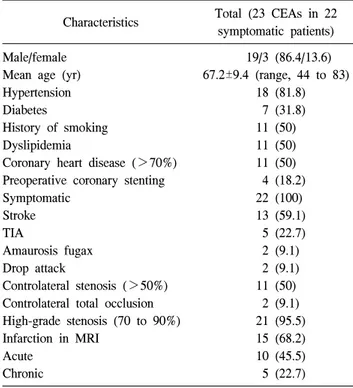
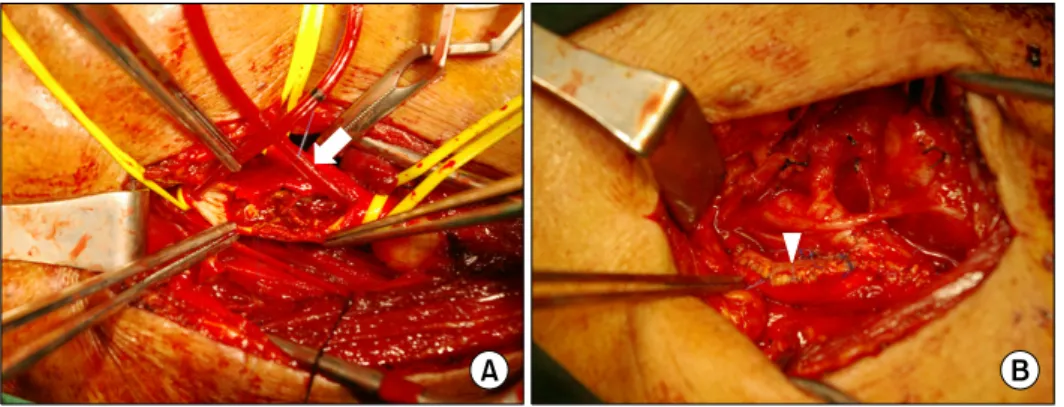
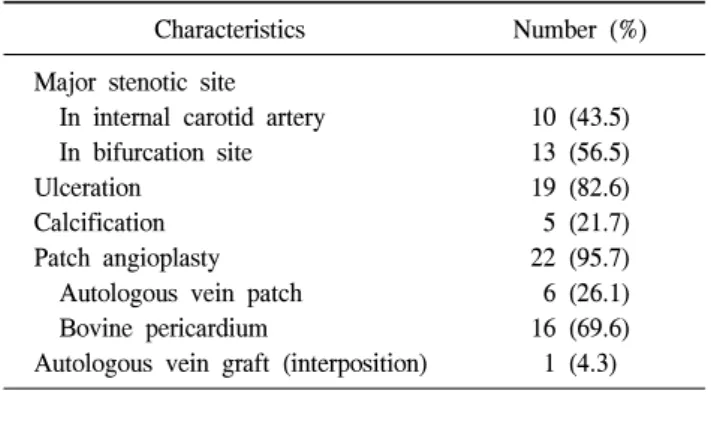
http://dx.doi.org/10.5090/kjtcs.2012.45.2.95 ISSN: 2233-601X (Print) ISSN: 2093-6516 (Online)
Department of Thoracic and Cardiovascular Surgery, Division of Neurology, Chonbuk National University Hospital, Chonbuk National University Medical School
Received: August 22, 2011, Revised: September 14, 2011, Accepted: October 16, 2011
Corresponding author: Jong Bum Choi, Department of Thoracic and Cardiovascular Surgery, Chonbuk National University Hospital, Chonbuk National University Medical School, 20 Geonji-ro, Deokjin-gu, Jeonju 561-712, Korea
(Tel) 82-63-250-1486 (Fax) 82-63-250-1480 (E-mail) jobchoi@chonbuk.ac.kr
C
The Korean Society for Thoracic and Cardiovascular Surgery. 2012. All right reserved.
CC