Immobilization Osteoporosis
Ghichan Kim
Department of Physicial Medicine and Rehabilitation, Kosin University College of Medicine, Busan, Korea
Immobilization osteoporosis is found in a number of diseases, such as complete motor paralysis and reversible immobilization. In general, immobilization-induced mechanical loading declines in bones are known to be among the key factors of osteoporosis in spinal cord injury, stroke, and bed rest patients.
However, recent research has also identified non-mechanical factors, including neural, endocrine, hormonal, nutritional, and iatrogenic factors. Prevention and treatment of immobilization osteoporosis can be divided into pharmacological and non-pharmacological methods. Pharmacologic treatments such as calcitonin, bisphosphonates, and oral phosphates have been applied to patients with spinal cord injury or under bed rest conditions to reduce bone loss. Recently, researchers have examined zoledronic acid, which has a positive impact on the prevention and treatment of acute stroke and spinal cord injury.
Zoledronic acid is an intravenous bisphosphonate given once a year, thereby bypassing gastrointestinal absorption/irritation problems. However, several questions still remain, including whether bone loss should continue in immobilized patients with spinal cord injury or stroke, how long bone mineral density levels can remain intact after treatment, and how long the treatment should continue. As such, more extensive research that studies these questions in the long term is needed.
Key Words: Immobilization, Osteoporosis, Bisphosphonates
Received: April 13, 2010 Revised: June 29, 2010 Accepted: July 8, 2010
Corresponding Author: Ghichan Kim, Department of Physicial Medicine and Rehabilitation, College of Medicine Kosin University, 34 Amnam-dong, Seo-gu, Busan 602-702, Korea Tel: +82-51-990-6639
E-mail: ghichan@hotmail.com
Immobilization osteoporosis is a secondary osteo- porosis which is connected with chronic diseases, exposures, or nutritional deficiencies that adversely affect bone metabolism. Immobilization has long been known as a cause of hypercalcemia and hypercalciuria, and prolonged immobilization has been associated with a marked reduction in skeletal mass.1-5
Diverse animal models and human conditions have been used to examine the impact of immobilization on bone loss. About thirty years ago,6 Hattner and McMillan classified the pathophysiology of immo- bilization osteoporosis into three types: 1) reduction of
stresses and strains secondary to weight-bearing and muscle tension; 2) neurally mediated influences; and 3) vascular changes. They concluded that the most important determinants appear to be mechanical strain and compression of bone by the interaction of muscles and gravity. In reality, many studies have found that mechanical forces can impact bone cell function,7,8 growth, modeling, remodeling, and mass. Similar fin- dings have been demonstrated on clinically paralyzed adults and chronically immobilized children.9,10 As mechanical force has emerged as an important patho- genesis of immobilization osteoporosis, it has been widely reported that physical activities including muscle exercise and weight-bearing have a positive impact on bone mineral content,11,12 as do various pharmacologic preparations.13,14
As such, this paper briefly reviews the clinical evidence and pathophysiology of immobilization osteo-
porosis and examines medical interventions for preven- tive treatment, focusing on both pharmacologic and non-pharmacologic treatments.
CLINICAL EVIDENCES
Bone atrophy induced by immobilization is found in a number of diseases such as complete motor paralysis and reversible immobilization. Of the various causes, we examined spinal cord injury, stroke, and temporary immobilization including application of a cast to treat fractures, therapeutic bed rest and prolonged voluntary bed rest.
1. Spinal cord injury (SCI)
SCI causes bone loss, which increases fracture risks.
A decline in bone mineral density (BMD) and bone mineral content (BMC) has been detected radiologically in the paralyzed limbs of patients as early as 6 weeks after SCI.15 Demineralization in SCI patients shows characteristics from other diseases. More specifically, the head remains intact in tetraplegic patients, while with paraplegia patients, the upper limbs remain intact, showing that demineralization affects only sublesional topography. Moreover, bone loss is known to be more common in SCI patients with high level lesion, complete injury, spasticity, long duration, and high age.16 However, body weight does not influence bone loss in SCI patients (unlike for able-bodied women).
The ability to stand or ambulate unaided does not increase bone mass nor reduce osteoporosis.17-19 It remains an open question how long bone loss may continue in the lower extremities in SCI patients. Some argue that bone loss ceases two years after an injury,15,20 while Bruin et al.21 report that it may last longer. Recent studies have found that significant bone loss may continue many years after an injury,22,23 and the time course of bone loss may differ by bone com- partment.24 As such, we believe that more research on the time course of bone loss in SCI patients is
necessary.
SCI may be accompanied not only by bone loss but also by changes of bone structure. Most researchers have found that the trabecular bones at both knees and the iliac crest deteriorate faster than the cortical bone and thus endure greater fracture risk.25-28
According to previous studies, the incidence of lower extremity fracture in SCI patients may range from 1%
to as high as 34%.29-33 In contrast, there have been few reports on vertebral fractures. Similar results were also found in bone mineralization studies. In the lower third of the femur and the upper third of the tibia, BMD reductions ranged from 24.5% to 70%,17,18,34,35 while the level of demineralization was relatively lower in the lumbar spine than in long bones.36 Most researchers believe that bone mass is well maintained in the vertebral column continued weight-bearing on paraple- gia.36 It is also interesting that in SCI patients, lower extremity fractures were more often found in the supracondylar area around the knee, rather than the hip or femoral shaft.31 However, the related mechanism is not yet well understood.
2. Hemiplegia and hemiparesis
Stroke results in extensive bone loss and increases fracture risk. Hamdy37 et al argued that deminerali- zation occurs within days of stroke onset and continues for four months, with no significant mineral loss thereafter. However, given other research that has found that demineralization lasts for a year,38,39 we believe that more work is necessary on this issue in SCI patients. Bone loss is more common on the paretic side,37,38 and BMD reduction can amount to 14% in the proximal femur and up to 17% in the upper extremities one year after stroke.40-42 Some studies have found that BMD on the non paretic side may increase with increased physical activity.42
In stroke patients, bone loss is more pronounced in the upper extremities compared to the lower. The severity of bone loss is greater in patients with the
ability to walk. For patients with walking disabilities, the level of bone loss increased in line with the duration of paralysis.43-45 Prince et al.46 argues that bone loss can be preventive in the absence of spasticity associated with a good functional level. However, it is difficult to reach such a conclusion because the correlation between functional level and spasticity can be a confounding factor.
The effects of sex and age on bone loss in stroke patients have not been extensively studied. Some researchers have found that female stroke patients endured greater bone loss than their male counter- parts.47,48 However, this research was limited by small sample size and the fact that its BMD measurement method is not yet recognized by the WHO.
Stroke patients are known to have 1.5-4 times greater fracture risks than a general population matched for age and sex.49,50 Ramnemark et al.18 examined 1,139 acute stroke patients for a median of 2.9 years and reported 154 fracture incidences in 120 patients, involving mostly the paretic side and hip fracture-s. According to this study, the most common cause of fracture was falling. Most falling incidents took place within six months of discharge and usually involved the paretic side.51,52 Pouwels53 et al. claim that hip/femur fracture risks are double stroke patients, with a high-risk group that includes patients who are female, younger than 71, and suffered strokes more recently.
3. Temporary immobilization
Temporary immobilization is nonpermanent immo- bilization caused by medical or therapeutic factors, voluntary bed rest, or space flight (ahypogravity state).
The study of the impact of simple bed rest54 was conducted on 34 patients who endured back pain from protrusion of the lumbar intervertebral disk. During the 27 day period, lumbar spine BMC declined by 0.9%
per week (range 11-24 weeks). Re-ambulation led to bone mineral gain in patients and bone conditions returned to nearly normal in four months. Pekka et al.55
argue that men with a history of tibial fracture show bone mass loss of 10~12% in the spine and 4~11%
in the knee. The study also found correlation between short immobilization time, low pain assessment, good muscle strength, and high functional scores of the injured extremity. Recent research on bone loss in space flight astronauts has drawn attention to hypo- gravity as a risk factor for osteoporosis. According to a research,56,57 bone mineral loss and renal stones were found during periods of bed rest and space flight and bone mineral loss progressed at a rate of 1~2% per month. In addition, recovery took three to four times longer.58 However, hypogravity states may be fraught with confounding factors, which must be taken into account when considering these results.
PATHOPHYSIOLOGY
Immobilization osteoporosis is not a single condition but a combination of multiple conditions and states and thus pathophysiological differences exist by condition.
In general, immobilization-induced mechanical loading declines in bones are known to be among the key factors of osteoporosis in SCI, stroke, and bed rest patients. However, recent research has identified non- mechanical factors, including neural, endocrine, hor- monal factor, and iatrogenic factors,57,59 and so associating the lack of mechanical loading with the various types of immobilization osteoporosis can be controversial.
1. Bone loss mechanisms
Mechanical stress is one of the determinants of bone morphology, BMD and bone strength. Therefore, disuse accelerates bone resorption, especially of cancellous bone, and the bone becomes atrophic and fragile.
Osteocytes embedded in the bone matrix respond to mechanical loads and changes in bone metabolism.60-62 The gap junction of the long processes of osteocytes plays an important role in transmitting mechanical loads
through intracellular (c AMP and c GMP) and extra- cellular (PGE2, IGF-I, ICF-II and TGF beta) signal transmitters to induce bone formation by osteoblasts, inhibit bone resorption by osteoclasts, or a combination of the two.63-65
Quantitative bone histological data were collected on iliac biopsies from spinal cord injury patients.66 The data showed that trabecular bone volume of immo- bilized patients decreases at a rate of 6% per month and is correlated with the duration of paralysis, up to 25 weeks after onset. After 25 weeks, trabecular bone volume remained constant at new, lower, steady state values. Osteoclastic resorption increased until 16 weeks after the start of immobilization and normalized in 40 weeks. A histomorphometric study was carried out on iliac bone samples from immobilized volunteers.67 As in the case of paraplegic patients, increased resorption and decrease formation were found, but there were no signs of trabecular bone volume loss. Accordingly, the impact on bone loss and cell kinetics in immobilized subjects was similar to that in paraplegic patients, but at a much slower pace. Osteoblastic activity or recruit- ment of trabecular bone is known to markedly decline in immobilized paraplegic patients68 with 30% of cases attributable to an increase in bone resorption and 70%
due to a decline in bone formation.69
Immobilization osteoporosis has a higher risk of occurrence in areas with a high proportion of trabecular bones. Bone loss patterns differ by region and such differences are attributable to the site specific cortical to trabecular bone ratio.70 Trabecular bone content is 66
~90% in vertebrae, 50% in the intertrochanteric region of the hip, 25% in the femoral neck, 25% in the distal radius, 1% in the mid-radius, and 5% in the femoral shaft.71 As the surface of trabecular bone is wider than that of cortical bone, trabecular bone is eight times more responsive to metabolic changes than cortical bone.72
2. Metabolic responses to immobilization 1) SCI patients
Early research found that urinary hydroxyproline (HP) and calcium reflect collagen turnover and demineralization, respectively, while serum calcium is related to elevated bone resorption and alkaline pho- sphatase is associated with bone formation activity.73 In the case of acute SCI patients whose post-injury period was less than six months, serum phosphate and ionized calcium significantly increases but serum calcium remains normal.74-77 This reflects the release of the mineral phase of bone tissue into the blood circulation, which resulted from accelerated bone resorption with decreased calcium absorption. However, in chronic SCI patients whose post-injury period spanned more than two years, serum total calcium significantly declined, while the serum concentration of ionized calcium was normal. Unlike at the acute stage, serum calcium declines at the chronic stage due to reduced serum albumin concentration but ionized calcium concentration remained normal thanks to a new balance of bone formation and resorption.78 Unlike adult SCI patients, children and adolescent patients often develop hypercalcemia even at the acute stage, as they are in a phase of rapid growth.79-81
The level of urinary calcium jumps right after injury and doubles to normal levels over an 8~10 week period. The concentration of calciuria stabilizes fifteen weeks later but remains higher than normal levels.
Urinary calcium retuns to normal seven months after injury. Urinary HP surgs right after injury and returns to normal after one year a similar pattern to urinary calcium.66,82,83 Such hypercalciuria conditions are attributed to increased osteoclastic bone resoprtion.
However, Maynard84 argues that SCI patients suffer renal functions declines and decreased tubular resorp- tion.
Exercise and ambulation can reduce hypercalciuria and strike a positive calcium balance, which shows that
immobilization is an important factor in negative calcium balance.85,86 However, some studies87 have found that the maximum urinary calcium level in SCI patients is two and four times higher than patients, who are under voluntary bed rest for a prolonged period, and passive weight bearing exercises or wheelchair activity did not drive down calciuria. Given this, the effectiveness of weight bearing exercises is still controversial. As calcium absorption of the gastroin- testinal tract declined in the acute phase of SCI patients,88 dietary calcium reduction is recommended as a way to prevent complications of hypercalciuria and reduce calcium excretion. However, this may lead to a negative calcium balance so that dietary restrictions on calcium are not applicable to SCI patients.
Since 1,25(OH)2 vitamin D formation is principally governed by parathyroid hormone (PTH) stimulation of renal 1-α hydroxylation of 25(OH) vitamin D, serum 1,25(OH)2 vitamin D levels may be decreased with the suppression of PTH. After acute SCI, the PTH –vitamin D axis is suppressed, with depressed PTH and 1,25(OH)2 vitamin D. A decline in serum PTH level was observed three weeks after SCI in a longitudinal study.89 Mechanick et al90 argue that in a cross sectional study of serum PTH and 1,25(OH)2 vitamin D level in SCI patients, PTH-vitamine D axis suppression is more frequent in complete injury compared to incomplete injury. Considering this data, the disruption of the PTH-vitamin D axis soon after SCI is unlikely to be involved in the pathogenesis of bone loss after injury.57 However, one study has reported mild secondary hyperparathyroidism91 in chronic SCI patients.
The researchers argued that secondary hyperparathy- roidism accelerated SCI-induced osteoporosis develop- ment. However, other research on chronic SCI patients reported no change92 or a decline93 in plasma PTH levels, which led us to believe these findings are insufficient to demonstrate secondary hyperparathyroi- dism in SCI induced osteoporosis.
On post-SCI period, bone tissue showed increased
turnover. In the mean time, there was an uncoupling between bone formation and resorption, which resulted in bone loss. SCI may cause the bone microenviron- ment to secret compounds that simulate osteoclasto- genesis. Demulder et al.94 reported that a higher number of osteoclast-like cells formed in iliac bone marrow cultures compared with sterna bone marrow cultures for paraplegic patients approximately six weeks after their lesion. IL-6 was found to be significantly higher in iliac conditioned media in most patients with paraplegia. Paraplegia may induce an increase in the capacity of progenitors to form osteoclast-like cells in the long-term bone marrow cultures. This may con- tribute to the dramatic bone loss after SCI.
According to research on bone resorption markers, urinary deoxypyridinoline (D-pyr) and urinary N- telopeptide of type I collagen was ten times higher than the upper normal limit in acute SCI patients and did not return to the normal level until the end of the research period.57 Pietschmann et al.90 argues that a month after SCI injury, urinary hydroxyproline/creatine ratios were significantly higher in the SCI group than a control group. Zehnder et al.95 investigated bone turnover biochemical markers in a cross sectional study of paraplegic men stratified by the time since injury:
less than 1 year (stratum I), 1 to 9 years (stratum II), 10~19 years (stratum III) and 20~29 years (stratum IV). Markers of bone resorption (D-pyr/Cr and Ca/Cr) dramatically increased in recently injured paraplegics, whereas bone resorption estimated by the D-pyr/Cr ratio remained elevated in 50% and 30% of patients in stratum II and strata III-IV, respectively. Both osteocal- cin and alkaline phosphatase are bone formation markers, but they reflect different aspects of bone formation.57 Although serum osteocalcin concentration increases in acute SCI patients, serum alkaline phosphatase remains stagnant until three months after injury.96 Such findings demonstrate that the imbalance between elevated bone resorption and normal bone formation post SCI was critical to bone loss and fracture.57
2) Hemiplegia and hemiparesis
A possible factor determining poststroke osteoporosis is the inhibition of PTH secretion after a stroke.
Previous studies97 have argued that stroke patients often suffer from vitamin D deficiency. The increase in serum calcium levels due to immobilization, suppresses PTH secretion and drives down the 1,25(OH)2 vitamin D level. Together with the decreased formation of 1,25(OH)2 vitamin D, hypercalcemia blocks secondary hyperparathyroidism and heightens the impact of vitamin D deficiency.98-100
Acute phase of stroke, serum pyridinoline (Pyr), serum procollagen type I C-terminal telopeptide (ICTP) and serum beta-2 microglobulin increased.101 This suggests that serum beta 2 microglobulin may be a good indicator of bone resorption in stroke patients. In the chronic phase of stroke, bed rest for 30 to 180 days induces an increase in urinary Pyr, urinary D-Pyr and serum ICTP. In contrast, serum alkaline phosphatase and serum carboxyterminal propeptide of human type I procollagen (PICP) remain normal.102
3) Temporary immobilization
Twelve weeks of bed rest resulted in decreased BMD of the femoral neck. Of bone metabolism markers, serum PTH and 1,25(OH)2 vitamin D decreased to normal levels, while, serum osteocalcin and serum bone specific alkaline phosphatase remained unchanged. Of bone resorption markers, there were visible increase in urinary HP, urinary D-Pyr and urinary type I collagen cross-linked N-telopeptides, which gradually decreased post-ambulation.103
PREVENTION AND TREATMENT
1. Non-pharmacologic treatments 1) Therapeutic exercise
Immobilization osteoporosis is mainly caused by a loss or reduction of mechanical stress on the bone. To apply such mechanical stress, early weight bearing and
assisted ambulation is commonly recommended. In addition, as immobilization osteoporosis is always associated with muscle atrophy and muscle weakness, it is necessary to complete therapeutic exercises to increase muscle volume. These exercises are also known to increase BMD and enhance joint mobility and soft tissue and thus improve microcirculation of the skeletal muscles.70
Opinions are divided over the efficacy of standing and walking as a weight bearing treatment within one year of SCI. Ben et al.104 argue that weight bearing-has a negligible effect on BMD soon after injury, but their study is limited by its. Relatively short treatment period and the treatment method compared to other studies.105,106 According to a prospective study by Alekna et al., when 54 traumatic SCI patients SCI patients underwent standing therapy for more than 1 hour per day, 5 days per week, BMD levels in the legs and pelvis significantly increased106. However weight bearing has been found to be ineffective in increasing BMD107-109 in the chronic period, especially more than one year after injury. Accordingly, post-injury weight bearing intervention should be more aggressive and conducted in a timelier manner.
Although there are few studies on the effects of physical exercise other than weight bearing through standing or walking, one study has tested two groups of young rats to compare weight bearing alone with weight bearing combined with physical exercise. They found that the group subjected to the combination of weight bearing and physical exercise showed an increase in trabecular thickness and bone mass and normalized long bone lengthening.110 Jones111 and Goktepe112 argue that clinical studies have found that exercise does not significantly impact BMD. In contrast, recent research by Miyahara113 claims that BMD levels are higher in patients who participate in sporting activities soon after their injury.
2) Electrical stimulation
Of four studies conducted during the early stage of the post-SCI injury period,114-117 three115-117 demon- strated the efficacy of electrical stimulation as a treatment option. Moreover, Dudley-Javoroski118 recen- tly argued in a prospective study that trained tibias showed less decline in BMD than untrained tibias when electrical stimulation was performed on the soleus muscle starting seven weeks after the injury for 30 min/day, 5 days/week for 4.8 years.
As with studies on the impact of weight bearing, there are conflicting results on the impact of electrical stimulation on chronic SCI patients. Most research has found improvements in osteoporosis when the training spanned 12 months or longer,119 were conducted at least per five times / week,120,121 or involved higher stimulus intensity.122 Such improvements are visible in trabecular bones, especially the distal femur or proximal tibia.
It has also been observed that the positive effect of electrical stimulation on bone mass remains only if the stimulation is continued in sufficient quantities.119,121 Accordingly, if electrical stimulation is frequently applied over the long term, it should prevent a further decline in bone mass.
Aside from electrical stimulation, spasticity, or involuntary muscle contraction, also affect residual muscular activity. Such spasms occur frequently with significant intensity and patients with spasticity are found to have visibly larger muscle mass than flaccid patients. Base on such observations, spasticity is believed to prevent bone loss.73 Some researchers123-125 have found that, in the case of SCI and stroke, patients with spasticity had a higher level of BMD than flaccid patients, buy the evidence is still weak.
2. Pharmacologic treatment
Pharmacologic treatments such as calcitonin, bisphos- phonate, and oral phosphate have been applied to patients with SCI or bed rest conditions to control bone loss. Recent research on various types of bisphos-
phonates has shown that they can have a positive impact on prevention and treatment of postmenopausal osteoporosis for women.
Calcitonin has been found to reduce hypercalcemia in acute SCI cases, but its impact was transient and more effective only in combination with etidronate or prednisone.126-129 The exclusive use of calcitonin did not achieve a decline in negative calcium or phosphorus balance in bed rest subjects. In contrast, oral phos- phorus is found to prevent hypercalciuria and reduce negative calcium balance.130-132
Vitamin D analogs are of clinical interest in the treatment of various forms of osteoporosis because they increase bone mass while having a greatly reduced ability to stimulate gut absorption of calcium.133 The administration of vitamin D analogs to long standing stroke patients can reduce risks of hip fracture and prevent a loss of BMD in hemiplegic sites.134 Bauman et al.135 found that 40 patients with complete chronic SCI conditions given a vitamin D analog for 24 months showed a significant increase in leg BMD compared to those who were not. According to the study, urinary N-telopeptide, a bone resorption marker, sharply dropped, but bone formation markers did not jump.
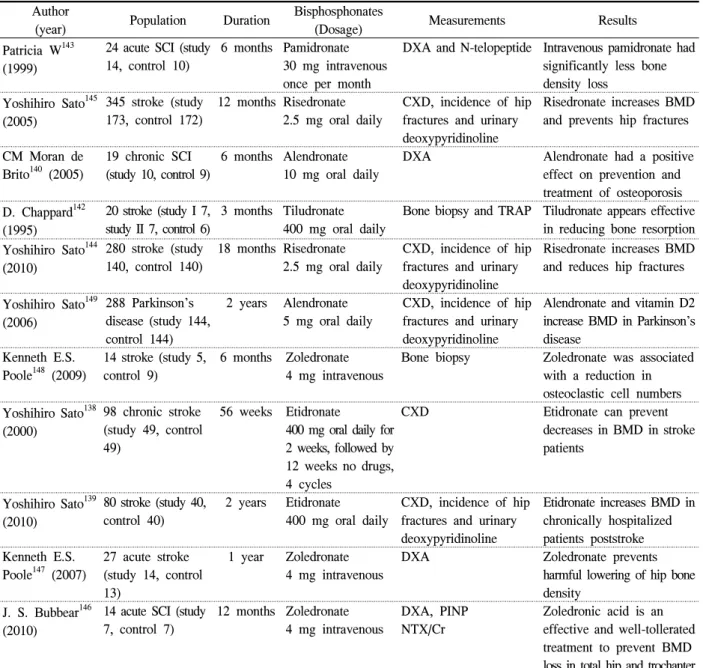
Bisphosphonates strongly inhibit bone resorption, and have been administered to treat primary osteoporosis and secondary osteoporosis, including immobilization osteoporosis, all over the world.70 Intermittent cyclic therapy with etidronate disodium increases vertebral BMD and prevents fractures in patients with post- menopausal osteoporosis136,137 and reduces bone resorp- tion in patients with acute stroke to prevent hip fracture.138 Etidronate disodium is a first generation bisphosphate and some studies have argued that despite its lower bone resorption compared to the 2nd or 3rd generation bisphosphonates, etidronate disodium should be recommended to severely disabled patients because it does not require sitting after taking the tablet.139 In recent years, various studies have been conducted on the effects of 2nd and 3rd generation bisphosphonate,
Author
(year) Population Duration Bisphosphonates
(Dosage) Measurements Results
Patricia W143 (1999)
24 acute SCI (study 14, control 10)
6 months Pamidronate 30 mg intravenous once per month
DXA and N-telopeptide Intravenous pamidronate had significantly less bone density loss
Yoshihiro Sato145 (2005)
345 stroke (study 173, control 172)
12 months Risedronate 2.5 mg oral daily
CXD, incidence of hip fractures and urinary deoxypyridinoline
Risedronate increases BMD and prevents hip fractures CM Moran de
Brito140 (2005)
19 chronic SCI (study 10, control 9)
6 months Alendronate 10 mg oral daily
DXA Alendronate had a positive effect on prevention and treatment of osteoporosis D. Chappard142
(1995)
20 stroke (study I 7, study II 7, control 6)
3 months Tiludronate 400 mg oral daily
Bone biopsy and TRAP Tiludronate appears effective in reducing bone resorption Yoshihiro Sato144
(2010)
280 stroke (study 140, control 140)
18 months Risedronate 2.5 mg oral daily
CXD, incidence of hip fractures and urinary deoxypyridinoline
Risedronate increases BMD and reduces hip fractures
Yoshihiro Sato149 (2006)
288 Parkinson’s disease (study 144, control 144)
2 years Alendronate 5 mg oral daily
CXD, incidence of hip fractures and urinary deoxypyridinoline
Alendronate and vitamin D2 increase BMD in Parkinson’s disease
Kenneth E.S.
Poole148 (2009)
14 stroke (study 5, control 9)
6 months Zoledronate 4 mg intravenous
Bone biopsy Zoledronate was associated with a reduction in osteoclastic cell numbers Yoshihiro Sato138
(2000)
98 chronic stroke (study 49, control 49)
56 weeks Etidronate
400 mg oral daily for 2 weeks, followed by 12 weeks no drugs, 4 cycles
CXD Etidronate can prevent
decreases in BMD in stroke patients
Yoshihiro Sato139 (2010)
80 stroke (study 40, control 40)
2 years Etidronate 400 mg oral daily
CXD, incidence of hip fractures and urinary deoxypyridinoline
Etidronate increases BMD in chronically hospitalized patients poststroke Kenneth E.S.
Poole147 (2007)
27 acute stroke (study 14, control 13)
1 year Zoledronate 4 mg intravenous
DXA Zoledronate prevents
harmful lowering of hip bone density
J. S. Bubbear146 (2010)
14 acute SCI (study 7, control 7)
12 months Zoledronate 4 mg intravenous
DXA, PINP NTX/Cr
Zoledronic acid is an effective and well-tollerated treatment to prevent BMD loss in total hip and trochanter DXA: Dual energy X-ray absorptionmeter, CXD: Computed X-ray densitometer, BMD: Bone mineral density TRAP: Tartrate resistant acid phosphatase, PINP: Procollagen I N-terminal peptide, NTX/Cr: N-telopeptide/creatinine ratio.
Table 1. The effect of bisphosphonates in patients with immobilization induced osteoporosis
such as alendronate,140,141 tiludronate,142 pamidronate,143 risedronate,144,145 zoledronate146-148 on osteoporosis pre- vention. Patricia143 studied the effects of pamidronate on 24 patients with acute stroke and argued that the use of intravenous pamidronate reduced bone density loss. In addition, when pamidronate was used to treat immobilization osteoporosis in patients with rheumatoid
arthritis (RA), it affected bone metabolism and osteoclastic activity in early RA patients and reduced interleukin 1, interleukin 6, tumor necrosis factor-alpha, and beta 2 microglobulin as well as the erythrocyte sedimentation rate and C-reactive protein, meaning it had both osteoporosis and antiarthritic effects.70 Risedronate is as effective in patients with immo-
bilization osteoporosis as etidronate disodium and it increases BMD levels and reduces hip fracture inci- dences in post-stroke elderly women145 and elderly men.144 Studies on alendronate and tiludronate140,142 have been conducted on a fewer number of patients, and were found to decrease osteoporosis risk.
Yoshihiro149 reported a study of 288 patients diagnosed with Parkinson's disease that found the use of alendronate with vitamin D2 increased BMD levels.
Bisphosphonates is the standard treatment for osteo- porosis. However, oral bisphosphonates have poor absorption and adverse effects such as gastrointestinal (GI) tract irritation. In contrast, zoledronic acid is a once-yearly intravenous bisphosphonate known to prevent and treat postmenopausal osteoporosis, increase bone mass in men with osteoporosis, and treat and prevent glucocorticoid-induced osteoporosis. It has a lasting impact through a 12 month dosing interval and avoids GI absorption/irritation problems. Kenneth et al.147 injected intravenous zoledronate 4 mg to 27 acute stroke patients within 35 days of injury, and during the one year observation period, the control group treated with an injection of calcium and vitamin D showed a 5.5%
decline in hip BMD, while the group treated with zoledronate showed no changes in hip BMD. They thus argue that zoledronate can prevent hip fracture in patients with acute stroke, at least for one year. In the case of SCI patients, zoledronate has early treatment effects in the hip, trochanter, and lumbar spine but only a marginal impact on the femur neck (146). According to recent studies, zoledronate can prevent immobilization osteo- porosis and hip fractures only in patients with severe disability. However, whether bone loss continue in immobilized SCI or stroke patients is still debatable, as are the questions of how long BMD levels can remain intact after treatment and how long the treatment should continue. As such, more extensive research should be conducted in this regard over longer periods.
REFERENCES
1. Albright F, Burnett CH, Cope O, Parson W. Acute atrophy of bone (osteoporosis) simulating hyper- parathyroidism. J Clin Endocrinol 1941;1:711-6.
2. Whedon GD. Disuse osteoporosis. In: Rodahl K, Nicholson JT, Brown EM (eds) Bone as a Tissue.
New York: McGraw Hill, 1960;67-99.
3. Whedon GD, Schorr E. Metabolic studies in paralytic acute anterior poliomyelitis. If: Alterations in calcium and phosphorus metabolism. J Clin Invest 1957;36:966-81.
4. Deitrick JE, Whedon GD, Schorr E. Effects of immobilization upon various metabolic and phy- siologic functions in normal men. Am J Med 1948;4:3-36.
5. Minaire P, Meunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biolo- gical data. Calcif Tissue Res 1974;17:57-73.
6. Hattner RS, McMillan DE. Influence of weightless- ness upon the skeleton: a review. Aerosp Med 1968;39(8):849-55.
7. Currey JD. The mechanical adaptations of bones.
Princeton University Press, 1984.
8. Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporosis and the bone mass effect of mechanical and non-mechanical agents.
Bone Mineral 1987;2:73-85.
9. Minaire P, Meunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res 1974;17:57-73.
10. Krolner B, Toft B. Vertebral bone loss: an unheeded side effect of therapeutic bed rest. Clin Sci 1983;64:537-540.
11. Dalen N, Olsson KE. Bone mineral content and physical activity. Acta Orthop Scand 1974;45:170-4.
12. Margulies JY, Simkin A, Leichter I, Bivas A, Steinberg R, Giladi M, et al. Effect of intense
physical activity on the bone mineral content in the lower limbs of young adults. J Bone Joint Surg 1986;68-A:1090-3.
13. Minaire P, Meunier P, Edouard C. et al. Acute osteoporosis in paraplegic patients: pathophysiology and effects of treatment with calcitonin and dichloromethylene diphosphonate. In: Menczel J, Robin GC, Makin M, Steinberg RJ. Wiley and sons (eds) Osteoporosis. Chichester, 1982;421-8.
14. Minaire P, Mallet E, Levernieux J, Schoutens A, Caulin F. Immobilization bone loss: preventing effect of calcitonin in several clinical models. In:
Christiansen C, Riis B (eds) Osteoporosis. Copen- hagen: Osteopress, 1987;603.
15. Chantraine A, Nusgens B, Lapiere CM. Bone remodeling during the development of osteoporosis in paraplegia. Calcif Tissue Int 1986;38:323-7.
16. Sheng-Dan J, Li-Yang D, Lei-Sheng J. Osteoporo- sis after spinal cord injury. Osteoporosis Int 2006;
17:180-92.
17. Sabo D, Blaich S, Wenz W, Hohmann M, Loew M, Gerner HJ. Osteoporosis in patients with paralysis after spinal cord injury: a cross sectional study in 46 male patients with dual-energy X-ray absorptiometry. Arch Orthop Trauma sug 2001;121:
75-8.
18. Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in weomen who have a complete spinal cord injury. J bone Joint Surg [Am] 2001;83:1195-200.
19. Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture and osteoporosis in paralyzed males. Arch Phys Med Rehabil 1993;74:73-8.
20. Biering-Sorensen F, Bohr HH, Schaat OP. Longi- tudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Inves 1990;20:330-5.
21. Brunin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E. Long-term changes in the tibia and radius bone mineral density following spinal cord
injury. Spinal Cord 2005;43:96-101.
22. Sabo D, Blaich S, Wenz W, Hohmann M, Loew M, Gerner HJ. Osteoporosis in patients with paraly- sis after spinal cord injury: a cross-sectional study in 46 male patients with dual-energy X-ray absorp- tiometry. Arch Orthop Trauma Surg 2001;121(Suppl 1-2):75-8.
23. Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 2004;34(Suppl 5):869-80.
24. Zehnder Y, Lüthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone meta- bolism, bone mineral density, quantitative ultra- sound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 2004;15(Suppl 3):180-9.
25. Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative hisological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res 1974;17:57-73.
26. Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J bone Miner Res 2004;19:48-55.
27. Szollar SM, Martin EME, Parthemore JG, Sartoris DJ, Deftos LJ. Demineralization in tetraplegic and paraplegic man over time. Spinal Cord 1997;35:
223-8.
28. Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free post- menopausal women. Osteoporos Int 2004.
29. Ragnarsson KT, Sell GH. Lower extremity frac- tures after spinal cord injury: a retrospective study.
Arch Phus Med Rehabil 1981;62:418-23.
30. Ingram RR, Suman RK, Freeman PA. Lower limb fractures in the chronic spinal cord injured patient.
Paraplegia 1989;27:133-9.
31. Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. AM J Surg 1962;13:732-9.
32. Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 1998;
36:790-6.
33. Frisbie JH. Fractures after myelopathy: the risk quantified. J Spinal Cord Med 1997;20:66-9.
34. Finsen V, Indredavik B, Fougner KJ. Bone mineral and hormone status in paraplegics. Paraplegia 1992;
30:343-7.
35. Garland DE, Adkins RH, Scott M, Singh H, Massih M, Stewart C. Bone loss at the os calcis compared with bone loss at the knee in individuals with spinal cord injury. J Spinal Cord Med 2004;
27:207-11.
36. Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients.
Bone 2000;27:350-9.
37. Hamdy RC, Moore SW, Cancellaro VA, Harvill LM. Long-term effects of strokes on bone mass.
Am J Phys Med Rehabil 1995;74:351-6.
38. Ramnermark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y. Progressive hemosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int 1999;9:269-75.
39. Sato Y, Kuno H, Kaji M, Ohshima Y, Asoh T, Oizumi K. Increased bone resorption during the first year after stroke. Stroke 1998;29:1373-7.
40. Jorgensen L, Crabtree NJ, Reeve J, Jacobsen BK.
Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: bone adaptation after decreased mechanical loading.
Bone 2000;27:701-7.
41. Lazoura O, Groumas N, Antoniadou E, Papadaki PJ, Papadimitriou A, Thriskos P, et al. Bone mineral density alterations in upper and lower
extremities 12 months after stroke: a longitudinal study. Osteoporos Int 2000;11:381-7.
42. Ramnemark A, Nyberg L, Lorentzon R, Olsson T, Gustafson Y. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke 1999;30:755-60.
43. Worthen LC, Kim CM, Kautz SA, Lew HL, Kiratli BJ, Beaupre GS. Key characteristics of walking correlate with bone density in individuals with chronic stoke. K Rehabil Res Dev 2005;42:761-8.
44. del Puente A, Pappone N, Mandes MG, Mantova D, Scarpa R, Oriente P. Determinants of bone mineral density in immobilization: a study on hemiplegic patients. Osteoporos Int 1996;6:50-4.
45. Sato Y, Maruoka H, Honda Y, Asoh T, Fujimatsu Y, Oizumi K. Development of osteopenia in the hemiplegic finger in patients with stroke. Eur Neurol 1996;36:278-83.
46. Prince RL, Price RI, Ho S. Forearm bone loss in hemiplegia: a model for the study of immobili- zation osteoporosis. J Bone miner Res 1988;3:305- 10.
47. Bainbridge NJ, Davie MW, Haddaway MJ. Bone loss after stroke over 52 weeks at os calcis:
influence of sex, mobility and relation to bone density at other sites. Age Ageing 2006;35:127-32.
48. Iwamoto J, Tsukimura T, Takeda T. Bone mineral density of metatarsus in hemiplegic subjects. Am J Phys Med Rehabil 1999;78:202-7.
49. Dennis MS, Lo KM, Mcdowall M, West T.
Fractures after stroke: frequency, types, and asso- ciations. Stroke 2002;33:728-34.
50. Ramnemark A, Nyberg L, Borssen B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int 1998;8:92-5.
51. Worthen LC, Kim CM, Kautz SA, Lew HK, Kiratli BJ, Beaupre GS. Key characteristics of walking correlate with bone density in individuals with chronic stroke. JK Rehabil Res Dev 2005;42:761-8.
52. Chiu KY, Pun WK, LuK KD, Chow SP. A prospective study on hip fractures in patients with
previous cerebrovascular accidents. Injury 1992;23:
297-9.
53. Sander P, Arief L, Bert L, Anthonius B, Cyrus C, Tjeerd S. Frank. V. Risk of hip/femur fracture after stroke: a population-based case-control study.
Stroke 2009;40:3281.
54. Krolner B, Toft B, Vertebral bone loss: An unheeded side effect of therapeutic bed res. Clin Sci 1983;64:537-40.
55. Kannus P, Jarvinen M, Sievane H, Oja P, Vuori I.
Osteoporosis in men with a history of tibial fracture. J Bone Miner Res 1994;9:423-9.
56. Ohshima H. Secondary osteoporosis update. Bone loss due to bed rest and human space flight study.
Clin Calci 2010;20:709-16.
57. Sheng-Dan J, Lei-Sheng J, Li-Yang D. Mechanisms of osteoporosis in spinal cord injury. Clinical Endocrinology 2006;65:555-65.
58. Ohshima H, Mukai C. Bone metabolism in human space flight and bed rest study. Clin Calci 2008;
18:1245-53.
59. Stefano C, Carlo C, Marco I, Maurizio B. Osteoporosis after stroke: a review of the causes and potential treatments. Cerebreasc Dis 2009;28:191-200.
60. Doty SB. Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 1981;33:509- 12.
61. Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int 1993;
53:S102-7.
62. Cowin SC, Moss-Salentijn L, Moss ML. Candi- dates for the mechanosensory system in bone. J Biomech Eng 1991;113:191-7.
63. Palumbo C, Palazzini S, Marotti G. Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990;11:401-6.
64. Rodan GA, Bourret LA, Harvey A, Mensi T.
Cyclic AMP and cyclic GMP: mediators of the mechanical effects on bone remodeling. Science 1975;189:467-9.
65. Duncan RL, Turner CH. Mechanotransduction and
the functional response of bone to mechanical strain. Calcif Tissue Int 1995;57:344-58.
66. Minaire P, Meunier F, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: Comparison with biological data. Calcif Tissue Int 1974;17:230-5.
67. Vico L, Chappard D, Alexandre C, Palle S, Minaire P, Morukov BV, et al. Etude hisologique quanti- tative de la masse et des activites cellulaires osseuses après undecubitus de 120 jours. Essai de protocols preventives. Ann Bil Clin 1987;45:145- 51.
68. Minaire P, Meunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biolo- gical data. Calcif Tissue Res 1974;17:57-73.
69. Rodan G, Thompson DD, Weinreb M. Characteri- zation and pharmacological control of immobiliza- tion induced bone resorption. In: Christiansen C, Riis B (eds) Osteoporosis. Copenhagen: Osteopress, 1987:762-3.
70. Shinjiro T, Natsuo Y. Disuse osteoporosis. J Med Invest 2001;48:147-56.
71. Einhorn TA. Bone strength: the bottom line. Calcif Tissue Int 1992;51:333-9.
72. Frost HM. Dynamics of bone remodeling. In: Frost HM (eds) Bone Biodynamics. Boston: Little brown, 1964;315-34.
73. Jenny KB. Immobilization osteopenia. In: Robert M, David F, Jennifer K (eds) Osteoporosis, 2nd edn. Elsevier, 2001;207-26.
74. Maïmoun L, Couret I, Micallef JP, Peruchon E, Mariano-Goulart D, Rossi M, et al. Use of bone biochemical markers with dual energy X-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism 2002;51:958-63.
75. Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 1998;83:415-22.
76. Bergmann P, Heilporn A, Schoutens A, Paternot J, Tricot A. Longitudinal study of calcium and bone metabolism in paraplegic patients. Paraplegia 1977;
15:147-59.
77. Stewart AF, Adler M, Byers CM, Segre GV, Broadus AE. Calcium homeostasis in immobiliza- tion: an example of resorptive hypercalciuria. N Engl J Med 1982;306:1136-40.
78. Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Parathormone and calci- tonin profiles in persons with long-standing spinal cord injury. Arch Phys Med Rehabil 1994;75:
766-9.
79. Demirel G, Yilmaz H, Paker N, Onel S. Osteo- porosis after spinal cord injury. Spinal cord 1998;
36:822-5.
80. Tsuzuku S, Ikegami Y, Yabe K. Bone mineral density differences between paraplegic and quadri- plegic patients: a cross-sectional study. Spinal Cord 1998;37:358-61.
81. Garland DE, Adkins RH, Kushwaha V, Stewart C.
Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 2004;27:202-6.
82. Bergmann P, Heilporn A, Schoutens A, Paternot J, Tricot A. Longitudinal study of calcium and bone metabolism in paraplegic patients. Paraplegia 1978;
15:147-59.
83. Pilchonery G, Minaire P, Milan JJ, Revol A.
Urinary elimination of glycosaminoglycans during the immobilization osteoporosis of spinal cord injury patients. Clin Orthop 1983;174:230-5.
84. Maynard, FM, Imai K. Immobilization hypercalce- mia in spinal cord injury. Archives of Physical Medicine and Rehabilitation 1977;58:16-24.
85. Kaplan PE, Roden W, Gilbert E, Richards L, Godschmidt JW. Reduction of hypercalciuria in tetraplegia after weight-bearing and strengthening exercises. Paraplegia 1981;19:289-93.
86. Kaplan PE, Gandhabadi B, Richards L, Godschmidt J. Calcium balance in paraplegic patients: influence
of injury duration and ambulation. Archives of Physical Medicine and Rehabilitation 1977;59:447- 50.
87. Claus-Walker J, Campos RJ, Carter RE, Vallbona C, Lipscomb HS. Calcium excretion in quadriple- gia. Arch Phys Med Rehabil 1972;53:14-20.
88. Zhou XJ, Vaziri ND, Segal JL, Winer RL, Eltorai I, Brunnemann SR. Effects of chronic spinal cord injury and pressure ulcer on 25(OH)-vitamin D levels. Journal of the American Paraplegia Society 1993;16:9-13.
89. Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, et al. Longitudinal study of bone turnover after acute spinal cord injury. Journal of Clinical Endocrinology and Metabolism 1998;83:
415-22.
90. Mechanick JI, Oomerantz F, Flanaga S, Stein A, Gordon WA, Ragnarsson KT. Parathyroid hormone suppression in spinal cord injury patients is asso- ciated with the degree of neurologic impairment and not the level of injury. Archives of Physical Medicine and Rehabilitation 1997;78:692-6.
91. Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism 1995;44:1612-6.
92. Pietschmann P, Pils P, Woloszczuk W, Maerk R, Lessan D, Stipicic J. Increased serum osteocalcin levels in patients with paraplegia. Paraplegia 1992;
30:204-9.
93. Vaziri ND, Pandian MR, Segal JL, Winer RL, Eltorai I, Brunnemann S. Parathormone and calci- tonin profiles in persons with long-standing spinal cord injury. Archives of Physical Medicine and Rehabilitation 1994;75:766-9.
94. Kohli A, Lamid S. Risk factors for renal stone formation in patients with spinal cord injury.
British Journal of Urology 1986;58:588-91.
95. Biering-Sorense F, Bohr HH, Schaadt OP.
Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. European Journal of
Clinical Investigation 1990;20:330-5.
96. Schmid A, Huonker M, Stahl F, Barturen JM, Konig D, Heim M, Lehmann M, Keul J. Free plasma catecholamines in spinal cord injured per- sons with different injury levels at rest and during exercise. Journal of the Autonomic Nervous System 1998;68:96-100.
97. Sato Y, Maruoka H, Oizumi K, Kikuyama M.
Vitamin D deficiency and osteopenia in the hemi- plegic limbs of stroke patients. Stroke 196;27:2183- 7.
98. Sato Ym Oizumi K, Kuno H, Kaji M. Effect of immobilization upon renal synthesis of 1,25- dihydroxy vitamin D in disabled elderly stroke patients. Bone 1999;24:271-5.
99. Sato Y, Kuno H, Asoh T, Honda Y, Oizumi K.
Effect of immobilization on vitamin D status and bone mass in chronically hospitalized disabled stroke patients. Age Ageing 1999;28:265-9.
100. Fujimatsu Y. Role of parathyroid gland on bone mass and metabolism in immobilized stroke patients. Kurume Med J 1998;45:265-70.
101. Sato Y, Kuno H, Kaji M, Oizumi K. Beneficial effect of intermittent cyclical etidronate therapy in hemiplegic patients following an acute stroke. J bone Miner Res 2000;15:2487-94.
102. Liu M, Tsuji T, Higuchi Y, Domen K, Tsujiuchi K, Chino N. Osteoporosis in hemiplegic stroke patients as studied with dual-energy X-ray absorptiometry. Arch Phys Med Rehabil 1999;80:
1219-26.
103. Zerwekh JE, Ruml LA, Gottschalk F, Pak CY.
The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J bone Miner Res 1998;13:1594-601.
104. Ben M, Harvey L, Denis S, Glinsky J, Goehl G, Chee S, Herbert RD. Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Aust J Physiother 2005;51:251-6.
105. De Bruin ED, Frey-Rindova P, Herzog RE, Dietz B, Dambacher MA, Strussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil 1999;
80:214-20.
106. Alekna V, Tamulaitiene M, Sinevicius T, Juocevicius A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years.
Spinal Cord 2008;46:727-32.
107. Biering-Sorensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Para- plegia 1988;26:293-301.
108. Saltzstein RJ, Hardin S, Hastings J. Osteoporosis in spinal cord injury: using an index of mobility and its relationship to bone density. J Am Paraplegia Soc 1992;15:232-4.
109. Goktepe AS, Tugcu I, Yilmaz B, Alaca R, Gunduz S. Does standing protect bone density in patients with spinal cord injury? J Spinal Cord Med 2008;31:197-201.
110. Sandrine Bourrin, Sabine Palle, Christian Genty, Christian Alexandre. Physical exercise duing remobilization restores a normal bone trabecular network after tail suspension-induced osteopenia in young rats. J Bone Miner Res 1995;10:820-8.
111. Jones LM, Legge M, Goulding A. Intensive exercise may preserve bone mass of the upper limbs in spinal cord injured males but does not retard demineralization of the lower body. Spinal Cord 2002;40:230-5.
112. Goktepe AS, Yilmaz B, Alaca R, Yazicioglu K, Mohur H, Gunduz S. Bone density loss after spinal cord injury. Elite paraplegic basketball players vs. paraplegic sedentary persons. Am J Phys Med Rehabil 2004;83:279-83.
113. Miyahara K, Wang D-H, Mori K, Takahashi K, Miyatake N, Wang B-L et al. Effect of sports activity on bone mineral density in wheelchair athletes. J Bone Moner Metab 2008;26:101-6.
114. Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Strussi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord- injured patients. Eur J Clin Invest 2003;33:412-9.
115. Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol 2006;95:2380-90.
116. Shields RK, Dudley-Javoroski S. Law LAF.
Electrical induced muscle contractions influence bone decline after spinal cord injury. Spine 2006;
31:548-53.
117. Clark JM, Jelbart M, Rischbieth H, Strayer J, Chatterton B, Schultz C, Marshall R. Physio- logical effects of lower extremity functional electrical stimulation in early spinal cord injury:
lack of efficacy to prevent bone loss. Spinal Cord 2007;45:78-85.
118. Dudley-Javoroski S, Shelds RK. Dose estimation and durveillance of mechanical loading interven- tions for bone loss after spinal cord injury. Phys Ther 2008;88:387-96.
119. Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Tramsborg G, Kjar M. Increased bone mineral density after prolonged electrical induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int 1997;61:22-5.
120. Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reserve osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 2000;81:1090-8.
121. Chen S-C, Lai C-H, Chan WP, Huang M-H, Tsai H-W, Chen J-JJ. Increases in bone mineral den- sity after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil 2005;27:1337-41.
122. Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 1996;19:
61-8.
123. Demirel G, Yilmaz H, Paker N, Onel S. Osteo- porosis after spinal cord injury. Spinal Cord 1998;36:822-5.
124. Biering-Sprensen R, Bohr H. Bone Mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia 1988;
26:293-301.
125. Panin N, Gorday WJ, Paul BJ. Osteoporosis in hemiplegia. Stroke 1971;2:41-47.
126. Merli GJ, McElwain GE, Adler AG, Martin JH, Roberts JD, Schnall B, Ditunno JF. Immobili- zation hypercalcemia in acute spinal cord injury treated with etidronate. Arch Intern Med 1984;
144:1286-8.
127. Carey D, Taisz L. Calcitonin therapy in prolonged immobilization hypercalcemia. Arch Phys Med Rehabil 1985;66:640-4.
128. Meythaler J, Stephen J, Tuel M, Cross L, Successful treatment of immobilization hyper- calcemia using calcitonin and etidronate. Arch Phys Med Rehabil 1993;74:316-9.
129. Minaire P, Depassio J, Berard E, Meunier PJ, Edouard C, Pilonchery G, Goedert G. Effects of clodronate on immobilization bone loss. Bone 1987;8:S63-8.
130. Hulley SB, Bogel JM, Donaldson CL, Effect of supplemental calcium and phosphorus on bone mineral changes in bed rest. J Clin Invest 1971;
50:2506-18.
131. Schneider V, McDonald J. Skeletal calcium homeostasis and countermeasures to prevent disuse osteoporosis. Calcif Tissue Int 1984; 36(Suppl):
S151-4.
132. Hantmann DA, Vogel JM, Donaldson CL, Friedman R, Goldsmith RS, Hulley SB. Attempts to prevent disuse osteoporosis by treatment with calcitonin, longitudinal compression and supple- mentary calcium and phosphate. J Clin Endocrinol Metab 1973;36:845-58.
133. Coburn JW, Tan AU Jr, Levine BS, Mazess RB, Kyllo DM, Knutson JC, Bishop CW. 1-α-
Hydroxyvitamin D2: a new look at an ‘old’
compound. Nephrol Dial Transplant 1996;11(Suppl 3):153-7.
134. Sato Y, Maruoka H, Oizumi K. Amelioration of hemiplegia-associated osteopenia more than 4 years after stroke by 1-alpha-hydroxyvitamin D3 and calcium supplementation. Stroke 1997;28(4):
736-9.
135. William AB, Spungen AM, Morrison N, Zhang RL, Schwartz E. Effect of a vitamin D analog on leg bone mineral density in patients with chronic spinal cord injury. J of Rehabilitation Research &
Development 2005;42:625-34.
136. Storm T, Thamsborg G, Steiniche T, Genant HK, Sørensen OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 1990;322:1265-71.
137. Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P, et al. Four-year study of intermittent cyclic etidronate treatment of post- menopausal osteoporosis: three years of blinded therapy followed by one year of open therapy.
Am J Med 1993;95:557-67.
138. Sato Y, Honda Y, Asoh T, Kikuyama M, Oizumi K. Hypovitaminosis D and decreased bone mine- ral density in amyotrophic lateral sclerosis. Eur Neurol 1997;37:225-9.
139. Sato Y, Honda Y, Iwamoto J. Beneficial effect of etidronate therapy in chronically hospitalized, disabled patients with stroke. J of stroke and cerebrovascular diseases 2010;19:198-203.
140. CM Moran de Brito, LR Battistella, ET saito and H Sakamoto. Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord 2005;43:341-8.
141. Sato Y, Iwamoto J, Kanoko T, Satoh K. Alendro-
nate and vitamin D2 for prevention of hip fracture in Parkinson’s disease: a randomized controlled trial. Movement disorders 2006;21:924-9.
142. Chappard D, Minaire P, Privat C, Berard E, Mendoza-Sarmiento J, Tournebise H, et al. Effects of tiludronate on bone loss in paraplegic patients.
J Bone Miner Res 1995;10:112-8.
143. Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil 1999;80:
243-51.
144. Sato Y, Iwanmoto J, Kanoko T, Satoh K. Rised- ronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke.
Arch Intern Med 2005;165:1743-8.
145. Sato Y, Iwamoto J, Kanoko T, Satoh K. Rised- ronate therapy for prevention of hip fracture after stroke in elderly women. Neurology 2005;64:
811-6.
146. Bubbear JS, Gall A, Middleton FI, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 2010.
147. Poole KE, Loveridge N, Rose CM, Warburton EA, Reeve J. A single infusion of zoledronate prevents bone loss after stroke. Stroke 2007;
38:1519-25.
148. Poole KE, Vedi S, Debiram I, Rose C, Power J, Loveridge N, et al. Bone structure and remodeling in stroke patients: early effects of zoledronate.
Bone 2009;44:629-33.
149. Sato Y, Iwamoto J, Kanoko T, Satoh K. Alendro- nate and vitamin D2 for prevention of hip fracture in Parkinson’s disease: a randomized controlled trial. Movement disorders 2006;21:924-9.
Peer Reviewers' Commentary
The author discussed clinical evidence and pathophysiology of immobilization osteoporosis and examines medical interventions for preventive pharmacologic and nonpharmacologic treatments.
Immobilization osteoporosis is found in a number of diseases, such as complete motor paralysis and reversible immobilization. Bone atrophy induced by immobilization is found in a number of diseases such as complete motor paralysis and reversible immobilization. Immobilization-induced mechanical loading declines in bones are known to be among the key factors of osteoporosis in SCI, stroke, and bed rest patients. However, recent research has identified non-mechanical factors, including neural, endocrine, hormonal factor, and iatrogenic factors.
Pharmacologic treatments such as calcitonin, bisphosphonates, and oral phosphates have been applied to patients with spinal cord injury or under bed rest conditions to reduce bone loss. Recent research has shown that they can have a positive impact on prevention and treatment of postmenopausal osteoporosis for women. Zoledronate can prevent immobilization osteoporosis and hip fractures only in patients with severe disability.
(정리: 편집위원회)