Korean J Vet Res(2016) 56(2) : 67~74 http://dx.doi.org/10.14405/kjvr.2016.56.2.67
67
<Original Article>
Water soluble tomato concentrate regulates platelet function via the mitogen-activated protein kinase pathway
Dahye Jeong
1, Muhammad Irfan
1, Evelyn Saba
1, Sung-Dae Kim
2, Seung-Hyung Kim
3, Man Hee Rhee
1,*
1
Department of Veterinary Medicine, College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea
2
Research Center, Dongnam Institute of Radiological and Medical Sciences, Busan 46033, Korea
3
Institute of Traditional Medicine & Bioscience, Daejeon University, Daejeon 34520, Korea (Received: March 10, 2016; Accepted: April 5, 2016)
Abstract : Tomato extract has been shown to exert antiplatelet activity in vitro and to change platelet function ex vivo, but with limitations. In this study, antiplatelet activity of water soluble tomato concentrate (Fruitflow I) and dry water soluble tomato concentrate (Fruitflow II) was investigated using rat platelets. Aggregation was induced by collagen and adenosine diphosphate and granule-secretion, [Ca2+]i, thromboxane B2, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) levels were examined. The activation of integrin αIIbβ3 and phosphorylation of signaling molecules, including mitogen-activated protein kinase (MAPK) and PI3K/Akt, were investigated by flow cytometry and immunoblotting, respectively. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were examined. Moreover, in vivo thrombus weight was tested by an arteriovenous shunt model. Fruitflow I and Fruitflow II significantly inhibited agonist induced platelet aggregation, adenosine triphosphate and serotonin release, [Ca2+]i, and thromboxane B2 concentration, while having no effect on cAMP and cGMP levels. Integrin αIIbβ3 activation was also significantly decreased. Moreover, both concentrates reduced phosphorylation of MAPK pathway factors such as ERK, JNK, P38, and PI3K/Akt. In vivo thrombus formation was also inhibited. Taken together, these concentrates have the potential for ethnomedicinal applications to prevent cardiovascular ailments and can be used as functional foods.
Keywords : blood platelet, cardiovascular disease, natural cardioprotective agent, thrombosis, tomato
Introduction
The prevalence of cardiovascular diseases in the devel- oped countries is increasing at a high pace [7]. Platelet hyperactivation is the causal factor for the growth and advancement of atherosclerosis and an important contributor in cardiovascular pathology [14]. Rupture of atherosclerotic plaque can lead to death by development of cardiovascular disease (CVD) [4]. Therapeutic antiplatelet agents have been proved to reduce the incidence CVD [15]. There is need to slow down the progression of these diseases along with focused attention on the influence of dietary compounds on the cardiovascular system [6]. Recently, increase in the pop- ularity of ethnomedicine and natural products has strength- ened interest in traditional remedies for CVD [10]. During the last few decades, consumption and popularity of toma- toes have been increased and previous literature suggests the overall health benefits of tomatoes and evidence to be con- sidered as cardiovascular protective food [1, 5]. Nutrients available in the tomato are accompanying theoretical or proven effects on reduced risk of degenerative diseases [28]
and cardiovascular system like cardioprotective effects of lycopene [1]. Previous studies have put forward a link between lower incidence of CVD and consumption of tomato in Med- iterranean countries [5, 23]. Dutta-Roy et al. [2] and O’Kennedy et al. [16] proved that platelet activity can be influenced by water tomato concentrates (Fruitflow I and Fruitflow II) in vitro and ex vivo. But the available data is insufficient to rec- ognize the underlying mechanism of action. In this study, we explored the effects of tomato ex vivo as well as in vivo in more detail using a rat model.
We hypothesize that observed cardioprotective benefits accredited to the tomato could be linked to reduce hyperactivity of platelets and the suppression of platelet function in vivo. This type of natural antithrombotic agent could have an application in the new era of ethnomedicine and prevention of CVD like ath- erosclerosis, myocardial infarction and coronary artery disease.
Materials and Methods
Chemicals preparation
Water soluble tomato concentrate (WSTC; Fruitflow I) and
*Corresponding author
Tel: +82-53-950-5967, Fax: +82-53-950-5955 E-mail: rheemh@knu.ac.kr
dry water soluble tomato concentrate (DWSTC; Fruitflow II) were obtained from DSM Nutritional product (Switzerland).
The Fruitflow II was diluted to the appropriate concentration immediately before all experiments were performed. Collagen and adenosine diphosphate (ADP) were purchased from Chrono- log Corporation (USA). Fura-2/AM was obtained from Sigma Chemical (USA). ATP Assay kit was obtained from Biomedical Research Service & Clinical Application (USA). TXB2 EIA kit was purchased from Enzo Life Sciences (USA). Serotonin EIA kit was purchased from LDN Labor Diagnostika Nord (Germany). Fibrinogen Alexa Fluor 488 conjugate was obtained from Molecular Probes (USA). Antibodies against phospho- p44/42, p44/42, phospho-p38, p38, phospho-SAPK/JNK, SAPK/
JNK, phospho-PI3K (p85), PI3K (p85), phospho-Akt and Akt were acquired from Cell Signaling Technology (USA). All chemicals were of reagent grade.
Animals and dosage
Male Sprague Dawley rats (6 weeks old) weighing from 240 to 250 g were obtained from Orient Bio (Korea). The animals were acclimated for 1 week before the experiments and maintained in an air-conditioned animal room with a 12/
12 h light/dark cycle at a temperature of 23 ± 2
oC and humidity of 50 ± 10%. The rats were randomly divided into 3 groups; one normal group, one Fruitflow I treated group and one Fruitflow II treated group (n = 10 for each group).
As based on tomato feeding of 3 g/day for human consump- tion [20], dose normalization for rats was done to 900 mg/kg and 45 mg/kg for Fruitflow I and Fruitflow II, respectively.
The rats were given oral doses of Fruitflow I and Fruitflow II once a day for 1 month. Normal group was administrated with vehicle control (physiological saline). At the end of 4th week, blood was collected from rats for experimentation and further processing (Fig. 1).
Platelet preparation
Whole blood was obtained after 2 h of last oral injection by heart puncture and platelet preparation was conducted as previ- ously described [18]. All experiments were carried out in accor- dance with the National Institutes of Health guidelines and approved by the Ethics Committee of the College of Veterinary Medicine, Kyungpook National University (2015-0094), Korea.
Platelet aggregation assay
Platelet aggregation was performed as previously described [11]. Aggregation was monitored by measuring light trans- mission using an aggregometer (Chrono-log Corporation, USA). Briefly, the washed platelets (3 × 10
8/mL) were pre- incubated at 37
oC for 2 min in the presence of 1 mM CaCl
2and stimulated with doses of collagen (1.25 µg/mL) and ADP (2.5 µM or 1.25 µM). The mixture was further incubated for 5 min with stirring and determined using the aggregometer.
[Ca
2+]
imeasurement
The intracellular calcium ion concentration ([Ca
2+]
i) was mea-
sured with Fura-2/AM as previously described [9] and Fura-2 fluorescence in the cytosol was measured with the spectro- fluorometer as previously described by Schaeffer and Blaustein [22] using following formula: [Ca
2+]
iin cytosol = 224 nM × ( F−min)/(Fmax−F), in which 224 nM is the dissociation con- stant of the Fura-2-Ca
2+complex, and min and max repre- sent the fluorescence intensity levels at very low and very high Ca
2+concentrations, respectively.
Adenosine triphosphate (ATP) release assay
ATP assay was performed as previously described [8].
After the aggregation reaction was terminated, the platelets centrifuged and the supernatants were used for the assay.
ATP release was measured in a luminometer (GloMax 20/20;
Promega, USA) using an ATP assay kit according to manu- facturer’s instructions.
Serotonin release assay
After terminating the platelet aggregation reaction, the mixture was immediately centrifuged at 12,000 × g for 5 min at 4
oC. The supernatant was collected and serotonin release was measured with a serotonin ELISA kit according to the manufacturer’s instructions.
Evaluation of thromboxane B2 (TXB2) generation Platelet aggregation assay was performed and the reac- tions were terminated by adding ice-cold 2.5 mM EDTA and 100 µM indomethacin. After centrifugation at 12,000 × g for 5 min at 4
oC, the supernatant was collected and the concen- tration of TXB2 was measured using a TXB2 EIA kit accord- ing to the manufacturer’s protocol.
Measurement of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) levels
After terminating the platelet aggregation reaction, cAMP or cGMP level was measured using a cAMP EIA kit or cGMP EIA kit according to the manufacturer’s protocol, respectively.
Assessment of fibrinogen binding to integrin α
IIbβ
3Fibrinogen Alexa Fluor 488 conjugate binding to washed
Fig. 1. Experimental scheme. WSTC, water soluble tomato con- centrate; DWSTC, dry water soluble tomato concentrate.platelets was quantified by flow cytometry as previously described [8]. The fluorescence of each platelet sample was analyzed using a FACS Caliber cytometer (BD Biosciences, USA), and the data were analyzed using CellQuest software (BD Biosiences).
Prothrombin time (PT)/activated partial thromboplas- tin time (aPTT)
aPTT and PT were measured using an Automated Coagula- tion Laboratory 100 Instrument (Instrumentation Laboratory, Italy). Briefly, the platelet-poor plasma (PPP) from rats treated Fruitflow I and Fruitflow II was incubated at 37
oC for 7 min. 100 µL of the incubated PPP was mixed with 50 mL of cephalin in the process plate, and coagulation started with the addition of 1 mM CaCl
2and 100 mL thromboplastin to the PPP for the aPTT and PT assays, respectively.
Ateriovenous shunt
The in vivo antithrombotic activity of Fruitflow I and Fruit- flow II was evaluated in a rat extracorporeal shunt model by the method of Umetsu and Sanai [26], with a little modifica- tion. Briefly, the ateriovenous shunt model was used and blood circulation in the cannula was carried out for 15 min, and thrombus weight was determined immediately.
Immunoblotting
Immunoblots were performed as described previously [3].
Briefly, after terminating the aggregation reaction, lysates were then prepared by solubilizing and centrifuging the platelets in sample lysis buffer. Protein concentration was determined using a bicinchoninic acid assay. Total cell pro- teins (35 µg) from the platelet lysate were separated using 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes in transfer buffer. The membranes were blocked in TBS-T containing 5% dry skim milk and incubated with primary antibody diluted in 5% bovine serum albumin solution.
The blots were then incubated with horseradish peroxidase- conjugated secondary antibody that was visualized by binding an enhanced chemiluminescence (Advansta, USA).
Statistical analysis
Data were analyzed with a one-way analysis of variance followed by a post hoc Dunnett’s test in order to measure sta- tistical significance of the differences observed (SAS Insti- tute, USA). All data are presented as the mean ± SE. P values of 0.05 or less were considered to be statistically significant.
Results
Effects on agonist-induced platelet aggregation The effect of WSTC (Fruitflow I) and DWSTC (Fruitflow II) on agonist-induced platelet aggregation was evaluated.
Our result showed that Fruitflow I (900 mg/kg) and Fruitflow II (45 mg/kg) dramatically inhibited platelet aggregations induced with collagen (1.25 µg/mL) to 32 and 33% and with ADP 2.5
µM to 24 and 14%, and with ADP 1.25 µM to 27 and 15%, respectively compared to normal control group 93 ± 10% (Fig. 2).
Effect on intracellular calcium concentration
Intracellular calcium release acts as a key role in platelet degranulation and aggregation [17]. Therefore, we examined whether Fruitflow I and Fruitflow II inhibit intracellular calcium concentration. As shown in Figure 3, Fruitflow I and Fruitflow II significantly reduced intracellular calcium mobilization by 85 ± 10% and 84 ± 10% respectively than control group.
Effect on granule secretion (ATP and serotonin release) Activated platelets release the contents of granules such as alpha and dense-granules and these granules content secre- tion enhances platelet activation including intracellular sig- naling pathway. We checked the effect of ATP and serotonin release of dense granules. These findings suggests that the Fruitflow I and Fruitflow II inhibited ATP release by 45 ±
Fig. 2. WSTC (Fruitflow I, 900 mg/kg) and DWSTC (Fruitflow II, 45 mg/kg) inhibited collagen and adenosine diphosphate (ADP) induced platelet aggregation ex vivo. Aggregation was quantified and expressed as a percentage. Each graph shows the mean± SE of at least four independent experiments performed.***p < 0.001 compared to the agonist control.
Fig. 3. The inhibitory effect of Fruitflow I (900 mg/kg) and Fruitflow II (45 mg/kg) on [Ca2+]i induced by collagen and ADP.
Washed platelets (3 × 108/mL) were incubated with a calcium fluorophore (5 µM, Fura-2/AM) and stimulated with collagen or ADP. The results are presented as the mean± SE of at least four independent experiments. **p < 0.01 and ***p < 0.001 vs. control.
5% and 46 ± 6% respectively (Fig. 4) as well as serotonin release by 75 ± 10% and 71 ± 10% (Fig. 5).
Effect on TXB2 production
Thromboxane A2 amplifies platelet activation during hemostasis as a mediator. As it is physiologically unstable, so we investigated its metabolite, TXB2 production effect. The result showed that Fruitflow I and Fruitflow II markedly decreased both ADP and collagen-induced TXB2 production by 82 ± 7% and 85 ± 6% respectively (Fig. 6).
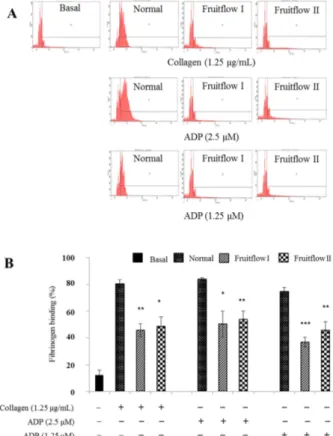
Fruitflow I and Fruitflow II inhibit fibrinogen binding to integrin α
IIbβ
3Binding of fibrinogen to integrin α
IIbβ
3which take pivotal role in the platelet activation and aggregation, induce out- side-in signaling leads to adhesion, spreading and complete aggregation [24]. So, we checked the effect of Fruitflow I
Fig. 4. Inhibitory effects of Fruitflow I (900 mg/kg) and Fruit- flow II (45 mg/kg) on granule secretion and ATP release.Washed platelets (3 × 108/mL) were pre-incubated for 2 min at 37oC in the presence of 1 mM CaCl2 and then stimulated with collagen or ADP. After terminating the aggregation, ATP release assay performed. Bar graph shows the mean± SE of at least four independent experiments. **p < 0.01 compared to the ago- nist control.
Fig. 5. Inhibitory effects of Fruitflow I (900 mg/kg) and Fruit- flow II (45 mg/kg) on serotonin release from dense granules.
After termination of aggregation reaction, serotonin release measured with ELISA kit. Bar graph shows the mean± SE of at least four independent experiments. **p < 0.01 and ***p <
0.001 vs. control.
Fig. 6. Inhibitory effect of Fruitflow I (900 mg/kg) and Fruit- flow II (45 mg/kg) on TXB2 production. After termination of aggregation reaction by adding ice-cold 2.5 mM EDTA and 100 µM indomethacin the mixture was centrifuged, the supernatant used and the concentration of thromboxane B2 (TXB2) mea- sured using a TXB2 EIA kit. Bar graph shows the mean± SE of at least four independent experiments. *p < 0.05 and **p <
0.01 vs. control.
Fig. 7. (A) The inhibitory effect of Fruitflow I (900 mg/kg) and Fruitflow II (45 mg/kg) on fibrinogen binding to integrin αIIbβ3. Washed platelets (3 × 108/mL) were pre-incubated for 2 min at room temperature in the presence of 0.1 mM CaCl2 and stimu- lated with collagen or ADP for 5 min and fibrinogen Alexa Fluor 488 (20 µg/mL), and then fixed with 0.5% paraformaldehyde at 4oC for 30 min. Representative FACS analysis results of four independent trials. (B) Bar graph summarizing the inhibitory effect of Fruitflow I and Fruitflow II on fibrinogen binding. *p
< 0.05, **p < 0.01 and ***p < 0.001 vs. control.
and Fruitflow II on fibrinogen binding to integrin α
IIbβ
3and the result showed that fibrinogen binding to integrin α
IIbβ
3reduced by 45 ± 5% and 38 ± 2% respectively than the con- trol group (Fig. 7).
Arteriovenous shunt thrombosis model
It is well established that the arteriovenous shunt thrombo- sis models have been used to evaluate in vivo antithrombotic effects [3, 26]. We therefore, investigated the effect of Fruit- flow I and Fruitflow II on extracorporeal shunts model throm- bus formation. As shown in Figure 8, Fruitflow I and Fruitflow II potently reduced the thrombus weight by 58 and 61%
respectively.
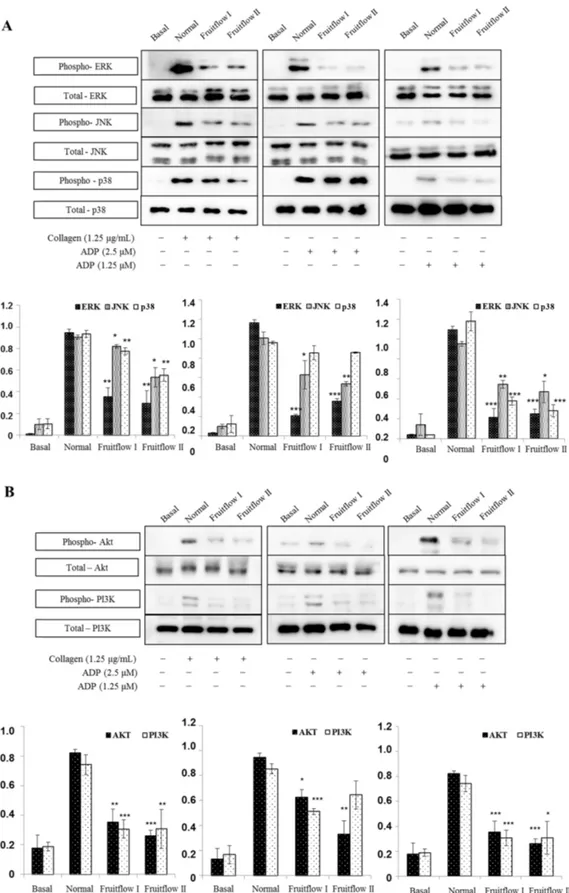
Fruitflow I and Fruitflow II attenuates agonist induced mitogen-activated protein kinase (MAPK) and PI3K/Akt phosphorylation
It is well-known that the phosphorylation of MAPKs (ERK, JNK and P38-MAPK) mediate platelet activation pathway and PI3K/Akt signaling pathway is another critical step for platelet activation and aggregation. Fruitflow I and Fruitflow II markedly blocked the phosphorylation MAPK’s and PI3K/
Akt, indicating that Fruitflow I and Fruitflow II mediate the MAPK’s and PI3K/Akt signaling pathway (Fig. 9).
Discussion
Previous studies proposed that prophylactic suppression of platelet activation can prevent prothrombotic state [25]. It slows down the progression of atherosclerosis and minimizes the risk of stroke and myocardial infarction, and side effects of prophylactic regimes outweigh their benefits [19]. A previous study demonstrated that Tomato extract inhibited collagen and ADP induced platelet aggregation ex vivo [16]. However, the underlying mechanism is not completely understood.
Therefore, in this study, we investigated the effect of WSTC (Fruitflow I) and DWSTC (Fruitflow II) on agonist-induced platelet activation ex vivo and thrombus formation in vivo.
Fruitflow I and Fruitflow II significantly inhibited platelet aggregation induced by collagen and ADP. These data suggested antiplatelet activity of Fruitflow I and Fruitflow II involved in glycoprotein VI and P2Y
12receptor signaling pathway. In order to demonstrate the inhibitory mechanism, downstream signaling components including calcium mobilization, gran- ule secretions, integrin α
IIbβ
3activation were examined. Platelet granule molecules play key roles in hemostasis, thrombosis, including activating other cells or cellular adhesion [29]. We therefore studied the role of Fruitflow I and Fruitflow II in the secretion of dense granules in platelets, by measuring ATP and serotonin release assay. It reduced ATP and serotonin releases on collagen and ADP induced platelet activation.
These results demonstrated that the antiplatelet effect of Fruitflow I and Fruitflow II can occur through its suppressive effect of platelet secretion.
It is well-known that cytosolic calcium play key role in platelet activation. Increasing calcium levels leads to several signaling pathways involved in actin-myosin interaction, pro- tein kinase C [13]. As a result of our Ca
2+mobilization study, Fruitflow I and Fruitflow II significantly decreased the intra- cellular calcium concentration on collagen and ADP induced platelet aggregation. Thromboxane A2 (TXA2) production from arachidonic acid via the cyclooxygenase pathway is an important positive feedback loop mechanisms for platelet activation [12]. A significant reduction of its production was observed by Fruitflow I and Fruitflow II treatment. Cyclic nucleotides such as cAMP and cGMP generation lead to inhibition of platelet aggregation but Fruitflow I and Fruit- flow II have no effect on cAMP and cGMP levels (data not shown).
Inhibitory potential of Fruitflow I and Fruitflow II was further confirmed by a clear suppression of granule secretion and integrin α
IIbβ
3activation (conjoint congregating step of platelet activation). The above findings propose that impaired α
IIbβ
3conformational changes may be induced by pretreat- ment with Fruitflow I and Fruitflow II on exposure to its high affinity fibrinogen binding site formerly called as inside-out signaling, followed by the platelet agonist interactions. Inversely, these findings also suggests that the outside-in signaling (the next step in fibrinogen binding followed by post-ligand occupancy proceedings) leading to platelet shape change and spreading may be weakened by given treatment. Number of evidences are available indicating that platelets are constantly exposed to a variety of activating factors, including collagen, fibrinogen, ADP, vWF, thrombin and thromboxane and inhibitory factors such as endothelial-derived NO, prostacyclin (PGI
2) and ADPase [21, 27] . Thrombotic or bleeding disorders can be developed by impairment of this equilibrium. Thus, a durable equilibrium between two opposing processes of platelet acti- vation and inhibition is thought to be critical for normal hemostasis. Our study suggests that pretreatment of activated
Fig. 8. In vivo effects of Fruitflow I (900 mg/kg) and FruitflowII (45 mg/kg) on thrombus formation. The ateriovenous shunt model was used and blood circulation in the cannula carried out for 15 mints, and thrombus weight determined immediately. Bar graph shows the mean± SE of at least three independent exper- iments. ***p < 0.001 vs. control.
Fig. 9. Fruitflow I (900 mg/kg) and Fruitflow II (45 mg/kg) attenuated the phosphorylation of mitogen-activated protein kinase (i.e.
ERK1/2, JNK and p38) (A) and PI3K/Akt (B). Cell proteins were extracted after aggregation termination and proteins separated using SDS-PAGE and transferred to polyvinylidene difluoride membranes which then probed to antibodies against total and phospho ERK1/
2, JNK and p38, and PI3K/Akt. All immunoblots were carried out in at least four independent experiments. *p < 0.05, **p < 0.01 and
***p < 0.001 vs. control.
platelets with Fruitflow I and Fruitflow II may contribute to the maintenance of this balance.
In the present study, observations indicate that Fruitflow I and Fruitflow II inhibits collagen and ADP-induced ERK1/2, JNK and p38-MAPK and potent inhibitory effect on PI3K/
Akt signaling, indicating modulation of both pathways may be involved in tomato’s anti-platelet activity. Our study indi- cate that tomato have GPVI and P2Y
12suppression potential, thus antagonism of these receptors may represents a novel therapeutic regime. PT and aPTT results did not show differ- ence between normal and treatment group suggesting that Fruitflow I and Fruitflow II do not induce changes to the integrity of extrinsic and intrinsic cascade of coagulation system (data not shown).
In accordance with previous findings on platelet hemosta- sis and pathophysiology of coagulation cascades [21, 27] we conclude the ex vivo capability of Fruitflow I and Fruitflow II to inhibit agonist induced aggregation, TXA2 production, granule secretion, α
IIbβ
3activation, [Ca
2+]
imobilization, via MAPK and PI3K/Akt phosphorylations and in-vivo throm- bus formation inhibition without affecting coagulation time, which illustrates the potential use of given compound as a nominee to be considered as an effective ethnomedicinal antithrombotic agent.
WSTC (Fruitflow I) and DWSTC (Fruitflow II) are potent inhibitors of agonist induced ex vivo platelet aggregation and granule secretion. In addition, it also significantly inhibited in vivo thrombus formation, while it had no effect on coagula- tion. Our findings indicate that Fruitflow I and Fruitflow II inhibit collagen and ADP-stimulated platelet function through modulation of signaling downstream via MAPK pathway.
Acknowledgments
This work was supported by Naam S&H (Korea).
References
1. Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW Jr. The tomato as a functional food. J Nutr 2005, 135, 1226-1230.
2. Dutta-Roy AK, Crosbie L, Gordon MJ. Effects of tomato extract on human platelet aggregation in vitro. Platelets 2001, 12, 218-227.
3. Endale M, Lee WM, Kamruzzaman SM, Kim SD, Park JY, Park MH, Park TY, Park HJ, Cho JY, Rhee MH.
Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol 2012, 167, 109-127.
4. Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD.
Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol 1998, 31, 352-358.
5. Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl
Cancer Inst 1999, 91, 317-331.
6. Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 2000, 72, 912-921.
7. Jackson SP. Arterial thrombosis-insidious, unpredictable and deadly. Nat Med 2011, 17, 1423-1436.
8. Jeon BR, Kim SJ, Hong SB, Park HJ, Cho JY, Rhee MH. The inhibitory mechanism of crude saponin fraction from Korean Red Ginseng in collagen-induced platelet aggregation. J Ginseng Res 2015, 39, 279-285.
9. Kamruzzaman SM, Endale M, Oh WJ, Park SC, Kim KS, Hong JH, Kwak YS, Yun BS, Rhee MH. Inhibitory effects of Bulnesia sarmienti aqueous extract on agonist- induced platelet activation and thrombus formation involves mitogen-activated protein kinases. J Ethnopharmacol 2010, 130, 614-620.
10. Kerver JM, Yang EJ, Bianchi L, Song WO. Dietary patterns associated with risk factors for cardiovascular disease in healthy US adults. Am J Clin Nutr 2003, 78, 1103-1110.
11. Lee HS, Kim SD, Lee WM, Endale M, Kamruzzaman SM, Oh WJ, Cho JY, Kim SK, Cho HJ, Park HJ, Rhee MH. A noble function of BAY 11-7082: inhibition of platelet aggregation mediated by an elevated cAMP-induced VASP, and decreased ERK2/JNK1 phosphorylations. Eur J Pharmacol 2010, 627, 85-91.
12. Lhermusier T, Severin S, Van Rothem J, Garcia C, Bertrand-Michel J, Le Faouder P, Hechler B, Broccardo C, Couvert P, Chimini G, Sié P, Payrastre B. ATP-binding cassette transporter 1 (ABCA1) deficiency decreases platelet reactivity and reduces thromboxane A2 production independently of hematopoietic ABCA1. J Thromb Haemost 2016, 14, 585- 595.
13. Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 2010, 30, 2341-2349.
14. Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization.
Am J Cardiol 2000, 86 (Suppl 2), 3J-9J.
15. Mehta SR, Yusuf S; Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Study Investigators.
The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme. Rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J 2000, 21, 2033-2041.
16. O’Kennedy N, Crosbie L, Whelan S, Luther V, Horgan G, Broom JI, Webb DJ, Duttaroy AK. Effects of tomato extract on platelet function: a double-blinded crossover study in healthy humans. Am J Clin Nutr 2006, 84, 561-569.
17. Oh WJ, Endale M, Park SC, Cho JY, Rhee MH. Dual roles of quercetin in platelets: phosphoinositide-3-kinase and MAP kinases inhibition, and cAMP-dependent vasodilator- stimulated phosphoprotein stimulation. Evid Based Complement Alternat Med 2012, 2012, 485262.
18. Park JY, Ji HD, Jeon BR, Im EJ, Son YM, Lee JY, Lee DH, Lee YC, Hyun E, Jia Q, Hong M, Park HJ, Rhee MH. Chlorin e6 prevents ADP-induced platelet aggregation by decreasing PI3K-Akt phosphorylation and promoting cAMP production. Evid Based Complement Alternat Med 2013,
2013, 569160.
19. Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF Jr, Smith SC Jr, Stone NJ, Taubert KA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation 2002, 106, 388-391.
20. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008, 22, 659- 661.
21. Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res 2007, 100, 1673-1685.
22. Schaeffer J, Blaustein MP. Platelet free calcium concentra- tions measured with fura-2 are influenced by the transmem- brane sodium gradient. Cell Calcium 1989, 10, 101-113.
23. Sesso HD, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular
disease in women. J Nutr 2003, 133, 2336-2341.
24. Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood 2004, 104, 1606- 1615.
25. Trip MD, Cats VM, van Capelle FJL, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med 1990, 322, 1549-1554.
26. Umetsu T, Sanai K. Effect of 1-methyl-2-mercapto-5-(3- pyridyl)-imidazole (KC-6141), an anti-aggregating compound, on experimental thrombosis in rats. Thromb Haemost 1978, 39, 74-83.
27. Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol 2008, 28, 403-412.
28. Willcox JK, Catignani GL, Lazarus S. Tomatoes and cardiovascular health. Crit Rev Food Sci Nutr 2003, 43, 1-18.
29. Xu XL, Huang YJ, Chen XF, Lin DY, Zhang W. 2,3,4,5- tetrahydroxystilbene-2-O-β-D-glucoside inhibits proliferation of vascular smooth muscle cells: involvement of NO/cGMP/
PKG pathway. Phytother Res 2012, 26, 1068-1074.
![Fig. 3. The inhibitory effect of Fruitflow I (900 mg/kg) and Fruitflow II (45 mg/kg) on [Ca 2+ ] i induced by collagen and ADP.](https://thumb-ap.123doks.com/thumbv2/123dokinfo/5262079.367278/3.892.466.810.127.338/fig-inhibitory-effect-fruitflow-fruitflow-induced-collagen-adp.webp)