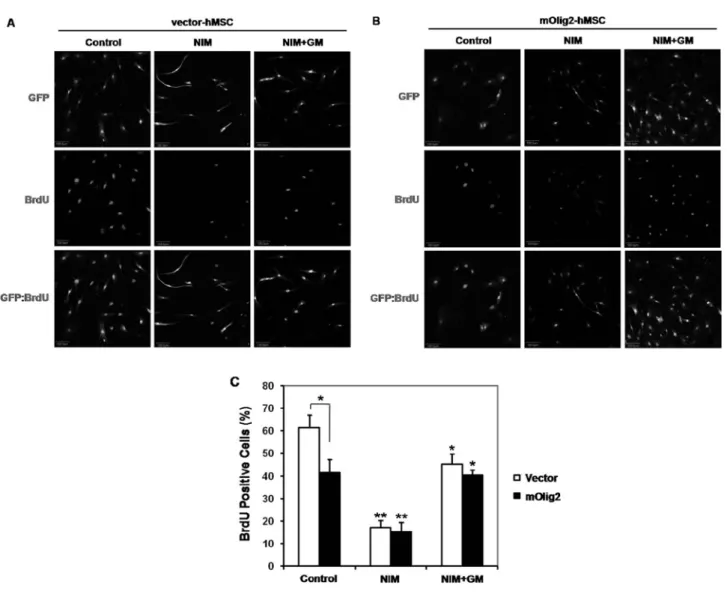
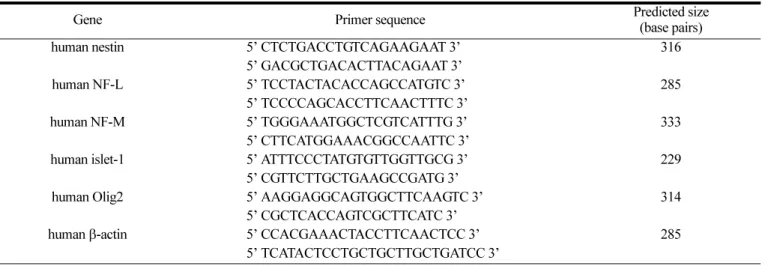
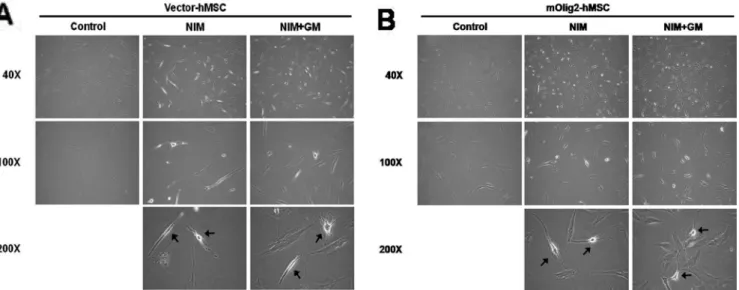
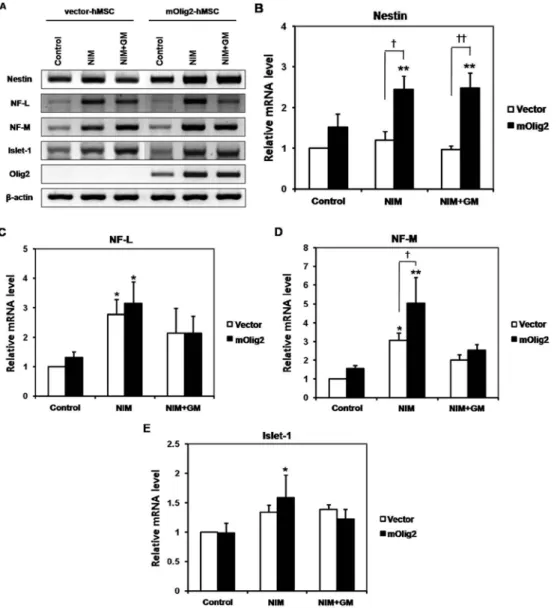
Induction of a Neuronal Phenotype from Human Bone Marrow-Derived Mesenchymal Stem Cells
전체 글
수치
관련 문서
Histopathologic findings of control group at 4 weeks show little bone- implant contact (BIC) around the implant (asterisks) and new-bone formation in the defect
First, after the Group Art Therapy, the scale of hopelessness depression in the test group decreased significantly in comparison with that before the group
In 4-week group, the group filled with bone graft with decortication revealed larger new bone formation area than shown in the group that had a defect area
Diagrams show the spatial distribution of individual trees and stand profile(a), crown projection(b) for Group of Quercus glauca... The comparison of
platensis under variously mixed medium conditions of low cost medium based on deep sea water.. platensis in the growth medium at 22 days
2) In between-group comparison before and after elastic band exercise, a significant difference was found in the percentage of body fat, fat-free
The 24hr proteinuria level were increased in kidney with cyclosporine-A group but significantly decreased in iNOS inhibitor and green tea polyphenol group..
Approved clinical use of bone marrow stem cells for myocardial infarction treatment... Cardiac