Received: June 27, 2017 Revised: August 14, 2017 Accepted: August 26, 2017.
Corresponding author: Jae Moon Yun
Department of Family Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4039, Fax: +82-2-766-3276, E-mail: jaemoon2@gmail.com
This research was supported by Hanmi Pharmaceutical Co., Ltd.
Copyright Ⓒ 2017 The Korean Academy of Clinical Geriatrics
This is an open access article distributed under the term s of the Creative Com m ons Attribution Non-Com m ercial License (http://creativecom m ons.org/licenses/by-nc/4.0) which perm its unrestricted non-com m ercial use, distribution, and reproduction in any m edium , provided the original work is properly cited.
Chronic Kidney Disease: A Risk Factor for Parkinson’s Disease
Soo Jin Wang1, Jae Moon Yun1, Dong Wook Shin2, Be Long Cho1, Ki Young Son1, Sang Hyuck Kim3, Ji Eun Lee1, Soo Min Jung1, A Young Eo1, Jeong Yean Yun1
1Department of Family Medicine, Seoul National University Hospital, Seoul, 2Department of Family Medicine, Samsung Medical Center, Seoul, 3Health Screening and Promotion Center, Bundang Seoul National University Hospital, Seongnam, Korea
Background: This study aims to reveal risk factors of Parkinson’s disease (PD) within Chronic kidney disease (CKD) patients.
A retrospective cohort was designed to clarify the association between PD and CKD through survival analysis.
Methods: Based on Korea’s National Health Insurance System (NHIS), the subjects were divided into non-CKD patients and newly diagnosed CKD patients between 2003 and 2004. From 2005 to 2013, a 9 years’ surveillance was performed to detect the occurrence of PD. The association between CKD and PD was assessed using survival analysis. Subjects were divided into 4 CKD stages based on the estimated glomerular filtration rate (eGFR): eGFR<15 (mL/min/1.73 m2), 15 to 29, 30 to 59 and 60 to 89, hazard ratios (HRs) with 95% confidence intervals (CIs) for each stages were assessed.
Results: CKD patients with an eGFR less than 15 had unadjusted HR of 2.79 (95% CI=1.25∼6.21, P<0.05) and adjusted HR of 2.60 (95% CI=1.25∼6.21, P<0.05) manifesting increased risk of PD. In male patients with eGFR less than 15, the risk was even higher in unadjusted HR of 4.21 (95% CI=1.75∼10.12, P<0.05) and adjusted HR of 3.71 (95%
CI=1.54∼8.91, P<0.05). However, no significant association was found within female patients.
Conclusion: In this study, a statistically significant association between CKD and PD was found. A notable increase in risk was found in male CKD patients with eGFR less than 15. Thus CKD resulting uremia could lead to increased risk of PDs.
Key Words: Renal insufficiency, Chronic, Parkinson disease
INTRODUCTION
Parkinson’s disease (PD) is a well acknowledged neuro- degenerative disease which has a prevalence of one percent in the population aged 60 over.1) The epidemiology of PD is known to be 150 to 200 per 100,000 and the number is increasing due to senescing population. It has been esti-
mated that the prevalence will increase two-fold by 2030.2-4) Although PD is non-life threatening, the mortality rate in- creases with progression of disease5,6) Subsequently, the hos- pitalization rate of patient’s with PD is rising.3,7) As a result, the rapidly increasing prevalence of PD within the aging population is drawing attention in both the medical and so- cio-economic domains.
PD can cause functional and psychological impairments which results a significant decrease in quality of life.8,9) Since there is no cure for PD, its management is mainly focused on early detection and prevention. In order to maintain ad- equate function for daily living, individualized treatment based on symptoms, disease severity, age and socio-economic background is required. A demand for prompt management of PD necessitates early detection of the disease.
A complete etiology of PD has not yet been proven.
Various factors encompassing genetic and environmental fac- tors have been suggested. Studies have revealed that mul- ti-factor interactions including patients’ age, sex, genetic predisposition and environment could lead to cellular damage.10,11) Environmental factors account for the long term exposure to metal or chemical substances.12-14) Recently, an association between certain medical condition and PD has drawn attention. Researchers are focusing on CKD as a risk factor for PD.15-17) Nonetheless, only two studies in Taiwan were conducted to reveal an association between PD and CKD. This study has limited the definition of PD to a disease code based on ICD-9 and the patients were limited to end stage renal disease (ESRD) only. Therefore, there re- mains a possibility that the results have been overestimated due to imprecise subject definition and secondary abnormal- ities resulting from uremia.
No literature has been published regarding an association between PD and decreased renal function. Thus, the authors aim to reveal an association between PD and CKD based on retrospective study.
MATERIALS AND METHODS
1. Study population
Korea’s National Health Insurance system (NHIS) has constructed a nationwide database of health and medical da- ta which were collected through stratified sampling. The da- tabase was established on disease, medical records and so- cio-demographic background. Thus, it is a highly repre- sentative and valuable resource which can be utilized in nu- merous studies. A NHIS-Senior cohort is a designed to ran- domly recruit 550,000 patients and comprised of 10% of
the population over 60 in 2002. Between 2002 and 2013, subjects’ claim information, medical screening, health sur- vey, medical institution and social acceptance survey was collected.
A total of 558,147 patients were enrolled in NHIS-Senior cohort (NHIS-2017-2-396) database. Out of 512,346 pa- tients between ages 60 to 80 years, a group of 506,809 pa- tients who have not been diagnosed with CKD prior to 2003 (2,488 patients) and PD prior to 2005 (3,769 patients) were selected. As a result, 506,089 patients were included in our study. The subjects’ stages of CKD could not be esti- mated with the diagnosis alone. Thus, subjects who were newly diagnosed with CKD from 2003 to 2004 (2,998 pa- tients) and those in the control group (503,091 patients) were compared. Within the newly diagnosed CKD patients, further analysis was performed between different stages of CKD based on estimated glomerular filtration rate (eGFR).
From 2005 to 2013, a 9-year period analysis was performed upon the incidence of PD.
Every subject information for retrospective analysis was coded. This study was approved from Institutional Review Board.
2. Definitions
PD was defined with a disease code of G20 according to ICD-10 (International Statistical Classification of Disease and Related Health Problems, 10th Revision). Patients who have been both diagnosed with PD (i.e. patients with G20 code) and prescribed with anti-Parkinson’s disease medication have been selected.
The anti-Parkinson’s disease medications were defined as available medications between 2005 and 2013 in Korea.
Enlisted medications include: amantadine, benztropine, bi- pertiden, bromocriptine, dihydroergocryptine, flunarizine, lev- odopa, lisuride, pergolide, procyclidine, ropinirole, selegiline, trihexyphenidyl, levodopa/benserazide, carbidopa/levodapa, parmipexole, entacarpone, levodopa/carbidopa/entacapone, tet- rabenazine, orphenadrine, rivastigmine, sulpiride/pyridoxine and piracetam.
In addition, code N18 (chronic kidney disease), I120 (hyper- tensive renal failure) and E142 (diabetic nephropathy) for
Table 1. Baseline characteristics of the study population in the cohort with and without chronic kidney disease
Variable Chronic kidney disease
Yes, Overall‡ No
Total 2,998 503,091
Age, mean (SD*) 67.78 (5.49) 67.32 (5.59) Sex
Female (%) 1,418 (47.30) 289,775 (57.60)
Male 1,580 213,316
Comorbidity
Hypertension (%) 2,530 (84.39) 215,985 (42.93) DM† (%) 1,927 (64.28) 98,955 (19.67) Dyslipidemia (%) 1,018 (33.96) 58,108 (11.55) Data are shown in mean±standard deviation for continuous varia- bles and n (%) for categorical variables. *SD: standard deviation.
†DM: diabetic mellitus. ‡Overall: group with diagnosis code.
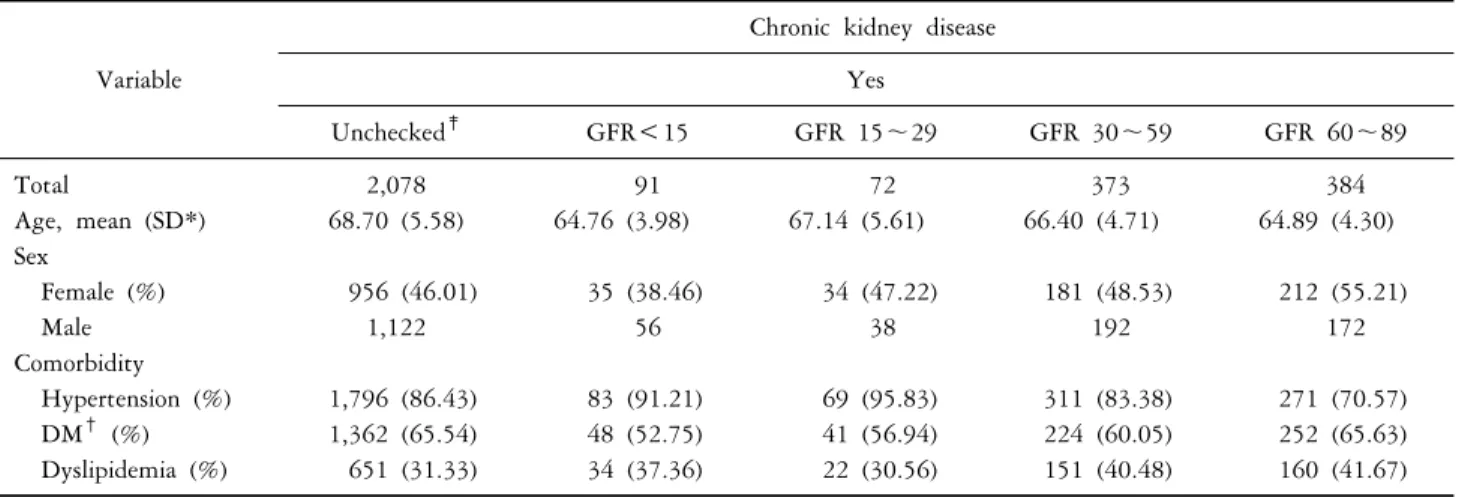
Table 2. GFR based stratification of baseline characteristics of the study population in the cohort with chronic kidney disease
Variable
Chronic kidney disease Yes
Unchecked‡ GFR<15 GFR 15∼29 GFR 30∼59 GFR 60∼89
Total 2,078 91 72 373 384
Age, mean (SD*) 68.70 (5.58) 64.76 (3.98) 67.14 (5.61) 66.40 (4.71) 64.89 (4.30)
Sex
Female (%) 956 (46.01) 35 (38.46) 34 (47.22) 181 (48.53) 212 (55.21)
Male 1,122 56 38 192 172
Comorbidity
Hypertension (%) 1,796 (86.43) 83 (91.21) 69 (95.83) 311 (83.38) 271 (70.57)
DM† (%) 1,362 (65.54) 48 (52.75) 41 (56.94) 224 (60.05) 252 (65.63)
Dyslipidemia (%) 651 (31.33) 34 (37.36) 22 (30.56) 151 (40.48) 160 (41.67)
Data are shown in mean±standard deviation for continuous variables and n (%) for categorical variables. *SD: standard deviation.
†DM: diabetic mellitus. ‡Unchecked: No data about GFR.
chronic kidney disease were selected according to ICD-10.
The NHIS has included creatinine as a screening parame- ter since 2009. Based on the data, authors have assessed subjects’ estimated glomerular filtration rate according to MDRD-eGFR [GFR (mL/min/1.73 m2)=175×(serum crea- tinine [mg/dL])−1.154×(age [years])−0.203×(0.742 if female)]
CKD patients were classified under KDOQI stages: eGFR<15 (mL/min/1.73 m2), eGFR 15 to 29 (mL/min/ 1.73 m2), eGRF 30 to 59 (mL/min/1.73 m2) and eGFR 60 to 89 (mL/min/ 1.73 m2). Stratification based on GFR was set on lowest eGFR value within the follow up periods.
Comorbidities include hypertension (ICD10 code: I10, I11, I12, I13, I15), diabetes mellitus (ICD10 code: E10, E11, E12, E13, E14) and dyslipidemia (ICD10 code: E78) which are based on data collected in 2005. Subjects’ age was recorded at the entry point of the cohort in 2002.
3. Statistical analysis
The data were evaluated in mean, standard deviation and frequency. ANOVA test and t-test were performed from continuous variable analysis. Kaplan-Meier analysis and the log rank test were used to compare the incidence of PD be- tween the CKD group and the non-CKD group. The haz- ard ratio (HR) of PD in relationship with CKD was derived from Cox proportional hazard regression models for time to event outcomes. HRs for each groups were separately ac- quired from stratification of subjects based on GFR. HR
with 95% confidence interval (CI) were estimated in the Cox models. Age, sex, hypertension, diabetes mellitus and dyslipidemia were used for adjustment. The incidence rates of PD were estimated in person-years (PY) and statistical significance was determined as the P value<0.05. Statistical nalysis were performed by using Stata version 14.0 (StataCorp, Texas, USA).
RESULTS
The cohort consists of 2,998 case subjects who were new- ly diagnosed with CKD between 2003 and 2004 and
Figure 1. Probability of developing PD for patients with (dash line) or without (solid line) CKD, using Kaplan-Meier analysis.
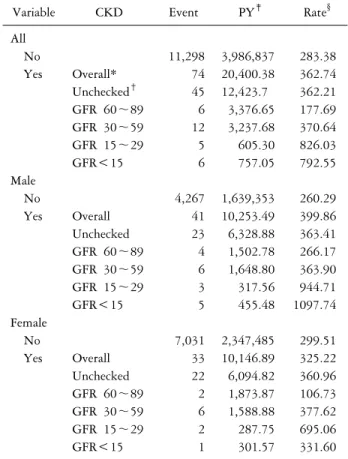
Table 3. Incidence rate of Parkinson’s disease in patients with and without chronic kidney disease
Variable CKD Event PY‡ Rate§
All
No 11,298 3,986,837 283.38
Yes Overall* 74 20,400.38 362.74
Unchecked† 45 12,423.7 362.21
GFR 60∼89 6 3,376.65 177.69
GFR 30∼59 12 3,237.68 370.64
GFR 15∼29 5 605.30 826.03
GFR<15 6 757.05 792.55
Male
No 4,267 1,639,353 260.29
Yes Overall 41 10,253.49 399.86
Unchecked 23 6,328.88 363.41
GFR 60∼89 4 1,502.78 266.17
GFR 30∼59 6 1,648.80 363.90
GFR 15∼29 3 317.56 944.71
GFR<15 5 455.48 1097.74
Female
No 7,031 2,347,485 299.51
Yes Overall 33 10,146.89 325.22
Unchecked 22 6,094.82 360.96
GFR 60∼89 2 1,873.87 106.73
GFR 30∼59 6 1,588.88 377.62
GFR 15∼29 2 287.75 695.06
GFR<15 1 301.57 331.60
*Overall: group with diagnosis code. †Unchecked: no data about GFR. ‡PY: person-years. §Rate: incidence rate, per 100,000 per- son-years (PY).
503,091 control subjects (Table 1). The mean follow up was 7.92 years long. A total of 4,007,238 person-year were observed. No notable difference was found between CKD patients’ age (67.78±5.49) and non-CKD patients’ age (67.32±5.59). Among CKD patients, male predominance was found (1,660 subjects, 52.70%). In contrast, female predominance (289,775 subjects, 57.60%) was found in non-CKD patients. Out of 2,998 CDK patients, 2,078 sub- jects had unavailable eGFR results (i.e. creatinine unchecked), 384 subjects had eGFR between 60∼89 (mL/min/1.73 m2), 373 subjects had eGFR between 30∼59 (mL/min/1.73 m2), 72 subjects had eGFR between 15∼29 (mL/min/1.73 m2) and 91 subjects had eGFR less than 15(mL/min/1.73 m2).
Comorbidities in CKD patients include hypertension (2,530 subjects, 84.39%), diabetes mellitus (1,927 subjects, 64.28%) and dyslipidemia (1,018 subjects, 33.96%). Proportions of comorbidities for each CKD classes based on eGFR were listed in Table 2. The percentages of hypertension, diabetes mellitus and dyslipidemia which are known CKD risk fac- tors were higher in CKD patients than non-CKD patients.
A total of 11,372 PD cases occurred (11,298 non-CKD patients and 74 CKD patients). Among the 74 CKD sub- jects, 6 subjects had eGFR 60∼89, 12 subjects had eGFR 30∼59, 5 subjects had eGFR 15∼29 and 6 subjects had eGFR less than 15. Table 3 displays the incidence rate of PD according to eGFR.
Kaplan Meier survival analysis revealed higher cumulated PD occurrence in CKD subjects (Figure 1). The CKD sub-
jects had a higher PD risk with an unadjusted HR of 1.29 (95% CI=1.03∼1.62, P<0.05). Even after adjusting for age, sex and comorbidities, the difference was statistically
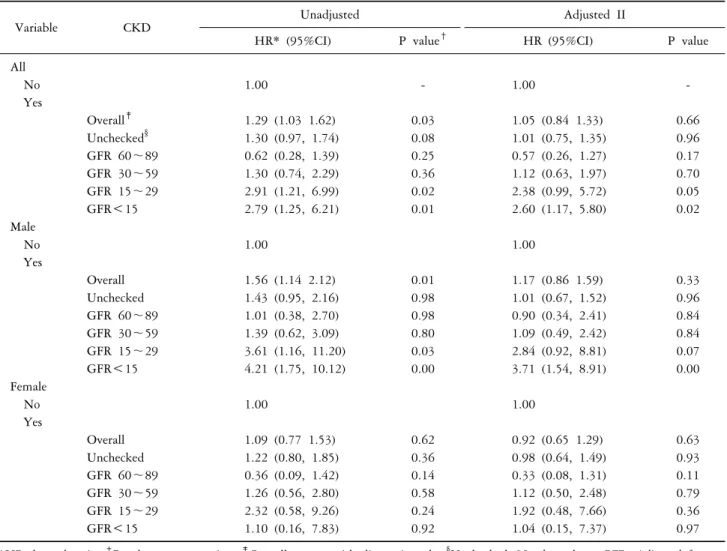
Table 4. Hazard ratio of Parkinson’s disease in patients with and without chronic kidney disease
Variable CKD Unadjusted Adjusted II
HR* (95%CI) P value† HR (95%CI) P value
All
No 1.00 - 1.00 -
Yes
Overall‡ 1.29 (1.03 1.62) 0.03 1.05 (0.84 1.33) 0.66
Unchecked§ 1.30 (0.97, 1.74) 0.08 1.01 (0.75, 1.35) 0.96
GFR 60∼89 0.62 (0.28, 1.39) 0.25 0.57 (0.26, 1.27) 0.17
GFR 30∼59 1.30 (0.74, 2.29) 0.36 1.12 (0.63, 1.97) 0.70
GFR 15∼29 2.91 (1.21, 6.99) 0.02 2.38 (0.99, 5.72) 0.05
GFR<15 2.79 (1.25, 6.21) 0.01 2.60 (1.17, 5.80) 0.02
Male
No 1.00 1.00
Yes
Overall 1.56 (1.14 2.12) 0.01 1.17 (0.86 1.59) 0.33
Unchecked 1.43 (0.95, 2.16) 0.98 1.01 (0.67, 1.52) 0.96
GFR 60∼89 1.01 (0.38, 2.70) 0.98 0.90 (0.34, 2.41) 0.84
GFR 30∼59 1.39 (0.62, 3.09) 0.80 1.09 (0.49, 2.42) 0.84
GFR 15∼29 3.61 (1.16, 11.20) 0.03 2.84 (0.92, 8.81) 0.07
GFR<15 4.21 (1.75, 10.12) 0.00 3.71 (1.54, 8.91) 0.00
Female
No 1.00 1.00
Yes
Overall 1.09 (0.77 1.53) 0.62 0.92 (0.65 1.29) 0.63
Unchecked 1.22 (0.80, 1.85) 0.36 0.98 (0.64, 1.49) 0.93
GFR 60∼89 0.36 (0.09, 1.42) 0.14 0.33 (0.08, 1.31) 0.11
GFR 30∼59 1.26 (0.56, 2.80) 0.58 1.12 (0.50, 2.48) 0.79
GFR 15∼29 2.32 (0.58, 9.26) 0.24 1.92 (0.48, 7.66) 0.36
GFR<15 1.10 (0.16, 7.83) 0.92 1.04 (0.15, 7.37) 0.97
*HR: hazard ratio. †P value cox regression. ‡Overall: group with diagnosis code. §Unchecked: No data about GFR. Adjusted for age (year, continuous), sex, Hypertension, Diabetic mellitus, Hyperlipidemia.
insignificant (Table 4). However, subjects with eGFR less than 15 showed a noticeable difference with unadjusted HR of 2.79 (95% CI=1.25∼6.21, P<0.05) and adjusted HR 2.60 (95% CI=1.17∼5.80, P<0.05). Especially in male patients with eGFR less than 15, a notable increase in risk was found with unadjusted HR of 4.21 (95% CI=1.75∼
10.12, P<0.05) and adjusted HR of 3.71 (95% CI=1.54∼
8.91, P<0.05). In contrast, female patients showed no dif- ference with statistical significance.
DISCUSSION
There was a no significant association between CKD and PD in patients diagnosed with CKD including early stages.
These results indicate that high blood pressure, diabetes
mellitus and dyslipidemia are actual risk factors of PD.
Thus, CKD could be a consequence from such diseases.
An adjusted HR increased to 2.60 (95% CI=1.17∼5.80, P<0.05) when the comparison was made between PD and CKD patients with eGFR under 15 (mL/min/1.73 m2). This result is consistent with previous studies where ESRD had an increased risk of PD.16) It is estimated that CKD could lead to increased cerebrovascular disease and uremic toxins which causes injury to the basal ganglia.18-23) A significant increase in the risk of PD was found in male subjects with HR of 3.71 (95% CI=1.54∼8.91, P<0.05). Whereas no significant association was found in female subjects. Some case control studies have suggested that toxicant exposure and head trauma may be risk factors for PD.18) A higher probability of occupational exposure to toxic substances and
traumatic head accidents in male subjects may have attrib- uted to the result.18) In addition, the neuroprotective func- tion of estrogen might have off-set the development of PD.18)
In CKD patients, the accumulation of various metabolites can cause complications in multiple organs. Major central nervous system complications include uremic encephalopathy, dementia, cerebrovascular disease and movement disorder.20) Uremia, thiamine deficiency, hemodialysis, hypertension, transplant rejection, electrolyte imbalance and medication are the known causes of uremic encephalopathy.21) Uremic ence- phalopathy can present various neurologic symptoms such as delirium, seizure, asterixis, myoclonus and coma.21) Cerebral cortical lesions can cause involuntary movement such as as- terixis and myoclonus.22) Involvement of bilateral basal gan- glia could lead to Parkinsonism (e.g. bradykinesia and pos- tural instability), dysarthria, dyskinesia and altered mental status.23) The literature has reported that patients with mo- tor disabilities due to uremia caused by CKD had vasogenic edema and cytotoxic edema.24,25) However, the exact patho- physiology of bilateral basal ganglia involvement resulting from uremia has not yet been found. One hypothesis states that prolonged diabetes mellitus causes microangiopathy to basal ganglia cells, making them vulnerable to uremic toxins. Consequently, basal ganglia cells are prone to edema caused by impaired metabolism and microangiopathies.26) Uremic encephalopathy has a complex pathophysiology which has not yet been fully understood. It has been suggested that the accumulation in metabolites, hormonal imbalance, disturbances of metabolism and imbalance between excitatory and inhibitory neurotransmitters, contribute to the develop- ment of uremic encephalopathy.21) Others have suggested metabolic acidosis in CKD patients as a cause.27) It is sup- ported by various findings that basal ganglia cells are vul- nerable to uremic toxins.28) Numerous publications have claimed that an increased frequency of hemodialysis has led to improvement in acutely developed Parkinson’s symptoms, and blood test results in CKD patients.29,30) The fact that our study is based on PD patients with ongoing medication, indicates that the patients are subject to both temporary (reversible) and permanent abnormalities.
Various factors including environment, medication and underlying diseases can contribute to the development of PD.10,11) Notably, reduced kidney function causing impair- ment of urea excretion could further increase the risk of PD. In addition, without prompt management of reversible uremic encephalopathy, permanent injury in the basal gan- glia can occur.
This is the first study which demonstrated the increased risk of PD in male patients with eGFR less than 15 (mL/min/1.73 m2). In order to achieve accurate diagnosis, the subjects were defined by both disease code and medi- cation status. In addition, this study analysis performed be- tween CKD classes based on eGFR.
Several limitations could be found. First, without a prop- er designation of disease codes, claim data cannot identify the correct disease. Thus, there is a high probability that early CKDs were unrecorded and underestimated. CKD pa- tients in the control group could also have countered the result. Second, the baseline study of eGFR did not coincide with the beginning of the study in 2002. Since GFR gen- erally decreases with time, it is likely that most CKD pa- tients have higher GFR results at the beginning of the study. Even though the result has revealed a statistically significant association at stage 5 CKD, it is possible that earlier stage CKD has influenced the development of PD.
Despite such limitations, this study is meaningful in several aspects. First, the study is based on a large population with long term follow ups. Second, in addition to disease code, reviewing anti-PD medication has enhanced the accuracy of diagnosis. Finally, we have found evidence that not only end-stage CKD, but also early CKD, could affect develop- ment of PD.
According to above limitations, future studies should be designed in prospective cohort or patient matching in order to increase comparison power.
In summary, the authors have confirmed no significant association could be found between PD and early CKD, however a significant association between PD and male pa- tients with eGFR less than 15 (mL/min/1.73 m2) through a retrospective cohort study. In a senescing society where the burden of PD is escalating, it’s believed that this study
could be meaningful for the purpose of risk evaluation.
REFERENCES
1. Behari M. Treatment of Parkinson’s disease: fighting the surging enemy. Neurology India 1999;47:259.
2. Schrag A, Ben-Shlomo Y, Quinn N. Cross sectional preva- lence survey of idiopathic Parkinson’s disease and Parkinson- ism in London. BMJ 2000;321:21-2.
3. Gil-Prieto R, Pascual-Garcia R, San-Roman-Montero J, Martinez-Martin P, Castrodeza-Sanz J, Gil-de-Miguel A.
Measuring the burden of hospitalization in patients with Parkinson's disease in spain. PLos One 2016;11:e0151563.
4. Dorsey E, Constantinescu R, Thompson J, Biglan K, Holloway R, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384-6.
5. Diem‐Zangerl A, Seppi K, Wenning GK, Trinka E, Ransmayr G, Oberaigner W, et al. Mortality in Parkinson's disease: a 20-year follow-up study. Mov Disord 2009;24:819-25.
6. Ishihara LS, Cheesbrough A, Brayne C, Schrag A. Estimated life expectancy of Parkinson’s patients compared with the UK population. J Neurol Neurosurg Psychiatry 2007;78:
1304-9.
7. Lindgren P, Von Campenhausen S, Spottke E, Siebert U, Dodel R. Cost of Parkinson’s disease in Europe. Eur J Neurol 2005;12:68-73.
8. Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assess- ment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009;24:1641-9.
9. Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 2007;22:1623-9.
10. Gorell JM, Peterson EL, Rybicki BA, Johnson CC. Multiple risk factors for Parkinson's disease. J Neurol Sci 2004;217:
169-74.
11. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 2011;26:1.
12. Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol 2011;26:547-55.
13. Gorell J, Johnson C, Rybicki B, Peterson E, Kortsha G, Brown G, et al. Occupational exposures to metals as risk fac- tors for Parkinson’s disease. Neurology 1997;48:650-8.
14. Gorell J, Johnson C, Rybicki B, Peterson E, Kortsha G, Brown G, et al. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology 1998;20:239-47.
15. Kim JH, Son KY, Shin DW, Kim SH, Yun JW, Shin JH, et al. Network analysis of human diseases using Korean na- tionwide claims data. J Biomed Inform 2016;61:276-82.
16. Wang IK, Lin CL, Wu YY, Chou CY, Lin SY, Liu JH, et al. Increased risk of Parkinson’s disease in patients with end-stage renal disease: a retrospective cohort study. Neuroep- idemiology 2014;42:204-10.
17. Lin HL, Lin HC, Chen YH. Increased risks of parkinsonism in the 3 years after chronic renal failure. Int J Clin Pract 2012;66:499-503.
18. Wooten G, Currie L, Bovbjerg V, Lee J, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry 2004;75:637-9.
19. Obrador GT, Pereira BJ. Systemic complications of chronic kidney disease: Pinpointing clinical manifestations and best management. Postgrad Med 2002;111:115-22.
20. Brouns R, De Deyn P. Neurological complications in renal failure: a review. Clin Neurol Neurosurg 2004;107:1-16.
21. Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, et al. Review on uremic toxins:
classification, concentration, and interindividual variability.
Kidney Int 2003;63:1934-43.
22. Raskin NH, Fishman RA. Neurologic disorders in renal failure. N Engl J Med 1976;294:204-10.
23. Wang HC, Brown P, Lees AJ. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov Disord 1998;13:952-7.
24. Lee PH, Shin DH, Kim JW, Song YS, Kim HS.
Parkinsonism with basal ganglia lesions in a patient with ure- mia: evidence of vasogenic edema. Parkinsonism Relat Disord 2006;12:93-6.
25. Dicuonzo F, Di Fede R, Salvati A, Palma M, De Mari M, Baldassarre GD, et al. Acute extrapyramidal disorder with bi- lateral reversible basal ganglia lesions in a diabetic uremic pa- tient: diffusion-weighted imaging and spectroscopy findings. J Neurol Sci 2010;293:119-21.
26. Wang HC, Cheng SJ. The syndrome of acute bilateral basal ganglia lesions in diabetic uremic patients. J Neurol 2003;
250:948-55.
27. Kumar G, Goyal MK. Lentiform Fork sign: a unique MRI picture. Is metabolic acidosis responsible? Clin Neurol Neurosurg 2010;112:805-12.
28. Jankovic J. The relationship between Parkinson’s disease and other movement disorders. In: Calne DB, editor. Drugs for the treatment of Parkinson’s disease. 1st ed. Berlin Heidelberg: Springer; 1989. p. 227-70.
29. Yoon CH, Im Seok J, Lee DK, An GS. Bilateral basal gan- glia and unilateral cortical involvement in a diabetic uremic patient. Clin Neurol Neurosurg 2009;111:477-9.
30. Cupidi C, Piccoli F, La Bella V. Acute reversible parkinson- ism in a diabetic-uremic patient. Clin Neurol Neurosurg 2006;108:601-3.