Received: February 3, 2010 Revised: February 10, 2010 Accepted: February 18, 2010
Corresponding Author: Heung Yeol Kim, Department of Obstetrics & Gynecology, College of Medicine, Kosin University, 34 Amnam-dong, Seo-gu, Busan 602-702, Korea
Tel: +82-51-990-6226, Fax: +82-51-990-3300, E-mail: hykyale@yahoo.com
* This study was supported by grant of Korea society of Osteoporosis (2009).
Effects of Combined Estrogen and Effervescent Fluocalcic Therapy on the Bone Mineral Density and Bone Metabolism of
Surgical Menopause Women with Osteopenia
Hoon Choi, Min Hyung Jung1, Il Woo Joo2, Eun Hee Kong3, Ji Young Lee5, Heung Yeol Kim4 Department of Obstetrics and Gynecology, College of Medicine, Inje University, Seoul,
Department of Obstetrics and Gynecology1, College of Medicine, Kyung Hee University, Seoul, Department of Family Medicine2, College of Medicine, Kwandong University, Seoul,
Department of Family Medicine3, Department of Obstetrics and Gynecology4, College of Medicine, Kosin University, Busan, Department of Obstetrics and Gynecology5, College of Medicine, Konkuk University, Seoul, Korea
수술적 폐경기 골감소증 여성에서 저용량 에스트로겐 요법과 Fluocalcic Effervescent 병합치료 후 골밀도 및 골전환 표지자의 변화
인제대학교 의과대학 산부인과학교실, 경희대학교 의과대학 산부인과학교실1, 관동대학교 의과대학 가정의학교실2, 고신대학교 의과대학 가정의학교실3, 고신대학교 의과대학 산부인과학교실4, 건국대학교 의과대학 산부인과학교실5
최 훈․정민형1․주일우2․공은희3․이지영5․김흥열4
목적: 골감소증의 치료 목적으로 흔히 사용되고 있는 저용량 에스트로겐과 불소를 병용 복용한 여성을 대상으로 병 용치료가 생화학적 표지자와 골밀도의 변화에 미치는 영향을 알아보고자 하였다.
연구대상 및 방법: 2007년 9월부터 2009년 12월까지 고신대학교 복음병원 산부인과와 가정의학과를 방문한 양성 질 환으로 인한 수술적 폐경기 골감소증 환자 200명을 4 치료군으로 나누어, 12개월 간 I군에서는 1일 Estradiol 1 mg (프로지노바Ⓡ 1, 쉐링 프라워)을 단독으로 경구 투여하였고 II군에서는 매일 1일 Estradiol 2 mg (프로지노바Ⓡ 2, 쉐링 프라워)을 단독으로 경구 투여하였다. III군에서는 매일 1일 Estradiol 1 mg (프로지노바Ⓡ 1, 쉐링 프라워)와 플루오칼 식(수도약품)을 경구 투여하였다. IV군에서는 매일 1일 Estradiol 2 mg (프로지노바Ⓡ 2, 쉐링 프라워)을 플루오칼식(수 도약품)을 경구 투여하였다. 치료 전후 혈중 osteocalcin, total alkaline phosphatase, 요중 deoxypyridnoline, 척추(L2-L4) 및 대퇴골 경부의 골밀도를 측정하였다.
결과: 척추골밀도는 III, IV군에서 12개월 치료 후 기저치에 비해 통계학적으로 유의하게 증가하였다(P<0.05). 치료군 간의 비교에서 제 I, II군에 비해 제 III, IV군에서 척추골밀도가 유의하게 증가하였다(P<0.05). 요중 deoxypyridinoline 는 III, IV군에서 12개월 치료 후 기저치에 비해 유의하게 감소하였다(P<0.05). 그러나 각 군 간의 차이는 없었다. 반 면 대퇴골 경부 골밀도, 혈청 osteocalcin, 혈중 total alkaline phosphatase 검사에서는 각 군 모두 6개월, 12개월 치료 후 기저치에 비해 통계학적 유의한 변화가 없었고, 각 군 간의 차이도 없었다.
결론: 골감소증 치료에 플루오칼식과 함께 저용량이나 고용량 여성호르몬과 동시에 사용하는 것이 여성호르몬 단독 으로 사용한 경우보다 더욱 더 골흡수를 감소시키고 골밀도의 증가를 나타내었다. 따라서 폐경 후 골감소증의 치료 에 여성호르몬과 플루오칼식을 동시에 사용하는 것이 여성호르몬을 단독으로 투여하는 것보다 치료의 효과를 더 높 일 수 있어 효과적일 것으로 생각된다.
중심단어: 골감소증, 플루오칼식, 여성호르몬
After menopause, osteopenia is caused incrementally by estrogen deficiency because bone turnover increases, and bone resorption is more activated than bone formation. In the case of natural menopause, much of osteopenia occurs during the first 5 years. The esti- mated fracture rate is 39.7%, and for femoral fractures, which is the major type of fracture, the rate is 17.5%
over the course of the remaining lifespan of women over 501,2. Osteopenia is defined as a decrease in bone density to between -1 and -2.5 on the Bone Mineral Density (BMD) test, and osteoporosis is defined as lower than -2.5 after menopause. 1 Osteopenia and osteoporosis are the most common diseases in old age.
Prevention is the best measure because their diagnosis and the treatment remains limited. Fractures after progress of osteopenia and osteoporosis do not heal.
According to the European Foundation for Osteo- porosis and Bone Disease (EFFO)’s report, osteopenia patients need to be treated for osteoporosis prevention3. Estrogen therapy has been used for the prevention and the treatment of osteopenia for 40 years in menopausal women4-6. Ettinger et al. and Cummings et al. pointed out that the relative risk of spine and femoral fractures in menopausal women with an E2 (estradiol) level under 5 pg/mL in the blood increased 2.5 times over those with an E2 level of more than 5 pg/ml, and therefore, the estrogen level in the blood should be increased7-8. Despite the advantages of estrogen therapy, some patients discontinue taking it because its side effects can include breast pain, cervix bleeding, weight gain, abdominal distension, breast cancer, and endometrial hyperplasia.
Thus, rather than being standardized, estrogen therapy has been individualized depending on personal risks9. Among these methods, low-dose estrogen therapy is the method in which patients take estrogen orally at a lower than standard dose. This is based on previous studies showing that side effects caused by estrogen could be reduced by decreasing the dose, and that the previously prescribed standard doses maintained
an unnecessarily high level of estrogen in the blood.
Some previous studies have reported that 0.3 mg or 0.45 mg conjugated equine estrogen (CEE), 1 mg or less than 2 mg Estradiol valerate, less than 50μg Transdermal estrogen, or a 20 mg or 25 mg Estradiol implant were effective10.
Fluorine is known to cause an increase in bone density by increasing the number of osteoblasts in bone metabolism11. The function of fluorine is affected by the level of fluorine in the blood. The increase in bone formation occurs at a level of 5~10μM12, and maintaining the blood level of fluorine is important. It was confirmed that the level of fluorine in the blood was maintained when Korean women took Fluocalcic† (ASTA; Disodium monofluorophosphate 100 mg+cal- cium carbonate 1,250 mg) twice a day12.
This study evaluated the effects of estrogen therapy alone and in combination with Fluocalcic on bone mineral density and bone metabolism in surgically menopausal osteopenic women. This study also evaluated the effects of estrogen dose, comparing a 1 mg dose of estradiol (low-dose) and Fluocalcic with a standard dose of 2 mg and Fluocalcic.
MATERIALS AND METHODS
1. Subjects
The subjects were 200 women who were diagnosed with osteopenia, confirmed by a BMD test with a T-value ranging from -1 to -2.5, after hysterectomy or bilateral ovariectomy as a treatment for benign uterine diseases between September 2007 and December 2009. Patients included had no history of estrogen therapy, and no menstruation for recent 6 months or more than 50 mIu/ml of follicle-stimulating hormone in the blood level and less than 25 pg/mL E2 in the blood. The patients had no problem with activities of daily living and no contraindications for estrogen therapy including acute hepatic disorder, chronic hepatic function failure, acute vascular disorder, breast
Table 1. Initial values of clinical characteristics
I II III IV
Age (yr) Menogausal age Weight FSH (mIu/mL) L 2-4 BMD
Femur neck BMD (g/cm2) Urinory Deoxypryridinoline Osteocalcin
Alkaline phospharase
50.23±4.93 48.21±4.53 58.21±4.62 121.23±38.02 0.893±0.391 0.864±0.148 9.31±2.05 17.27±1.03 92.81±9.23
51.36±5.33 48.98±3.51 58.55±6.05 116.51±26.01 0.887±0.261 0.832±0.032 8.39±1.02 16.68±2.37 9.24±8.76
53.5±4.12 49.12±3.78 56.78±3.58 118.43±28.53 0.842±0.287 0.812±0.052 8.78±1.87 17.21±3.01 86.23±11.03
52.89±3.98 48.63±2.43 57.23±5.24 126.21±36.32 0.813±0.310 0.795±0.098 9.56±2.31 16.35±2.04 83.25±7.65 Group - I: 0.30 mg CEE group, II: 0.625 mg CEE group, III: 0.3 mg CEE group+Fluorocalcic group, IV: 0.625 mg CEE group+Fluorocalcic group. Values are expressed in Mean±SD
cancer, or vaginal bleeding for unknown reasons.
2. Methods
All patients were stratified into four groups as follows: 1 mg per day estradiol (ProgynovaⓇ 1, Schering-Plough Corp) was administered orally to Group I, 2 mg estradiol (ProgynovaⓇ 1,) to Group II, 1 mg estradiol (ProgynovaⓇ 1) and Fluocalcic (ASTA Corp.) to Group III, and estradiol 2 mg (ProgynovaⓇ 1) and Fluocalcic to Group IV.
BMD testing and mammography were performed, and fasting bone metabolism markers were checked as baseline data before starting estrogen therapy. Bone density and fasting bone metabolism markers were checked every 6 months thereafter. Patients who changed medications or doses, did not maintain follow-up for at least 1 year, or temporarily discon- tinued medication were excluded from this study.
1) Bone Mineral Density (BMD) test
The BMD of the L2-L4 (L-lumbar spine) from anterior and posterior views and of the femoral neck, which are the parts most frequently fractured in those with osteoporosis, and which have the greatest effect on prevalence and mortality, were measured by dual- energy x-ray absorptiometry (DEXA) with a QDR-4500 (Hologic Inc., Waltham, MA, USA).
2) Measuring Serum Osteocalcin
5 mL blood was centrifuged at 3000 xg for 20 minutes to extract serum using the NovoCalcin kit (Metra Biosystems, Inc, Mountain View, CA, USA).
The intrassay error was 8.3%, and interassay error was 8.6%.
3) Measuring Serum Total Alkaline Phosphatase 5 mL blood was centrifuged in 3000 xg for 20 minutes to extract serum with the NovoCalcin kit (Metra Biosystems). Phenol was produced from phenyl-D-sodium phosphatase by hydrolysis. It was condensed with 4-aminoantiphyline with catalytic oxidizer, generating red-colored quinon. Next, the activity level was calculated with colorimetric analysis (Kind-King method). Intrassay error was 11.8%, and interassay error was 3.9%.
4) Measuring Urinary Deoxypyridinoline (Dpd) Patients had fasted from the night before and collected their urine between 8:00 and 10:00 a.m. after the first urination at 6:00 a.m. on the test day. Measu- ring urinary Dpd was performed with the Pyrilink-D kit (Metra Biosystems). This test is monoclonal antibody enzyme-linked immunosorbent assay (ELISA) in the micro filter plate. Urine was left for 30 min to remove suspension and diluted 10 times by buffer solution. The
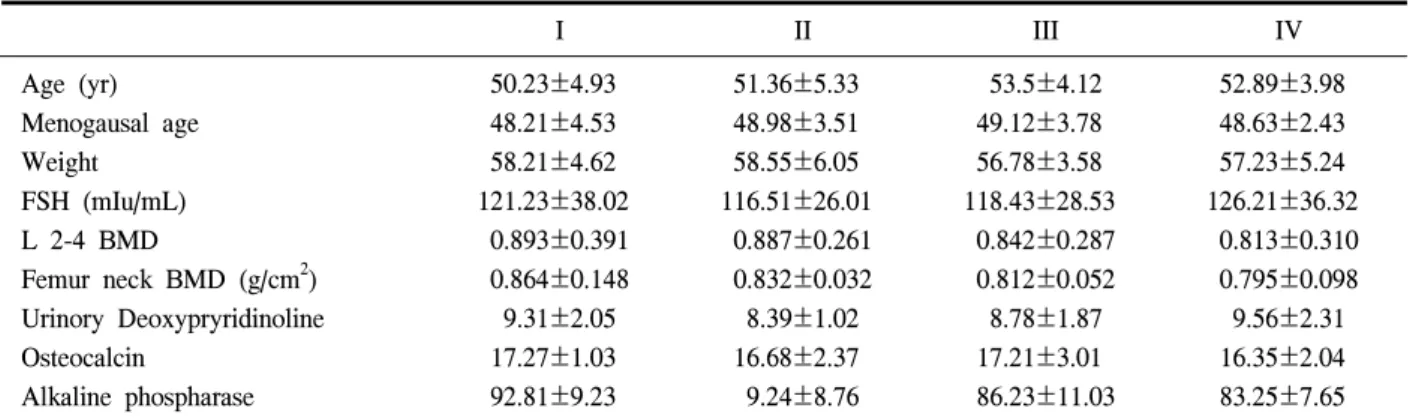
Fig. 1. Changes in the levels of bone mineral density of surgically menopausal women according to study group (Group I: 0.3 mg CEE, Group II: 0.625 mg CEE, group III: 0.3 mg CEE+Fluocalcic, Group IV: 0.625 mg CEE+Fluocalcic). Values are expressed as a ratio to initial values. Significance: * P<0.05
intrassay error was 4.2%, and interassay error was 6.3%.
3. Statistical Analysis
All data are shown with mean±standard deviation.
Data analysis was performed by SPSS (version 10.0) with IBM PC. ANOVA was used to compare each group, and a post-hoc test performed. P<0.05 was considered statistically significant (Table 1).
RESULTS
The change in bone density and bone mechanism markers after estrogen therapy was calculated as a ratio to baseline levels. Data are shown with mean±
standard error of mean.
1. Change in Bone Density in the L2 and L4 (Fig. 1)
Bone density measurements of the lumbar spine were performed at 6 months and 12 months. The ratios of bone density to baseline data were 1.018±0.027 and 1.031±0.045 in Group I, 1.023±0.028 and 1.038±
0.012 in Group II, 1.025±0.016 and 1.041±0.031 in Group III, and 1.032±0.024 and 1.053±0.028 in Group
IV. There were significant increases in bone density compared to baseline data at 12 months in Groups III and IV (P<0.05). There were also significant increases in bone density in Groups III and IV comparing with Group I and II (P<0.05).
2. Change in Bone Density in the femoral neck (Fig. 1)
Bone density measurements of the femoral neck were performed at 6 months and 12 months. The ratios of bone density to baseline data were 1.010±0.027 and 1.014±0.045 in Group I, 1.015±0.028 and 1.019±
0.012 in Group II, 1.016±0.023 and 1.021±0.019 in Group III, and 1.019±0.018 and 1.027±0.032 in Group IV. There were no statistically significant increases in bone density compared to baseline data at 6 and 12 months. There were no significant differences in bone density among groups.
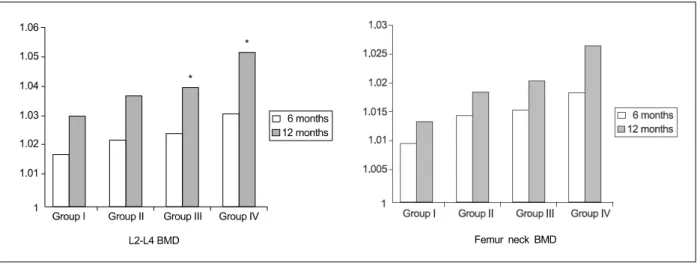
3. Change in Serum Osteocalcin (Fig. 2) Serum osteocalcin measurements were performed at 6 months and 12 months. The ratios of serum osteo- calcin to baseline data were 0.97±0.03 and 0.93±0.04 in Group I, 0.95±0.03 and 0.92±0.02 in Group II, 0.91
±0.03 and 0.86±0.03 in Group III, and 0.89±0.02 and
Femur neck BMD
*
*
Fig. 2. Changes in the levels of bone metabolism of surgically menopausal women according to study group (Group I: 0.3 mg CEE, Group II:
0.625 mg CEE, Group III: 0.3 mg CEE+
Fluocalcic, Group IV: 0.625 mg CEE+Fluo- calcic). Values are expressed as a ratio to initial values. Significance: * P<0.05
0.82±0.02 in Group IV. There were no significant increases in serum osteocalcin compared with baseline data at 6 and 12 months. There were no significant
increases in serum osteocalcin among groups.
4. Change in Serum Total Alkaline Phos- phatase (Fig. 2)
Serum total alkaline phosphatase measurements were performed at 6 months and 12 months. The ratios of serum total alkaline phosphatase to baseline data were 0.95±0.03 and 0.93±0.05 in Group I, 0.94±0.02 and 0.92±0.04 in Group II, 0.93±0.02 and 0.91±0.04 in Group III, and 0.93±0.02 and 0.91±0.04 in Group IV.
There were no significant increases in serum total alkaline phosphatase compared with baseline data at 6 and 12 months. There were no significant increases in serum total alkaline phosphatase among groups.
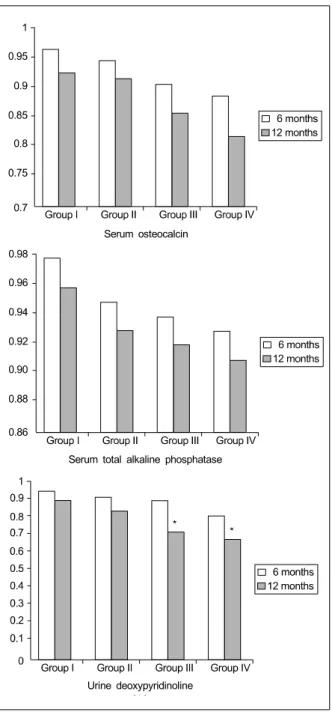
5. Change in Urinary Deoxypyridinoline (Fig. 2)
Urinary deoxypyridinoline measurements were per- formed at 6 months and 12 months. The ratios of urinary deoxypyridinoline with baseline data were 0.93
±0.03 and 0.85±0.02 in Group I, 0.91±0.02 and 0.73
±0.03 in Group II, 0.82±0.04 and 0.69±0.06 in Group III, and 0.93±0.02 and 0.91±0.04 in Group IV. There were statistically significant increases in urine deoxy- pyridinoline compared with baseline data at 12 months in Groups III and IV (P<0.05). However there were no significant differences in urine deoxypyridinoline among groups.
DISCUSSION
Human pituitary gonadotropic hormone is increased by failure of the hypothalamic-pituitary feedback mechanism caused by a decrease in ovary function because steroid hormone formation and the number of primordial follicles decrease after menopause. Although menopausal symptoms including hot flashes, urogenital organ atrophy, osteoporosis, and cardiovascular disor- ders may not appear during the first 1~2 years after natural menopause, because of the continued existence
Serum osteocalcin
Serum total alkaline phosphatase
Urine deoxypyridinoline
of a few remaining follicles that can respond to follicle stimulating hormone, these symptoms will appear after surgical menopause because of a decrease in estrogen13. Thus, appropriate treatments are needed to promote better quality of life in postmenopausal women because as the population ages, women will live one third of their lives after menopause.
Bone is active and reproductive tissue. Bone meta- bolism consists of bone resorption by osteoclasts and bone formation by osteoblasts, and bone is remodeled with a balance of mineral metabolism. Bone resorption and bone formation are balanced in the general population, but as they get older, bone formation decreases compared to bone resorption. In particular, osteoblast function decreases, and osteoclast function increases after menopause. This accelerates bone re- sorption14.
Osteopenia is defined as a decrease in bone mass scuh that only slight trauma can cause fractures. This has been confirmed in radiological findings, and radiation permeability increases without pathological findings. Bone density decreases because of estrogen deficiency, the decrease of bone formation, hyper- parathyroidism, and other reasons. As a result, one of every two women in their 60s could be diagnosed with osteopenia, and most women would be diagnosed with osteopenia by 85 years of age15.
Osteoporosis is defined as a decrease in bone mass to below the fracture threshold and a decrease in physical strength so that even slight trauma can easily cause fractures. The reasons for osteoporosis are known to be age, heredity, calcium deficiency, smoking, the lack of exercise, and hypoestrogenemia, which is the major cause of osteoporosis14. Fractures caused by osteoporosis occur in the spine, wrist, and femur. As people get older, the risk of fractures increases. The risk of fractures in Caucasian women caused by osteoporosis over their lifespan is up to 30%. In particular, 50% of women in their 70s experience fractures caused by osteoporosis16.
The mechanism of osteopenia after menopause is not yet clear; however, it is supposed that the reason is the increase of bone resorption by osteoclasts caused by a decrease of estrogen in the ovaries17. 1~3% of bone mass is lost per year after menopause. Thus, after several years the risk of fractures has increased more than two-fold2. The risk of fractures by osteoporosis can be reduced up to 50~60% with treatment18. However, increasing bone mass in women with osteoporosis is difficult. For prevention and treatment of osteopenia after menopause, it is necessary to identify the group at highest risk for osteoporosis.
Appropriate treatments for patients with osteopenia are important18,19.
Up to this point, among the medications for preven- tion and treatment of osteopenia, medications that suppress bone resorption have been in the majority.
According to a previous study by Lindsay et al., 0.625 mg CEE was the minimum dose for prevention of bone loss in the spine and the ossa antebrachii, so 0.625 mg CEE had been recommended as a standard dose to prevent osteoporosis after menopause. However, it was suggested that 0.31 mg CEE could be a minimum dose with biochemical evidence, namely the decrease in calcium excretion in the urine, and with DEXA, which can measure BMD more exactly20. Ettinger et al.
reported first that a combination of 0.31 mg CEE and 1,500 mg calcium was effective for preventing osteo- penia after menopause21. Gallagher et al. reported that a combination of 0.3 mg CEE, 1.0 g calcium, and 10 mg medroxyprogesterone acetate (MPA) could prevent osteo- penia in the cortical bone and the trabecular bone as well as CEE alone22. MacLennan et al. suggested that a change in treatment from 0.625 mg CEE and 10 mg MPA to 0.3 mg CEE and low-dose progestogen (2.5 mg MPA, 30μg levonorgestrel, and 350μg norethis- terone) could maintain bone density at 12 months, so it could be a substitute for existing menopause treat- ment23. Webber et al. suggested that 0.3 mg CEE and 2.5 mg MPA could be used selectively for patients with
a high risk of breast cancer24. Mizunuma et al. reported that low-dose estrogen therapy (0.31 mg CEE and 2.5
mg MPA) was effective in the increase of bone density in the lumbar spine25. Recker et al. reported that 0.3 mg CEE, 2.5 mg MPA, and 1,000 mg calcium could prevent bone mass during the period of the acceleration of bone loss after menopause. At the same time, bone density was increased in the spine with 25-hyhdroxyvitamin D26. The Women’s Health, Osteo- porosis, Progestin, Estrogen (HOPE) study reported that 0.45 mg CEE and 1.5 mg MPA showed positive out- comes. In the our previous studies, the group taking 0.3 mg CEE showed an increase in bone density, but the effect of bone density was less than in the group taking 0.625 mg CEE.
Sodium fluoride is known to induce cell proliferation with MPA kinase activity by suppression of phospho- tyrosyl-protein phosphatase in osteoblasts27. However, the level of fluorine in the blood should be monitored because side effects of overdose include osteosclerosis or renal function failure28. Fluorine is absorbed through a mucous membrane in the upper gastrointestinal tract, and the level of fluorine reaches the maximum after 30 to 60 minutes when administered orally.
50% of the fluorine is absorbed in bone tissue, and the rest is excreted in the urine29. However, the function of fluorine in osteopenia treatment has yet to be explained theoretically. According to studies in the 1990s, while fluorine increased bone density in the spine, it was concluded that treatment with fluorine was negative because fluorine could not decrease the frequency of spinal fractures, and increased the frequency of fractures elsewhere30. Other studies since 1995 have reported the opposite results--that bone density in the spine increased, and there were no findings of increasing fractures in the spine or other parts with 50 mg of fluorine (NaF) a day31. In recent studies, the argument for fluorine has remained.
Reginster et al. reported that the frequency of fractures in the spine and other parts decreased with sodium
monofluorophosphatase (20 mg/day MFP), which was a third generation drug, after 4 years32. On the other hand, Meunier et al. reported that administering MFP (150~200 mg/day) versus NaF (50 mg/day) showed no difference in effect on prevention of fractures in the spine compared with a control group, which was treated with calcium and vitamin D33. However, in a later study (Meunier et al.), patients diagnosed with serious osteoporosis were treated for 2 years, which was too short a treatment period. Although fluorine increased bone density in the spine, the frequency of fractures was not decreased because the cortical bone was weakened, and the frequency of fractures in other parts increased despite of the increase in the bone density in the trabecular bone by fluorine15. Many researchers have reported that NaF or MFP could decrease the frequency of fractures in the spine, but could not decrease the frequency of fractures elsewhere
32,34,35. In this study, the groups administered fluocalcic and 0.3 mg or 0.625 mg estradiol showed a greater increase of bone density than in the groups admini- stered estradiol only. The group that received fluocalcic and low-dose estradiol showed a similar increase in bone density in the lumbar spine as the group that received fluocalcic and a standard dose of estradiol.
Pyd and Dpd act as new markers, which are bone resorption markers. 3-hydroxy pyridinium crosslinks, which are made from the cartilage tissue, form Pyd and Dpd. This synthesis depends on the maturation of collagen; thus, they are made continuously during the bone formation and resorption cycle36. The reason Dpd was used as a bone resorption marker was that collagen was the unique source of Dpd, and urine did not particularly interfere with Dpd. It was reported that urinary excretion of pyridinium crosslinks in post- menopausal women reached its maximum within 1 year after menopause36, and then decreased after 1 year. In this study, all of the four groups showed a decrease in urine Dpd, and this fit with other results. It may be that the increasein pyridinium crosslinks is caused by the
increase in the bone turnover rate by estrogen defi- ciency. In addition, the bone loss by menopause could be prevented with early treatment because the excretion of urine pyridinium crosslinks is decreased by appro- priate treatment for osteopenia. In this study, combined treatment with low-dose estradiol and fluocalcic was effective in the prevention of bone loss.
Osteocalcin, which consists of 3 gamma-carboxyglu- tamic acid (GLA), is a non-collagen protein with a small molecular weight of 4.9 kd, and is found in bone and tooth tissue. Osteocalcin, which is synthesized in osteoblasts, is combined with bone tissue stromata, and part of it is excreted into the blood. It can be measured with a radioimmunoassay37. Serum osteocalcin, which is the one of the bone proteins produced by osteoblasts, is useful in evaluating the activity of osteoblasts and bone formation. It is known that serum osteocalcin in women increases with age beginning in the 30s. In particular, it increases over two-fold after menopause, and can bedecreased to its premenopausal level with estrogen38. Postmenopausal women, who had no treatment, received follow-up bone density tests for 2~4 years. As a result, serum osteocalcin was reported to be the most reliable biochemical marker of the bone turnover rate39. This marker can be an outstanding indicator of bone turnover when bone resorption and bone formation are balanced, and can be an indicator of bone formation when bone resorption and bone formation are not balanced. Osteocalcin, which is separated from bone resorption, may reflect bone resorption39. In this study, osteocalcin was decreased in all the groups, and the reason was assumed to be that the number of patients was small.
Serum alkaline phosphatase increased in cases of Paget Syndrome or hyperparathyroidism. However, the test sensitivity is low, and it was reported that there was no significant difference in osteopenia or osteo- porosis patients26. Likewise, the present study showed no statistical difference in serum alkaline phosphatase.
In this study, the changes in biochemical markers
were not significant; there were no differences in serum osteocalcin and total alkaline phosphatase compared to baseline data. The exception was urinary Dpd, which showed a decrease in Groups III and IV at 12 months after treatment. Other studies on combined treatments have shown various results. Hong et al. reported that there was no change in osteopenia and osteoporosis patients administered a combined treatment40. Reginster et al. reported that bone formation markers increased for 4 years, but bone resorption markers showed no significant changes32. Alexandersen et al. reported that bone formation and bone resorption markers were decreased after a combined treatment35. The reasons for different results could be variation in the number of the patients, MFP doses, or test methods.
Considering all these results, it can be concluded that combined treatment with estradiol and Fluocalcic is more effective for treatment of postmenopausal symp- toms and the maintenance of bone density compared with estradiol only.
CONCLUSION
To evaluate the effects of combined treatment with estrogen and fluorine on bone biochemical markers and bone mineral density in 200 surgically menopausal osteopenic women, patients were stratified into 4 groups for 12 months; patients in Group I took 0.3 mg CEE (0.3 mg Premarin Tab, Wyeth Corp.) orally per day, 0.625 mg CEE (0.625 mg Premarin Tab, Wyeth) in Group II, 0.3 mg CEE and Fluocalcic in Group III, and 0.625 mg CEE and Fluocalcic in Group IV. Serum osteocalcin, total alkaline phosphatase, urinary deoxy- pyridinoline, and BMD in L2-L4 and the femoral neck were measured pre and post treatment. The results were as follows:
The BMD of the lumbar spine was significantly increased at 12 months after estrogen therapy in Groups III and IV (P<0.05). The BMD of the lumbar spine in Groups III and IV was also significantly higher than in
Groups I and II (P<0.05). Urinary deoxypyridinoline as significantly decreased at 12 months after estrogen therapy in Groups III and IV compared with baseline data (P<0,05). However, there were no significant differences among groups.
On the other hand, there were no differences in the BMD of the femoral neck, serum osteocalcin, or serum total alkaline phosphatase compared to baseline data.
There were no differences among groups. Combined treatment with Fluocalcic and low-dose or high-dose estrogen (estradiol) decreased bone resorption and increased bone density compared to estrogen only.
Therefore, combined treatment with low-dose estrogen (estradiol) and Fluocalcic is more effective for osteo- penia in postmenopausal women because it could increase compliance with treatment.
REFERENCES
1. Min BK, Ku BS. A study on menopause in Korean women. Kor J Obstet Gynecol 1985;28:966-72.
2. Michaelsson K, Baron JA, Farahmand BY, Johnell O, Magnusson C, Persson PG. Hormone replace- ment therapy and risk of hip fracture: population based case-control study. BMJ 1998;20:1853-63.
3. Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. Guidelines for diagnosis and manage- ment of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporosis Int 1997;7:390-406.
4. Lee CJ, Kim HY, Park UD. The Effects of Hormone Replacement Therapy on Serum Osteo- calcin, Serum Calcium, Serum Alkaline Phospha- tase, and Urine Calcium of Postmenopausal Women.
Korean J Obstet Gynecol 1997;40:2733-40.
5. Kim HY, Park ED. Impacts of Added Progestogen on the Bone Mineral Densities and Bone Meta- bolism of Postmenopausal women undergoing Estrogen Replacement Therapy. Korean J Obstet Gynecol 1998;41:564-75.
6. Yang HI, Kong EH, Cha HS, Choi YS, Eo WK, Kim KC, et al. Long term effects on oral proges- togen (medroxyprogesterone acetate) on the bone mineral densities and the level of serum lipid metabolism during estrogen replacement therapy in postmenopausal women. J Korean Acad Fam Med 1999;20:1000-11.
7. Ettinger B, Pressman A, Sklarin P. Associations between low levels of serum estradiol. bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab 1998;83:2239-43.
8. Cummings SR, Palermo L, Browner W. Monitoring osteoporosis therapy with bone densitometry: mis- leading changes and regression to the mean.
Fracture Intervention Trial Research Group. JAMA 2000;283:1318-21.
9. Grodstein F, manson JE, Colditz GA. A prospec- tive, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000;133:933-41.
10. Heikkinen J, Vaheri R, Ainulainen P. Long term continuous combined hormone replacement therapy in the prevention of postmenopausal bone loss: A comparison of high-and low-dose estrogen-progestin regimens. Osteoporos Int 2000;11:929-37.
11. Farley JR, Wergedal JE, Baylink DJ. Fluoride directly stimulates proliferation and alkaline phos- phatase activity of born forming cells. Science 1983;222:330-2.
12. Chung HY, Kim SW, Han KO, Han IK, Min HK, Yun HK, et al. Serum Fluoride Level in Normal Adult Women and Changes in Serum Fluoride Level after Disodium Monofluorophosphate Admi- nistration. J Korean Soc Endocrinol 1997;12:565- 70.
13. Corden RS. Follicular status at menopause. Human Reprod 1987;2:617-23.
14. Emans SJ, Graec E, Hoffer FA, Gundberg C, Ravnilkar V, Wood ER. Estrogen deficiency in
adolescents and young adults: impact on bone mineral content and effects of estrogen replacement therapy. Obstet Gynecol 1990;76:585-92.
15. Riggs BL, Hodgson SF, O'Fallon WM, Chao EY, Wahner HW, Muhs JM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990;
322:802-9.
16. Melton LJ, Chrischilles EA, Copper C, Lane AW, Riggs BL. Perspective how many women have osteoporosis. J Bone Miner Res 1992;7:1005-10.
17. Borderie D, Cherruau B, Dougados M, Ekindjjan OG, Roux C. Biochemical markers as predictors of bone mineral density changes after GnRH agonist treatment. Calcif Tissue Int 1998;6:21-5.
18. Weinstein MC, Schiff I. Cost-effectiveness of hormone replacement therapy in the menopause.
Obstet Gynecol Surv 1983;38:445-55.
19. Kim HY, Park ED. The effects of 1alpha- hydroxyvitamin D3 on the Bone Mineral Density and Bone Metabolism in Postmenopausal Women.
Korean J Obstet Gynecol 1998;41:829-38.
20. Lindsay R, Hart DM, Clark DM. The minimum effective dose of estrogen for prevention of post- menopausal bone loss. Obstet Gynecol 1984;63:
759-63.
21. Ettinger B, Genant HK, Cann CE. Menopausal bone loss can be prevented by low dose estrogen with calcium supplement. J Comput Assist Tomogr 1985;9:633-9.
22. Gallagher JC, Kable WT, Goldgar D. Effect of progestin therapy on cortical and trabecular bone:
Comparsion with estrogen. Am J Med 1991;90:
171-8.
23. MacLennan AH, Henry D, Hills S. Oral oestrogen replacement therapy versus placebo for hot flushes.
In: Farquhar C, Cooke I, Barlow D, eds. Menstrual Disorders Module of the Cochrane Database of Systematic Reviews. Oxford: Cochrane Library Update Software; 2000
24. Webber CE, Blake JM, Chambers LF, Roberts JG.
Effects of 2 tears of hormoen replacement upon bone mass, serum lipid and lipoprotein. Maturis 1994;19:13-23.
25. Mizunuma H, Okano H, Soda M, Kagami I, Miyamoto S, Tokizawa T, et al. Prevention of post- menopausal bone loss with minimal uterine bleeding using low dose continuous estrogen/progestin the- rapy: a 2-year prospective study. Maturitas 1997;
27:69-76.
26. Recker RR, Davies KM, Dowd RM. Effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. Ann Intern Med 1999;130:
897-904.
27. Lau KW. Inhibition of phosphotyrosine dephos- phorylation leads to increased phosphotyrosine phosphorylation of MAP kinase, MAP kinase activity, and human bone cell proliferation. Bone 1995;16(Suppl 1):96-100.
28. Resch H, Libanati C, Talbot J, Tabuenca M, Farley S, Bettica P. Pharmacokinetic profile of a new fluoride preparation: Sustained-release monofluoro- phoshate. Calcif Tissue int 1994;54; 7-11.
29. Baylink DJ. Serum fluoride levels In; Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, New York: Raven Press 3;
262-263, 1998
30. Resch H, Libanati C, Talbot J, Tabuenca M, Farley S, Bettica P. Pharmacokinetic profile of a new fluoride preparation: Sustained-release monofluoro- phoshate. Calcif Tissue Int 1994;54;7-11.
31. Pak CY, Sakhaee K, Adams-Huet B, Piziak V, Peterson RD, Poindexter JR. Treatment of post- menopausal osteoporosis with slow-release sodium fluoride. Final report of a randomized controlled trial. Ann Intern Med 1995;123:401-8.
32. Reginster JY, Meurmans L, Zegels B, Rovati LC, Minne HW, Giacovelli G, et al. The effect of sodium monofluorophosphate plus calcium on ver-
tebral fracture rate in postmenopausal women with moderate osteoporosis. A randomized, controlled trial. Ann Intern Med 1998;129:1-8.
33. Meunier PJ, Sebert JL, Reginster JY, Briancon D, Appelboom T, Netter P, et al. Fluoride salts are no better at preventing new vertebral fractures than calcium-vitamin D in postmenopausal osteoporosis:
the FAVO study. Osteoporos Int 1998;8:4-12.
34. Ringe JD, Setnikar I. Monofluorophosphate com- bined with hormone replacement therapy in post- menopausal osteoporosis. An open-label pilot efficacy and safety study. Rheumatol Int 2002;
22:27-32.
35. Alexandersen P, Riis BJ, Christiansen C. Mono- fluorophosphate combined with hormone replace- ment therapy induces a synergistic effect on bone mass by dissociating bone formation and resorption in postmenopausal women: a randomized study. J Clin Endocrinol Metab 1999;84:3013-20.
36. Black D, Duncan A, Robbins SP. Quantitative analysis of the pyridinium crosslinks of collagne in urine using on paired reverse D phase high- performace liquid chromatography. Anal Biochem 1998;169:197-204.
37. Slovik DM, Gundberg CM, Neer R. Clinical evaluation of bone turnover by serum osteocalcin measurement in a hospital setting. J Clin Endo- crinol Metabl 1994;59:228-36.
38. Crilly RG, Jones MM, Horsman A, Nordin BE.
Rise in plasma alkaline phosphatase at the meno- pause. Clin Sci (Lond) 1980;58:341-2.
39. Adriana BCM, Rosemary H, Richard E. Monitoring Alendronate therapy for Osteoporosis. J Bone Miner Res 1999;14:602-8.
40. Hong JR, Kim HY. Changes of markers of bone turnover after 6-month treatment with HRT and Fluocalcic in postmenopausal osteoporotic women.
Kosin Medical Journal 2003;18;11-6.
■ ABSTRACT ■
Objective: To evaluate the effects of estrogen therapy and fluocalcic on the bone mineral density and bone metabolism in surgically menopausal osteopenic women.
Methods: This prospective randomized clinical trial examined the effects of conjugated equine estrogen and fluocalcic in combination and separately, on bone mass density (BMD) and biochemical markers of bone turnover in 200 women with low bone mass. Treatment included 1 mg estradiol (Group I, n=50), 2 mg estradiol (Group II, n=50), 1 mg estradiol plus fluocalcic (Group III, n=50), and 2 mg estradiol plus fluocalcic (Group IV, n=50) for 12 months. Bone density measurements were performed at 6 and 12 months at the lumbar spine and femur neck. Biochemical markers of bone turnover were also measured every 6 months.
Results: The BMD of the lumbar spine increased significantly during the treatment in Group III and Group IV (P<0.05), while the BMD of the femur neck increased slightly, but not significantly, during treatment in all groups. In addition, urinary deoxypyridinoline decreased significantly in Group III and Group IV at 12 months of treatment (P<0.005). On the other hand, serum osteocalcin and total alkaline phosphatase decreased slightly, but not significantly, in all groups during the treatment.
Conclusion: The combined treatment with estradiol and fluocalcic is more effective in surgically menopausal women with osteopenia because it decreases bone biochemical markers and increases BMD.
Key Words: Osteopenia, Estradiol, Fluocalcic