ABSTRACT
Purpose: To compare between sodium acetate (SA) and sodium chloride (SC) in parenteral nutrition (PN) with associated metabolic acidosis and neonatal morbidities in preterm infants.
Methods: Preterm infants below 33 weeks gestational age, and with a birth weight under 1,301 g were enrolled and further stratified into two groups: i) <1,000 g, or ii) ≥1,000 g in birth weight. The subjects were randomized to receive PN containing SA or SC within the first day of life. The results of routine blood investigations for the first 6 days of PN were collated, and the neonatal outcomes were recorded upon discharge or demise.
Results: Fifty-two infants entered the study, with 26 in each group: 29 infants had extremely low birth weight (ELBW). There were no significant differences in birth weight, gestation, sex, exposure to chorioamnionitis and antenatal steroids, surfactant doses and duration of mechanical ventilation between groups. The SA group had significantly higher mean pH and base excess (BE) from days 4 to 6 than the SC (mean pH, 7.36 vs. 7.34; mean BE −1.6 vs. −3.5 [p<0.01]), with a two-fold increase in the mean BE among ELBW infants. Significantly fewer on SA required additional bicarbonate (n=4 vs. 13, p=0.01). The rate of bronchopulmonary dysplasia (BPD) was approximately four-fold lower in SA than SC (n=3 vs. 11, p<0.01). No significant differences were observed in necrotizing enterocolitis, patent ductus arteriosus, retinopathy of prematurity, cholestatic jaundice, and mortality between groups.
Conclusion: The use of SA in PN was associated with reduced metabolic acidosis and fewer BPD.
Keywords: Acidosis; Bronchopulmonary dysplasia; Infant, premature; Parenteral nutrition;
Sodium acetate; Sodium chloride
INTRODUCTION
Despite recent advances in the field of neonatology, it is still very much a challenge for neonatologists to provide optimal nutritional support for extremely preterm and very low birth weight (VLBW) infants. Early nutritional support, which is primarily delivered through parenteral nutrition (PN) during the first week, is essential in the management of this population. Full enteral feeding is generally delayed due to the prematurity of gastrointestinal
Original Article
Received: Sep 24, 2019 1st Revised: Nov 27, 2019 2nd Revised: Jan 10, 2020 3rd Revised: Mar 2, 2020 Accepted: May 1, 2020 Correspondence to Fook-Choe Cheah
Department of Paediatrics, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latiff, Bandar Tun Razak, 56000 Kuala Lumpur, Malaysia.
E-mail: cheahfc@ppukm.ukm.edu.my Copyright © 2020 by The Korean Society of Pediatric Gastroenterology, Hepatology and Nutrition
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://
creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ORCID iDs Adli Ali
https://orcid.org/0000-0001-5615-5966 Ee-Yan Ong
https://orcid.org/0000-0002-3008-4255 Birinder Kaur Sadu Singh
https://orcid.org/0000-0001-7485-2503 Fook-Choe Cheah
https://orcid.org/0000-0002-1137-6295
Adli Ali ,1 Ee-Yan Ong ,2 Birinder Kaur Sadu Singh ,2 and Fook-Choe Cheah 1
1Department of Paediatrics, Faculty of Medicine, University Kebangsaan Malaysia, Kuala Lumpur, Malaysia
2Department of Pharmacy, University Kebangsaan Malaysia Medical Center, Kuala Lumpur, Malaysia
Comparison Between Sodium Acetate
and Sodium Chloride in Parenteral
Nutrition for Very Preterm Infants on
the Acid-Base Status and Neonatal
Outcomes
Funding
This study was funded by the Universiti Kebangsaan Malaysia Medical Centre Fundamental Research Grant (FF-209-2010).
Conflict of Interest
The authors have no financial conflicts of interest.
function; these infants have poor sucking and swallowing reflexes, and there are concerns that a liberal feeding strategy may lead to complications, such as feeding intolerance or necrotizing enterocolitis (NEC) [1,2].
The neonatal kidney has a limited capacity to excrete and conserve sodium and other electrolytes, which results in infants undergoing natriuresis and diuresis in the first 48–72 hours of life. Furthermore, preterm neonates have a limited tubular capacity to reabsorb sodium, which leads to further sodium urinary losses [3,4]. As a result, sodium supplementation, which is achieved mainly via PN in the first week, is necessary in order to replace these losses.
In most parts of the world, sodium supplementation in PN has largely been in the form of sodium chloride (SC) [2]. However, due to decreased solute excretion, secondary to a lower glomerular filtration rate, VLBW infants are unable to process the chloride load efficiently;
thus, sodium replacement solely by SC predisposes infants to high cumulative chloride intake which may lead to hyperchloremia [3-10]. Previous studies have shown that there is a significant linear correlation between blood chloride concentration and metabolic acidosis in preterm infants [7,8]. In addition, hyperchloremic acidosis is associated with several clinical situations, such as renal tubular acidosis and renal failure, following intravenous administration of SC solution [11-15]. Metabolic acidosis is a common problem after infusion of chloride-based PN, as a result of hyperchloremic acidosis [7,8,16]. Indeed, previous investigators have reported an incidence of hyperchloremia ranging from 77 to 88% in preterm neonates receiving SC-based PN [7,8]. This may further compound the problem of metabolic acidosis, which occurs more commonly in the very preterm infant. Metabolic acidosis is associated with intraventricular hemorrhage (IVH) and pulmonary hypertension [17-20], which could further lead to increased ventilator support and bicarbonate therapy [7]. For mitigating the problem of hyperchloremic metabolic acidosis, the recent European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) guideline recommends the use of chloride-free electrolyte solutions, such as sodium acetate (SA) or sodium lactate (SL) in PN for preterm infants [21].
Some investigators have shown that SA is similar to bicarbonate in its ability to restore blood pH and plasma bicarbonate in patients suffering from metabolic acidosis due to acute chloremia [22,23]. SA is also considered a safe alternative for SC in PN and has been widely used by various neonatal intensive care units (NICU) across Europe, the USA, and Australia [1,2,7]. Nonetheless, only a few trials have evaluated the role of SA and its clinical significance when used in PN, especially in the VLBW infant population. Due to the limited evidence for SA in PN and its clinical impact, SC has remained the major mineral content in PN for many NICUs worldwide, including our unit, until recently. Changes in the practice of neonatology, particularly in ventilator and nutrition management makes it timely for us to reassess and evaluate the significance of SA in PN for the increasing population of VLBW and ELBW infants of lower gestations that are currently managed in the NICU.
Our study compared the occurrence of metabolic acidosis and incidence of neonatal co- morbidities among very preterm infants randomized to receive either SA or SC in PN.
MATERIALS AND METHODS
Design
This was a double-blind randomized controlled trial (RCT) conducted at the NICU of the Universiti Kebangsaan Malaysia Medical Centre from November 2010 to February 2012. This study was approved by the Hospital Research Ethics Committee (project reference: UKM- FF-209-2010) and registered as a clinical trial in the National Medical Research Registry (NMRR-12-1459-13857).
Patients
Our study population comprised preterm infants of less than 33 weeks' gestation, with a birth weight less than 1,301 g who were admitted to our NICU. Written informed consent was obtained for each recruited infant prior to the initiation of PN. Infants with an inborn error of metabolism, severe metabolic alkalosis, severe liver failure, and syndromic infants with multiple congenital abnormalities and severe perinatal asphyxia were excluded from the study.
Randomization
Infants who met the inclusion criteria and whose parents consented to participate were randomized to one of the two intervention groups (SA, or the standard SC) prior to the preparation and commencement of PN. Randomization was performed by pharmacists at the Sterile Preparation/Parenteral Unit of this hospital upon enrolment of each case. The randomization process was performed according to block randomization of four, using sealed, opaque envelopes that were sequentially numbered. For multiple births, each infant was individually randomized.
Intervention
PN was prepared based on the ESPEN/ESPGHAN Guidelines on Pediatric Parenteral Nutrition, Clinical Nutrition that was reviewed in 2006 [1]. Upon enrolment of each infant in the study, PN was prepared to include the assigned intervention of either SA or SC. Other PN details followed the unit protocol as ordered by the attending doctor using an online inpatient pharmacy system. The assigned SA or SC in PN was only known to the pharmacist who prepared the PN; the pharmacist was unaware of the infant's clinical state. Each assigned intervention was concealed in the form of similar appearing compounded bags of PN. The type of sodium used was the only masked item in the list of compounded ingredients. This was to allow adjustment of contents, if necessary, by the attending team of doctors. The PN trial code number assigned to each individual, and the corresponding SA or SC intervention status was kept by the pharmacist-in-charge. Breaking of the study code was possible upon request by the consultant in-charge for clinical indications, such as severe alkalosis.
Outcome measures
All relevant maternal and infant demographic data, blood investigation results, and clinical outcomes were recorded. The primary outcomes, pH, and base excess (BE) were recorded from pre-PN, and days 1–6 of PN. Blood samples were obtained from an in-dwelling arterial catheter or by capillary sampling. For day 1 samples, the latest blood gas within 6 to 12 hours of initiation of PN was used. On subsequent days (day 2 to day 6), if several samples were taken throughout the day, the first recorded value after 5 o'clock in the morning was used in order to standardize the sampling time before the nursing morning shift changeover.
Samples for blood gas analyses were obtained in an adjoining room within the NICU using an ABL 700 Series Blood Gas Analyzer (Serial Number-1902-441R0134N0) (Radiometer,
Copenhagen, Denmark), which was calibrated daily. Routine blood investigation was performed on days 1, 3, and 6 of PN, as per the unit protocol. The outcome data were obtained from the case notes upon patient discharge or death. Data related to PN, total fluid, and enteral feed intakes were obtained and collated at frequent intervals during the first week, and later as necessary throughout the NICU stay for each infant. Neonatal co- morbidities were determined by the attending clinician according to internationally accepted definitions and methods. Hemodynamically significant patent ductus arteriosus (PDA) was confirmed by echocardiography [24], and required medical intervention within the first 14 days of life. Bronchopulmonary dysplasia (BPD) was diagnosed when the infant remained on respiratory support and had classical chest radiographic changes at 28 days of life [25].
Other conditions reportable in this study included infants with severe intraventricular IVH of at least grade three classification [26], NEC of at least stage two according to the modified Bell criteria [27], and retinopathy of prematurity (ROP) of stage two and above [28,29]. Sepsis was diagnosed when clinical signs were present, with or without blood culture confirmation, supported by laboratory or radiological markers, and when the infant required antimicrobial therapy. Cholestatic jaundice was defined when the infant exhibited clinical signs plus elevated liver enzymes, and was diagnosed by the evidence of direct hyperbilirubinemia (direct bilirubin of more than 1 mg/dL or more than 20% of the total bilirubin) [30].
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Co., Armonk, NY, USA). Chi-square test was used to compare categorical variables, and the Student's t-test and Mann-Whitney U-test were used for normally distributed and skewed quantitative data, respectively. A p-value <0.05 was considered as statistically significant.
Sample size
Metabolic acidosis was compared in two groups of interventions (i.e., SA PN and SC/
standard PN) to calculate the sample size. pH and BE were used as the primary measurement of metabolic acidosis. Using one of the primary outcomes (pH), the appropriate sample size was calculated utilizing the PS; Power and Sample Size Calculation (version 3.1.6 software, Vanderbilt University, Nashville, TN, USA) [31]. A total of 26 infants were required in each intervention group to achieve the desired power of 80%, with a total of 52 infants.
RESULTS
Out of 61 potentially eligible infants, a total of 53 infants were enrolled in the study; 27 infants were randomized to the SA group and 26 infants to the SC group. One infant from the SA group was excluded from the analysis as the infant died before the initiation of PN. Two patients, one in each group, died during the study period, both on day 5 of PN. Data from 52 infants were analyzed (Fig. 1).
There were no significant differences in the birth characteristics between the two groups.
The mean birth weight and gestational age were similar for both groups. There were also no significant differences in the completion of antenatal steroids, the requirement of surfactant and mechanical ventilation, and exposure to chorioamnionitis (Table 1).
There were no significant differences in the commencement of PN, total sodium, and total fluids received during the study period between the two groups (Table 2). Total chloride
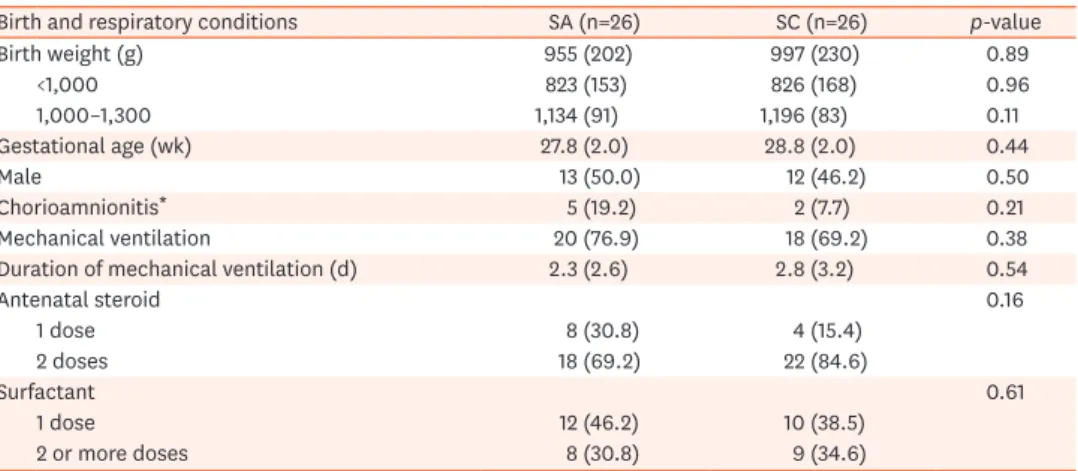
received in the SC group was expectedly and significantly higher (p-value=0.04), while the SA group had significantly higher levels of serum bicarbonate (p-value<0.01). This may attribute to the SA group requiring less bicarbonate therapy (p-value=0.01). There were no significant difference in the mean cumulative results over the 6 days of the study period for pH, BE, and pCO2 (Table 2). However, when the daily trends of the pH, BE, and pCO2 over the 6-day period were further analyzed, infants who received SA had a significantly higher mean pH and BE from days 4 to 6 than the SC group (mean pH, 7.36 vs. 7.34; mean BE, −1.6 vs. −3.5 [p<0.01]) (Fig. 2). Sub-group analysis of the ELBW infants showed a two-fold increase in their mean BE over the whole period, but there were no significant differences in pCO2 between groups, and also the subgroups of ELBW infants.
Sodium acetate
(n=27) Died before PN
initiated (n=1) Received intervention
as allocated (n=26) Sodium chloride
(n=26)
Completed trial (n=25)
Died within trial (n=1)
*
Received intervention as allocated
(n=26)
Completed trial (n=25)
Died within trial (n=1)
*
Randomisation Registered patients below 33 weeks gestational age,
birth weight <1.3 kg (n=61)
Not eligible for study (n=8)
1 No consent 3 Syndromic multiple
anomalies
4 Did not receive PN prior to 72 hours of life
Fig. 1. Enrolment of the study population.
PN: parenteral nutrition. *Died on day 5, during the intervention trial period.
Table 1. Clinical characteristics of infants who receive SA or SC in PN
Birth and respiratory conditions SA (n=26) SC (n=26) p-value
Birth weight (g) 955 (202) 997 (230) 0.89
<1,000 823 (153) 826 (168) 0.96
1,000–1,300 1,134 (91) 1,196 (83) 0.11
Gestational age (wk) 27.8 (2.0) 28.8 (2.0) 0.44
Male 13 (50.0) 12 (46.2) 0.50
Chorioamnionitis* 5 (19.2) 2 (7.7) 0.21
Mechanical ventilation 20 (76.9) 18 (69.2) 0.38
Duration of mechanical ventilation (d) 2.3 (2.6) 2.8 (3.2) 0.54
Antenatal steroid 0.16
1 dose 8 (30.8) 4 (15.4)
2 doses 18 (69.2) 22 (84.6)
Surfactant 0.61
1 dose 12 (46.2) 10 (38.5)
2 or more doses 8 (30.8) 9 (34.6)
Values are presented as mean (SD) or number (%).
SA: sodium acetate, SC: sodium chloride, PN: parenteral nutrition.
*Clinical or histologic chorioamnionitis.
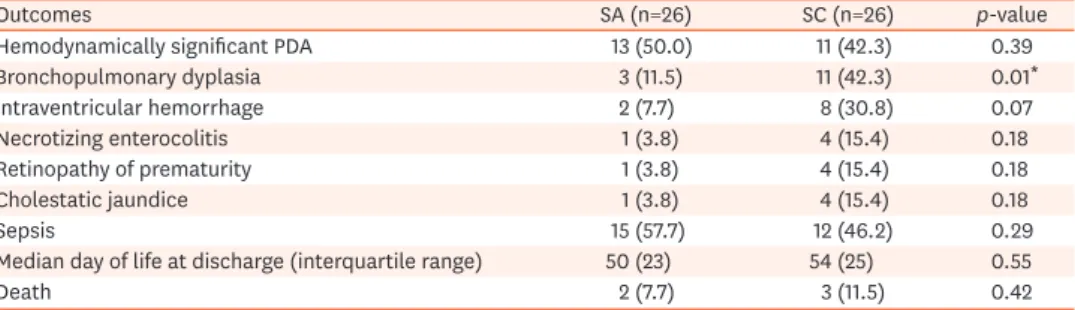
The number of infants with BPD was almost four-fold lower in the SA group than in the SC group (n=3 vs. 11, respectively; p-value<0.01). There were no statistical differences in hemodynamically significant PDA, IVH, ROP, NEC, cholestatic jaundice, and mortality between the groups (Table 3).
Table 2. Comparison between the fluid intake, acid-base balance, and blood biochemistry between infants who received SA and SC in PN over 6 days
Fluids, therapy and blood biochemistry SA (n=26) SC (n=26) p-value
Postnatal age when PN was initiated (d) 1.54 (0.58) 1.69 (0.55) 0.34
Total sodium received (mmoL/kg) 24.1 (12.0) 24.4 (10.8) 0.57
Total chloride received (mmoL/kg) 13.9 (11.5) 29.9 (13.8) 0.04§
Average total fluid intake (mL/kg/day) 128.8 (9.5) 130.3 (10.2) 0.63
Enteral feeding at D6 (mL/kg/day) 24.4 (14.4) 21.5 (12.0) 0.44
pH* 7.360 (0.025) 7.340 (0.007) 0.11
Base excess* −1.5 (1.3) −2.5 (0.3) 0.13
pCO2 (mmHg*) 45.9 (1.7) 44.9 (1.4) 0.26
Pre-PN serum sodium (mmoL/L) 136.5 (7.1) 137.7 (7.4) 0.54
Serum sodium (mmoL/L†) 134.7 (3.6) 135.1 (4.0) 0.48
Serum bicarbonate (mmoL/L†) 26.0 (3.3) 20.8 (3.6) <0.01§
Serum lactate (mmoL/L†) 1.5 (0.4) 1.8 (0.5) 0.23
Infants given additional bicarbonate therapy‡ 4 (15.4) 13 (50.0) 0.01§ Values are presented as mean (SD) or number (%).
SA: sodium acetate, SC: sodium chloride, PN: parenteral nutrition.
The data, except for ‘postnatal age when PN was initiated’, are the averages from 25 of 26 infants in each group, as one infant died in each group on day 5.
*Average of all results obtained over 6 days of PN. †Sample at the completion of 6 days of PN. ‡During the first 14 days of life. §Statistically significant.
7.450
7.400
7.350
7.300
7.250
pH
Daily pH trend Sodium acetate
Sodium chloride
1 2 3 4 5 6
Days
A
B 1.000
0.000 1.000 2.000 3.000 4.000
Baseexcess
Daily base excess trend Sodium acetate
Sodium chloride
1 2 3 4 5 6
Days
Fig. 2. pH (A) and base excess (B) trends in blood samples from infants on sodium acetate or sodium chloride in parenteral nutrition. Values for pH and base excess for D6 were from 25 infants instead of 26, for each group, because one infant from each group died on day 5.
DISCUSSION
Despite the increasing use of SA in PN for VLBW infants, especially in developed countries since a RCT more than 20 years ago, limited studies have investigated the benefits of SA in PN [7]. Unlike this earlier RCT, we used SA entirely as the source of sodium to replace SC in the intervention arm of our study [7]. Furthermore, only a few studies have focused on the use of SA in PN among VLBW and ELBW infants. The RCT by Peters et al. [7] investigated SA use on hyperchloremia and its relationship with acid-base status in very preterm infants. Based on contemporary recommendations, our study population was started on PN much earlier, from the first day rather than day 3 of life in a previous study [7]. Thus, replenishing sodium in the form of chloride earlier in PN solutions potentially leads to higher cumulative chloride intake. The development of hyperchloremic metabolic acidosis in the first few days of life may be more hazardous in predisposing to complications, such as IVH. Indeed, the study by Peters et al. [7] showed that the chloride group were more acidotic with lower pH and BE than the acetate group (pH of 7.30 vs. 7.35 and BE of −5.7 vs. −2.1, respectively) on day 5 of life and the third day of PN. Our study showed that the blood pH and BE rose to normal values after 3 days of PN in the SA group. Of note, pCO2 showed no significant differences between groups, and the acid-base status was more rapidly improved when SA was used. In another study, significantly more neonates given SC-only based PN developed hyperchloremia, which showed a significant linear correlation with metabolic acidosis. [32] These findings were similar to the study by Richards et al. [8] that showed higher infusion rates of chloride in PN increased the incidence of metabolic acidosis.
We also analyzed the acid-base status of the ELBW subgroup of infants and noted an even greater improvement in its balance in the SA group. Improvement of the acid-base balance in the SA group could be explained by the reduction in the total chloride received and the alkalization effects of acetate metabolism in vivo, thus reducing hyperchloremic metabolic acidosis [5,13,16,23,33,34]. The use of bicarbonate therapy, although rarely practiced now, was significantly lower in the SA group, consistent with the results by Peters et al. [7]. Peters et al. [7] also reported that the median duration of mechanical ventilation was longer in the SC versus the SA group (12 days vs. 4.5 days, respectively), which may indicate sicker infants in the SC group. In contrast, in an era of earlier extubation and non-invasive ventilation, our study subjects had a much shorter mean duration of ventilation, and there was no significant difference between the SA and SC groups.
Table 3. Clinical outcomes and neonatal co-morbidities of preterm infants who received SA or SC in PN
Outcomes SA (n=26) SC (n=26) p-value
Hemodynamically significant PDA 13 (50.0) 11 (42.3) 0.39
Bronchopulmonary dyplasia 3 (11.5) 11 (42.3) 0.01*
Intraventricular hemorrhage 2 (7.7) 8 (30.8) 0.07
Necrotizing enterocolitis 1 (3.8) 4 (15.4) 0.18
Retinopathy of prematurity 1 (3.8) 4 (15.4) 0.18
Cholestatic jaundice 1 (3.8) 4 (15.4) 0.18
Sepsis 15 (57.7) 12 (46.2) 0.29
Median day of life at discharge (interquartile range) 50 (23) 54 (25) 0.55
Death 2 (7.7) 3 (11.5) 0.42
Values are presented as number (%) unless specified.
SA: sodium acetate, SC: sodium chloride, PN: parenteral nutrition, PDA: patent ductus arteriosus.
*Statistically significant.
A novel discovery from our study was the significantly lower occurrence of BPD in the acetate group. The occurrence of severe IVH was also lower but did not reach statistical significance. When we conducted this study to determine the benefits of SA, we hypothesized that increased acidosis in VLBW infants receiving SC may be associated with a greater incidence of IVH, and perhaps also a longer duration of mechanical ventilation that may result in increased BPD [17,19]. Although the SA group had a higher pH and BE after day 3, there was no significant difference in the cumulative pH in both groups, and no severely low pH cases that were predisposed to the risk of severe IVH. The duration of mechanical ventilation was also similar in both groups. As such, we could not ascertain the effects of a higher baseline pH and BE after day 3 and their potential protective benefits. Furthermore, neonatal co-morbidities were secondary outcome measures, and our study was not sufficiently powered to confirm these findings. Future randomized trials are required to determine if SA in PN results in lower BPD and IVH rates. However, based on in vitro studies, we speculate that there may be additional protective effects from SA with reported anti-inflammatory and antioxidant properties [35]. Diseases associated with prematurity, including BPD, periventricular leukomalacia (PVL), IVH, ROP, and NEC have been linked to free radical-mediated damage to the tissues and organs of the preterm infant, resulting in cell injury and inflammation [17,18,36,37]. Ishiguro et al. [35] studied the efficacy of acetate in murine models of inflammatory diseases and found that acetate inhibited Nuclear Factor of Activated T-cell (NFAT) by interfering with the interaction between NFAT and importin β1 in T cells, suggesting that acetate acted as an anti-inflammatory agent. NFAT is a critical transcription factor in cytokine gene expression in activated T cells [35,38]. NFAT is required in combination with calcineurin in the regulation of perinatal lung maturation, function, and the transcription of surfactant protein D, which plays a critical role in host defense, surfactant homeostasis, and pulmonary immunomodulation. Dysregulation of these processes results in pulmonary immaturity and surfactant deficiency, predisposing to lung disease of prematurity and BPD [38,39].
Although the pathogenesis of IVH and PVL in preterm infants is largely multifactorial, free radical-mediated cellular injury has been proposed as a key pathogenetic factor in cerebral white matter injury in this population [18,36,37]. The susceptibility of immature oligodendrocytes to oxidant-mediated injury and the benefits of free radical scavengers in protecting these cells have been demonstrated in experimental studies [36,37]. The anti- inflammatory properties of SA [35] that may decrease the incidence of BPD, and perhaps also severe IVH, need further evaluation. Future trials are also needed to investigate whether the protective benefits of SA include reducing other complications of prematurity, such as NEC and PVL.
Although the use of SA appeared to confer greater advantages, it is more expensive and less widely available in many centers, especially in the developing world. The alternative is SL, which is also in the latest ESPGHAN recommendation [21]. Clinical trials to explore the use of SL in PN for preterm infants, and comparisons with SA in cost-effectiveness to reduce morbidities, such as BPD are eagerly awaited.
In conclusion, SA but not SC in PN for preterm infants was associated with less metabolic acidosis and a lower incidence of BPD. The use of SA as the sodium component in PN is recommended in the first week at least, especially in preterm infants of VLBW for improved acid-base status and neonatal outcomes. In fact, chloride-free solutions should be used in early PN for VLBW babies to reduce the risk of hyperchloremic metabolic acidosis.
Adequately powered translational studies focusing on the direct anti-inflammatory effects of SA to limit the common neonatal co-morbidities in very preterm infants are anticipated.
ACKNOWLEDGEMENTS
We wish to convey our gratitude to the staff of the Pharmacy Department of the Universiti Kebangsaan Malaysia Medical Centre for their diligent assistance rendered in this study. We also thank Shirley Szu-Ying Seah, Drs. Suerialoasan Navanesan and Tian-Lee Tan (Faculty of Medicine, Universiti Kebangsaan Malaysia) for their help in the preparation of this manuscript.
REFERENCES
1. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 2005;41 Suppl 2:S1-87.
PUBMED | CROSSREF
2. Valentine CJ, Puthoff TD. Enhancing parenteral nutrition therapy for the neonate. Nutr Clin Pract 2007;22:183-93.
PUBMED | CROSSREF
3. Barnett HL, Vesterdal J. The physiologic and clinical significance of immaturity of kidney function in young infants. J Pediatr 1953;42:98-119.
PUBMED | CROSSREF
4. Heller H. The renal function of newborn infants. J Physiol 1944;102:429-40.
PUBMED | CROSSREF
5. Ekblad H, Kero P, Takala J. Slow sodium acetate infusion in the correction of metabolic acidosis in premature infants. Am J Dis Child 1985;139:708-10.
PUBMED
6. Groh-Wargo S, Ciaccia A, Moore J. Neonatal metabolic acidosis: effect of chloride from normal saline flushes. JPEN J Parenter Enteral Nutr 1988;12:159-61.
PUBMED | CROSSREF
7. Peters O, Ryan S, Matthew L, Cheng K, Lunn J. Randomised controlled trial of acetate in preterm neonates receiving parenteral nutrition. Arch Dis Child Fetal Neonatal Ed 1997;77:F12-5.
PUBMED | CROSSREF
8. Richards CE, Drayton M, Jenkins H, Peters TJ. Effect of different chloride infusion rates on plasma base excess during neonatal parenteral nutrition. Acta Paediatr 1993;82:678-82.
PUBMED | CROSSREF
9. Kermorvant-Duchemin E, Iacobelli S, Eleni-Dit-Trolli S, Bonsante F, Kermorvant C, Sarfati G, et al. Early chloride intake does not parallel that of sodium in extremely-low-birth-weight infants and may impair neonatal outcomes. J Pediatr Gastroenterol Nutr 2012;54:613-9.
PUBMED | CROSSREF
10. Iacobelli S, Kermorvant-Duchemin E, Bonsante F, Lapillonne A, Gouyon JB. Chloride balance in preterm infants during the first week of life. Int J Pediatr 2012;2012:931597.
PUBMED | CROSSREF
11. Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth 2008;101:141-50.
PUBMED | CROSSREF
12. McCague A, Dermendjieva M, Hutchinson R, Wong DT, Dao N. Sodium acetate infusion in critically ill trauma patients for hyperchloremic acidosis. Scand J Trauma Resusc Emerg Med 2011;19:24.
PUBMED | CROSSREF
13. Skutches CL, Sigler MH, Teehan BP, Cooper JH, Reichard GA. Contribution of dialysate acetate to energy metabolism: metabolic implications. Kidney Int 1983;23:57-63.
PUBMED | CROSSREF
14. Tsai IC, Huang JW, Chu TS, Wu KD, Tsai TJ. Factors associated with metabolic acidosis in patients receiving parenteral nutrition. Nephrology (Carlton) 2007;12:3-7.
PUBMED | CROSSREF
15. Vinay P, Prud'Homme M, Vinet B, Cournoyer G, Degoulet P, Leville M, et al. Acetate metabolism and bicarbonate generation during hemodialysis: 10 years of observation. Kidney Int 1987;31:1194-204.
PUBMED | CROSSREF
16. Sugiura S, Inagaki K, Noda Y, Nagai T, Nabeshima T. Acid load during total parenteral nutrition:
comparison of hydrochloric acid and acetic acid on plasma acid-base balance. Nutrition 2000;16:260-3.
PUBMED | CROSSREF
17. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723-9.
PUBMED | CROSSREF
18. Kenet G, Kuperman AA, Strauss T, Brenner B. Neonatal IVH--mechanisms and management. Thromb Res 2011;127 Suppl 3:S120-2.
PUBMED | CROSSREF
19. Low JA, Lindsay BG, Derrick EJ. Threshold of metabolic acidosis associated with newborn complications.
Am J Obstet Gynecol 1997;177:1391-4.
PUBMED | CROSSREF
20. Cooke RW. Factors associated with periventricular haemorrhage in very low birthweight infants. Arch Dis Child 1981;56:425-31.
PUBMED | CROSSREF
21. Jochum F, Moltu SJ, Senterre T, Nomayo A, Goulet O, Iacobelli S, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Fluid and electrolytes. Clin Nutr 2018;37(6 Pt B):2344-53.
PUBMED | CROSSREF
22. Cash RA, Toha KM, Nalin DR, Huq Z, Phillips RA. Acetate in the correction of acidosis secondary to diarrhoea. Lancet 1969;2:302-3.
PUBMED | CROSSREF
23. Watten RH, Gutman RA, Fresh JW. Comparison of acetate, lactate, and bicarbonate in treating the acidosis of cholera. Lancet 1969;2:512-4.
PUBMED | CROSSREF
24. Kluckow M, Evans N. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J Pediatr 1995;127:774-9.
PUBMED | CROSSREF
25. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline- membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357-68.
PUBMED | CROSSREF
26. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529-34.
PUBMED | CROSSREF
27. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1-7.
PUBMED | CROSSREF
28. Cryotherapy for Retinopathy of Prematurity Cooperative Group and the National Eye Institute.
Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol 1988;106:471-9.
PUBMED | CROSSREF
29. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991-9.
PUBMED | CROSSREF
30. Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2004;39:115-28.
PUBMED | CROSSREF
31. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program.
Control Clin Trials 1990;11:116-28.
PUBMED | CROSSREF
32. Cheng K. Relationship between chloride status and metabolic acidosis in preterm neonates requiring PN.
67th Annual Meeting, British Paediatric Association 1995:59 (abstract).
33. Eliahou HE, Feng PH, Weinberg U, Iaina A, Reisin E. Acetate and bicarbonate in the correction of uraemic acidosis. Br Med J 1970;4:399-401.
PUBMED | CROSSREF
34. Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest 1979;64:708-13.
PUBMED | CROSSREF
35. Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Goto H. Acetate inhibits NFAT activation in T cells via importin beta1 interference. Eur J Immunol 2007;37:2309-16.
PUBMED | CROSSREF
36. O'Donovan DJ, Fernandes CJ. Free radicals and diseases in premature infants. Antioxid Redox Signal 2004;6:169-76.
PUBMED | CROSSREF
37. Zia MT, Csiszar A, Labinskyy N, Hu F, Vinukonda G, LaGamma EF, et al. Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: role of NAD(P)H oxidase. Stroke 2009;40:2191-8.
PUBMED | CROSSREF
38. Davé V, Childs T, Xu Y, Ikegami M, Besnard V, Maeda Y, et al. Calcineurin/Nfat signaling is required for perinatal lung maturation and function. J Clin Invest 2006;116:2597-609.
PUBMED | CROSSREF
39. Davé V, Childs T, Whitsett JA. Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells. J Biol Chem 2004;279:34578-88.
PUBMED | CROSSREF