The BMPs expression and histomorphometric study of ${\beta}-TCP$ / rhBMP-2 Grafting on the rabbit cranial bone defects
전체 글
수치
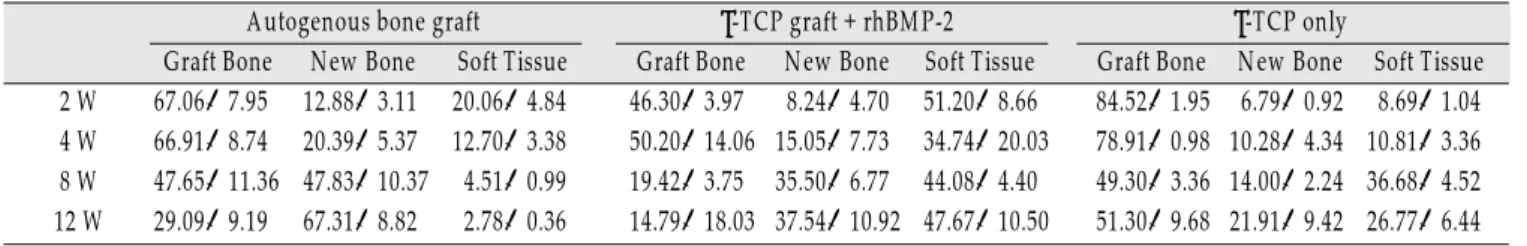
관련 문서
Histopathologic findings of control group at 4 weeks show little bone- implant contact (BIC) around the implant (asterisks) and new-bone formation in the defect
The grafted particulated tooth driven allografts were partially surrounded with new bone matrix and fairly supported graft associated new bone formation with some
Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: a 2-year prospective study.. Corinaldesi G, Pieri F, Sapigni
The aim of this study was to compare the effect on bone regeneration relative to maintenance period of PTFE membrane in rabbit calcarial defects.. Eight adult
In 4-week group, the group filled with bone graft with decortication revealed larger new bone formation area than shown in the group that had a defect area
Histologic evaluation of early human bone response to different implant surfaces2. Histologic evaluation of human bone integration on machined and
Higher power view of the box area of the left figure showed poor new-bone formation around the implant (asterisk) and low BIC. Villanueva
The result of using PLGA nanofiber membrane with bone graft material showed that the PLGA nanofiber membrane in the experimental group of 2 weeks were ten times more new