrol Dial Transplant, 2010; 25(5): 1588-95.
8. Duncan ND, Singh S, Cairns TD, et al. Tesio-Caths provide effective and safe long-term vascular access. Nephrol Dial Transplant, 2004; 19(11): 2816-22.
9. Liu F, Bennett S, Arrigain S, et al. Patency and complications of translumbar dialysis catheters. Semin Dial, 2015; 28(4):
E41-7.
10. Stavropoulos SW, Pan JJ, Clark TW, et al. Percutaneous
transhepatic venous access for hemodialysis. J Vasc Interv Radiol, 2003; 14(9 Pt 1): 1187-90.
11. Younes HK, Pettigrew CD, Anaya-Ayala JE, et al.
Transhepatic hemodialysis catheters: Functional outcome and comparison between early and late failure. J Vasc Interv Radiol, 2011; 22(2): 183-91.
12. Murthy R, Arbabzadeh M, Lund G, et al. Percutaneous transrenal hemodialysis catheter insertion. J Vasc Interv Radiol, 2002; 13(10): 1043-6.
13. Law WP, Cheung CY, Chan HW, et al. Hemodialysis catheter insertion using transrenal approach. Hemodial Int, 2015;
19(4): E14-6.
14. Denny DF. Central venous access via the hemizygous vein. In: Trerotola SO, Savader SJ, Durham JD. VENOUS INTERVENTIONS. Fairfax, VA: Society of Vascular and Interventional Radiology, 1995; 507-10.
15. Jaber MR, Thomson MJ, Smith DC. Azygos vein dialysis catheter placement using the translumbar approach in a patient with inferior vena cava occlusion. Cardiovasc Intervent Radiol, 2008; 31 Suppl 2: S206-8.
16. Kaminski R, Strozecki P, Kosinski A, et al. Left superior intercostal vein as the last resort for hemodialysis vascular access. J Vasc Access, 2016; 17(1): e5-6.