Received: June 16, 2015 / Accepted: June 27, 2015 Address for correspondence: Du-Shin Jeong, MD, MPH
Department of Neurology and Clinical Epidemiology, College of Medicine Soonchunhyang University, 31 Suncheonhyang 6-gil, Dongnam-gu, Cheonan 330-930, Korea
Tel: +82-41-570-2290, Fax: +82-41-579-9021, E-mail: Jbrain@schmc.ac.kr
A Correlation between Plasma Homocysteine and Polyneuropathy in Parkinson’s Disease
In-Uk Song, MD
a, Young-Do Kim, MD
a, Dushin Jeong, MD, MPH
baDepartment of Neurology, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon;
bDepartment of Neurology and Clinical Epidemiology, College of Medicine Soonchunhyang University, Cheonan, Korea
KEYWORDS Homocysteine, Parkinson’s disease, Polyneuropathy
Background: Recently, polyneuropathy has been been described in higher proportions for patients with Parkinson’s disease (PD) than in normal population. This finding was hypothesized to be related to the elevation of plasma homocysteine, following the management of PD with levodopa. We conducted this study to clarify the clinical value of elevated plasma homocysteine in PD patients for their relation to polyneuropathy.
Methods: A total of 37 PD patients without neuropathy (PD control) and 41 PD patients with polyneuropathy (PDP), who were recruited for this study, were compared with age and sex matched 48 healthy controls. All PD patients performed electrophysiological tests, including nerve conduction study, to diagnose polyneuropathy. Plasma homocysteine levels were measured in all subjects and compared between each groups.
Results: The homocysteine of PDP showed higher homocysteine level than those of PD control and healthy controls. However, there was no significant difference in homocysteine levels between PD control and healthy controls. In each group of PD control and PDP, there were no intercorrelations between daily levodopa dose, duration of PD symptoms and PD treatment or motor severity with homocysteine levels.
Conclusions: We could cautiously assume that plasma homocysteine level may be related with the involvement of peripheral nerve of PD patients in this study. The pathophysiologic role of homocysteine and the relationship between plasma homocysteine level and levodopa in PDP need to be confirmed.
Introduction
Parkinson’s disease (PD) is a hypokinetic movement dis- order with cardinal motor features of bradykinesia, resting tremor and rigidity. However, non-motor symptoms, includ- ing sensory disorders, cognitive impairment, sleep disorder and autonomic disturbance, are receiving greater attention.
1Of these non-motor symptoms, sensory disorders (numbness, tingling, burning, aching, coldness and heat) are a well-rec- ognized non-motor manifestation of PD that affects 40% to
75% of patients during their illness.
2Polyneuropathy has re-
cently been described in significantly higher proportions in
patients with PD than in the control subjects, despite the
poorly understood sensory disorders in PD and the diffi-
culties of treatment.
1,3Furthermore, a coexisting poly-
neuropathy in PD patients is of great clinical relevance, as it
could further aggravate the gait and the balance, which has
been already compromised. This finding was hypothesized to
be related to the elevation of plasma homocysteine, due to the
deficiency or alterations in the metabolism of cobalamin, fol-
lowing the management of PD with levodopa.
4,5Therefore, we conducted this study to clarify the influence and clinical significance of the elevated plasma homocysteine in PD pa- tients with polyneuropathy. We also studied the role of levo- dopa exposure for polyneuropathy and relationship between the elevated homocysteine and motor severity of PD patients.
Materials and Methods
The study was approved by the local ethics committee (OC13RISI0008), and each patient gave us their written in- formed consent for participation. A series of consecutive pa- tients were admitted to the Movement disorder and Parkinson’s disease Unit of the Department of Neurology be- tween October 2013 and December 2014. A total of 78 PD patients (37 PD patients without neuropathy (PD control) and 41 PD patients with polyneuropathy [PDP]), who were re- cruited for this study, were compared with 48 healthy con- trols to confirm the value of homocysteine as a potential cause of polyneuropathy associated with PD patients. All PD patients and healthy controls were matched for age and gender. The healthy controls did not have any history or symptoms of PD, complaint of sensory disturbance and other neurological and medical diseases such as diabetes, tuber- culosis, hepatitis, thyroid-related disease, chronic infectious disease or rheumatoid disease. In addition, the nerve con- duction study of healthy controls showed normal findings.
All PD patients were diagnosed, according to the UK Parkinson’s Disease Society Brain Bank Clinical Diagnosis criteria,
6and underwent for nerve conduction study to diag- nose polyneuropathy. Polyneuropathy in this study was de- fined as damage or disease affecting peripheral nerves in roughly the same areas on both sides of the body, featuring sensory disturbance such as numbness, pins-and-needles, sensory loss, and burning pain associated with abnormal electrophysiologic (NCV/EMG) findings. Most of patients begins in the hands and feet and may progress to the arms and legs; and sometimes to other parts of the body where it may affect the autonomic nervous system. The nerve conduction study measured motor conduction of the both median, ulnar, peroneal, and tibial nerve and sensory conduction of the both median, ulnar, superficial peroneal and sural nerves. Both F-responses and H-reflexes were also measured. Patients
were asked to provide history of systemic illnesses such as diabetes, tuberculosis, hepatitis, thyroid-related disease, lep- rosy, chronic infectious disease or rheumatoid disease, rec- reational alcohol use, toxic exposures, and any family history of neuropathy in order to exclude other possible causes of polyneuropathy. Prior to neuro-electrophysiological exami- nation, a complete neurological examination by an experi- enced neurologist and the magnetic resonance image of the brain was performed in all subjects to exclude neurological diseases other than PD, such as brain tumor and cere- brovascular disease. All patients identified within this study had routine blood work to rule out the possible causes for polyneuropathy. This included a complete blood count, elec- trolytes, creatinine, liver enzymes, thyroid function tests, fasting glucose, hemoglobin A1C, erythrocyte sedimentation rate, vitamin B12, folic acid, thiamine, hepatitis B, hepatitis C, HIV test, VDRL test, antinuclear antibody and rheumatoid factor. The lifetime dose and the duration of intake of L-dopa were determined for each patient by a retrospective chart re- view, and had been verified by retrospective self- reporting by each patient in all cases. Motor severity of patients with PD was evaluated, according to the staging system by Hoehn and Yahr (H & Y stage).
7Plasma homocysteine level was measured, routinely, in all patients and healthy controls.
Venous blood samples from patients and healthy controls,
who had fasted for 8 to 10 h, were collected in tubes that con-
tained ethylenediaminetetraacetic acid. The samples were
immediately separated by centrifugation at 3,000 rpm for 10
min. The separated sera were kept at −70 °C until the labo-
ratory evaluation. Laboratory data were evaluated by an ex-
aminer who was blind to the clinical details and patient
evolution. Statistical analysis was performed using the SPSS
software package, version 17.0. The results were expressed
as the mean±standard deviation. Analysis of variance
(ANOVA) with post-hoc test (Tukey test) was used to com-
pare continuous variables in the group of PD control, the
group of PDP and the healthy controls group. The correla-
tions between homocysteine and age, daily levodopa dosage
or duration of PD were analyzed with Spearman’s rank order
correlation. Independent t-tests were used to compare con-
tinuous variable between PD control and PDP. Statistical sig-
nificance was assumed at the 5% level.
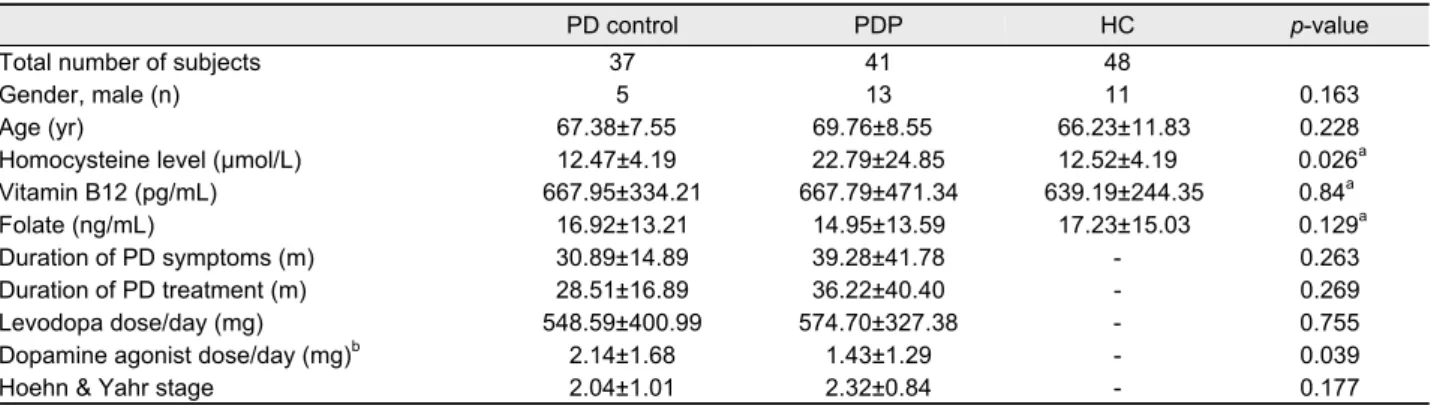
Table 1. Baseline characteristics of all the subjects with PD and healthy controls
PD control PDP HC p-value
Total number of subjects 37 41 48
Gender, male (n) 5 13 11 0.163
Age (yr) 67.38±7.55 69.76±8.55 66.23±11.83 0.228
Homocysteine level (µmol/L) 12.47±4.19 22.79±24.85 12.52±4.19 0.026a
Vitamin B12 (pg/mL) 667.95±334.21 667.79±471.34 639.19±244.35 0.84a
Folate (ng/mL) 16.92±13.21 14.95±13.59 17.23±15.03 0.129a
Duration of PD symptoms (m) 30.89±14.89 39.28±41.78 - 0.263
Duration of PD treatment (m) 28.51±16.89 36.22±40.40 - 0.269
Levodopa dose/day (mg) 548.59±400.99 574.70±327.38 - 0.755
Dopamine agonist dose/day (mg)b 2.14±1.68 1.43±1.29 - 0.039
Hoehn & Yahr stage 2.04±1.01 2.32±0.84 - 0.177
Values are presented as mean±SD unless otherwise indicated.
aPost-hoc comparison of serum homocysteine: PD control versus PDP; bAll patients have taken only pramipexole.
PD, idiopathic Parkinson's disease; PD control, PD without neuropathy; PDP, PD with polyneuropathy; HC, healthy control.
Results
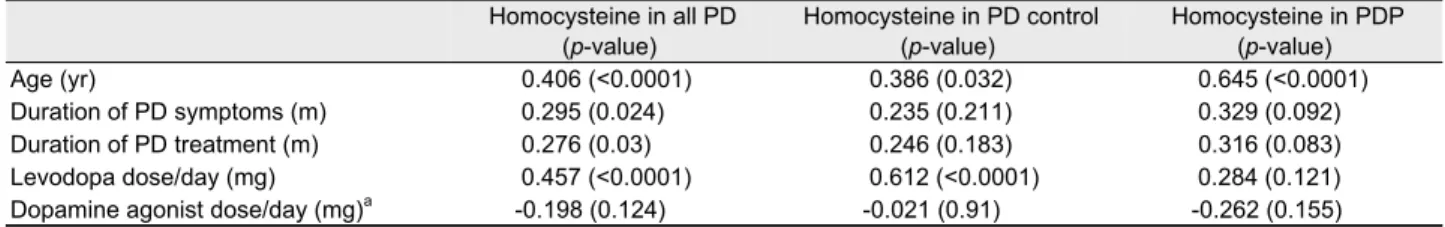
A total 78 PD patients (37 patients with PD control and 41 patients with PDP) and 48 healthy controls were included in this study. The mean age was 67.38±7.55 years in the PD control, 69.76±8.55 years in the PDP and 66.23±11.83 years in the healthy controls. The homocysteine levels were sig- nificantly increased in PDP compared to PD control and healthy controls (p=0.026; p=0.006). However, there was no significant difference in serum homocysteine level between PD control and healthy control. Daily dopamine agonist dose was significantly larger in the PD control than those in the PDP (p=0.039). However, there were no significant differ- ences between PD control and PDP for the duration of PD symptoms and treatment, daily levodopa dose and motor se- verity of PD evaluated by using H & Y stage. The baseline characteristics of all subjects are presented in Table 1. In the Spearman’s rank order correlation, the correlation coefficients showed that plasma homocysteine levels in the PD control were significantly correlated with daily levodopa dose but had no correlation with duration of PD symptoms as well as duration of PD treatment (r=0.612; p<0.0001). Whereas, ho- mocysteine levels in PDP group did not show any correlation with daily levodopa dose, duration of PD symptoms or dura- tion of PD treatment (Table 2). Age factor was related to ho- mocysteine level in both groups.
Discussion
In this study, we ascertained a significant correlation be-
tween homocysteine levels and polyneuropathy in PD patients. Recently, it has been discovered that pharmacother- apeutic management of PD with levodopa is associated with elevated homocysteine level.
3,8,9Homocysteine may increase the susceptibility to mitochondria toxins, contribute to free rad- ical formation, exert glutaminergic-associated neurotoxicity, induce inflammation, and impair DNA repair mechanism.
3Namely, elevated homocysteine levels may be a risk factor for stroke, coronary artery disease, and neurodegenerative disease including dementia because homocysteine has various neuro- nal and endothelial cellular toxicities.
3,6,10,11Therefore, in- creased homocysteine levels have been suggested to have a role in progression of disease or development of cognitive impair- ment in PD patients.
Homocysteine accumulation may also occur with defi-
ciency or alteration in the metabolism of cobalamin, which
can lead to polyneuropathy.
9Previous studies suggested that
elevated homocysteine levels in levodopa-treated PD might
be caused by levodopa treatment rather than by the disease
itself.
3,4,8,12,13The present study also showed that plasma ho-
mocysteine levels in PDP were elevated more than those in
the PD control and controls. However, the present study
showed no difference between plasma homocysteine levels in
PD control and those in the controls. Furthermore, there are
no differences of daily levodopa dose and duration of levodo-
pa treatment between PDP and PD control in spite of sig-
nificant difference of plasma homocysteine levels between
these two groups. Spearman’s rank order correlation analysis
also demonstrated that there is no significant correlation be-
tween plasma homocysteine levels and daily levodopa dose
Table 2. Spearman's rank order correlation coefficients between homocysteine and other variables
Homocysteine in all PD
(p-value)
Homocysteine in PD control (p-value)
Homocysteine in PDP (p-value)
Age (yr) 0.406 (<0.0001) 0.386 (0.032) 0.645 (<0.0001)
Duration of PD symptoms (m) 0.295 (0.024) 0.235 (0.211) 0.329 (0.092)
Duration of PD treatment (m) 0.276 (0.03) 0.246 (0.183) 0.316 (0.083)
Levodopa dose/day (mg) 0.457 (<0.0001) 0.612 (<0.0001) 0.284 (0.121)
Dopamine agonist dose/day (mg)a -0.198 (0.124) -0.021 (0.91) -0.262 (0.155)
Values are Spearman's rank order correlation coefficients and p-value in parentheses.
aAll patients were used pramipexole as dopamine agonist.
PD, idiopathic Parkinson's disease; PD control, PD without neuropathy; PDP, PD with polyneuropathy.
in PDP (p=0.121), whereas there is significant correlation be- tween plasma homocysteine levels and daily levodopa in PD control (Table 2). Previous reports demonstrated occurrence of neuropathy in PD patients without exposure to levodopa and indicated that Meissner corpuscles loss was associated with levodopa use but epidermal nerve fibers loss occurred regardless of levodopa use.
14-16These reports suggested that peripheral deafferentation induced by epidermal nerve fibers loss could play a role in the pathogenesis of the neuropathy in PD without exposure to levodopa. And this suggestion was consistent with our findings that neuropathic involvement in PD could occur in PD regardless of plasma homocysteine levels related to levodopa use.
We cautiously assumed that elevated plasma homocysteine levels were not induced by both high daily levodopa dosage and duration of levodopa exposure but might be the result caused by different characteristics of generic factors in PD patients. Whereas, we could confirm that the involvement of peripheral nerve and plasma homocysteine levels were not correlated with motor severity of PD as well as the duration of the disease, which is in accordance with other previous studies.
3,4,9Age showed positive correlation with plasma ho- mocysteine levels, but this was irrelevant to the presence of polyneuropathy in PD patients. In conclusion, this study demonstrated that plasma homocysteine levels were higher in PDP compared to PD control. The PD itself might not be a contributing factor for developing polyneuropathy, because we did not find any difference in homocysteine levels be- tween PD control and healthy control group. Based on above-mentioned findings, we could cautiously assume that plasma homocysteine level may be related with the involve- ment of peripheral nerve of PD patients in this study, al- though we could only ascertain that plasma homocysteine levels were higher in PDP compared to PD control. However,
we were unable to confirm clear pathophysiologic role of plasma homocysteine in PDP and the relationship between plasma homocysteine level and levodopa in PDP by this study. Therefore, we suggest that large study and longitudinal study need to be performed in the future to confirm clear pathophysiology and pathogenesis of involvement of periph- eral nerve in PD patients.
REFERENCES
1. Park A, Stacy M. Non-motor symptoms in Parkinson's disease. J Neurol 2009;256 Suppl 3:293-298.
2. Schestatsky P, Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Tolosa E, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 2007;69:2162-2169.
3. Toth C, Breithaupt K, Ge S, Duan Y, Terris JM, Thiessen A, et al.
Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol 2010;68:28-36.
4. Rajabally YA, Martey J. Neuropathy in Parkinson disease: preva- lence and determinants. Neurology 2011;77:1947-1950.
5. Urban PP, Wellach I, Faiss S, Layer P, Rosenkranz T, Knop K, et al. Subacute axonal neuropathy in Parkinson's disease with coba- lamin and vitamin B6 deficiency under duodopa therapy. Mov Disord 2010;25:1748-1752.
6. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181- 184.
7. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-442.
8. Postuma RB, Lang AE. Homocysteine and levodopa: should Parkinson disease patients receive preventative therapy? Neurology 2004;63:886-891.
9. Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D.
Neuropathy as a potential complication of levodopa use in Parkinson's disease. Mov Disord 2008;23:1850-1859.
10. Song IU, Kim JS, Park IS, Kim YD, Cho HJ, Chung SW, et al.
Clinical significance of homocysteine (hcy) on dementia in Parkinson's disease (PD). Arch Gerontol Geriatr 2013;57:288-291.
11. Shin HW, Sohn YH. Hyperhomocysteinemia in patients with Parkinson's disease and relationship to vitamin B level. J Mov
Disord 2009;2:33-36.
12. Muller T, Jugel C, Ehret R, Ebersbach G, Bengel G, Muhlack S, et al. Elevation of total homocysteine levels in patients with Parkinson's disease treated with duodenal levodopa/carbidopa gel. J Neural Transm 2011;118:1329-1333.
13. O'Suilleabhain PE, Bottiglieri T, Dewey RB Jr, Sharma S, Diaz- Arrastia R. Modest increase in plasma homocysteine follows lev- odopa initiation in Parkinson's disease. Mov Disord 2004;19:
1403-1408.
14. Rajabally YA, Martey J. Levodopa, vitamins, ageing and the neu- ropathy of Parkinson's disease. J Neurol 2013;260:2844-2848.
15. Ceravolo R, Cossu G, Bandettini di Poggio M, Santoro L, Barone P, Zibetti M, et al. Neuropathy and levodopa in Parkinson's dis- ease: evidence from a multicenter study. Mov Disord 2013;28:
1391-1397.
16. Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, et al. Sensory deficit in Parkinson's disease: evi- dence of a cutaneous denervation. Brain 2008;131:1903-1911.