Two-year Clinical Outcomes Following Everolimus-eluting Stent Use for Off-label Versus On-label Indications: From the Korean Multicenter Drug-eluting Stent Registry
전체 글
수치
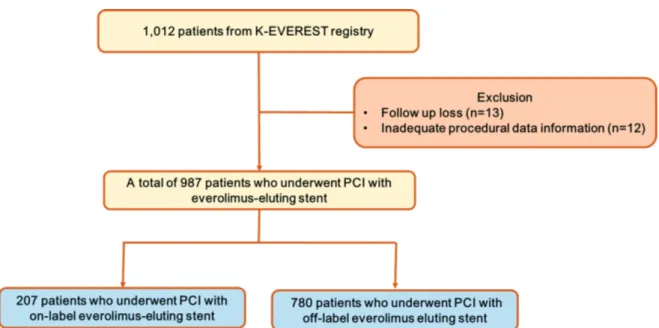
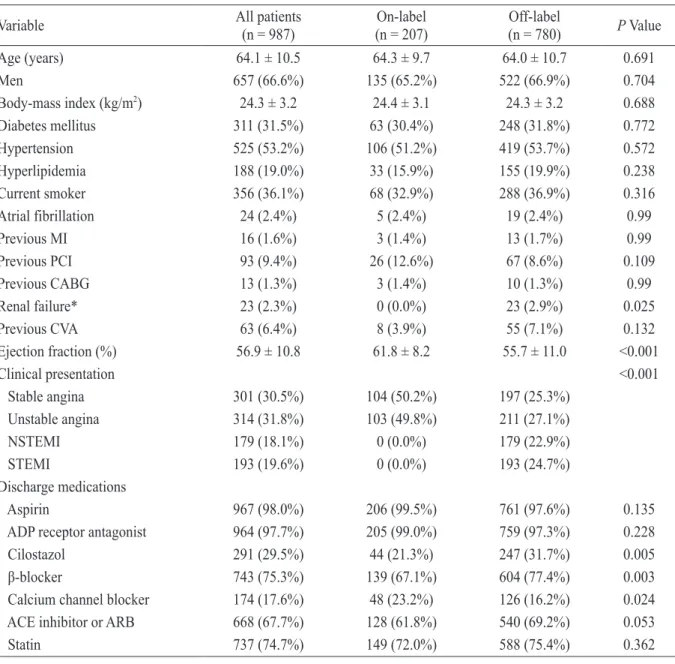
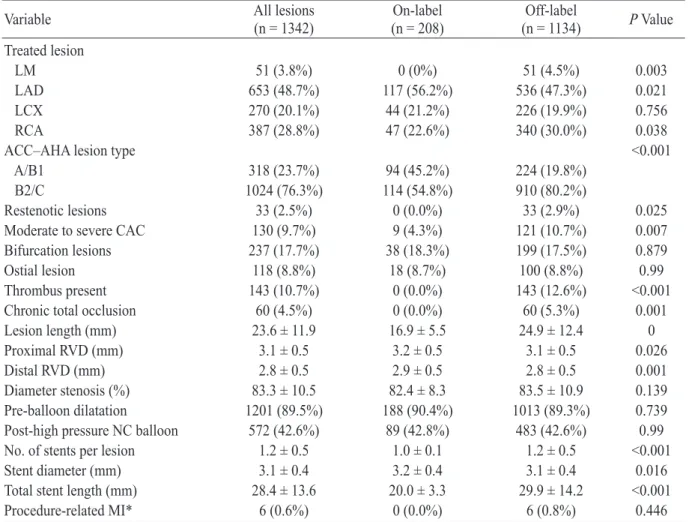
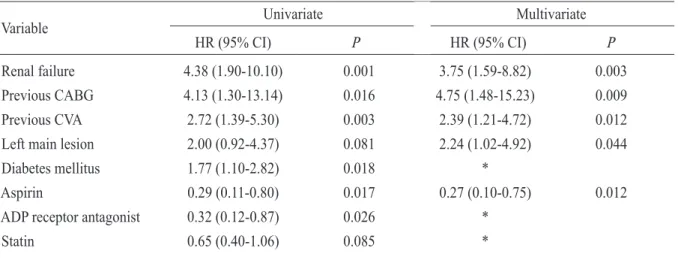
관련 문서
Conclusion: In Neer type II distal clavicle fracture treatment, both groups showed satisfactory result in clinical and radiological outcomes, but the hook plate
Even though the cartoon is very crucial as a source content in the one-source multi-use era, the Korean cartoon industry is still in the poor condition due to
A study on the characteristics of bacteria isolated from cultured flounder( Paralichthys olivaceus )showing disease symptoms in Jeju area of Korea.. Drug
Although visceral fat adiposity has known to be associated with clinical, pathologic, and oncologic outcomes in patients with colorectal cancer (CRC), the
Methods: This was a multicenter, open-label, observational study using the Patient Perception of Intermittent Catheterization (PPIC) questionnaire and
Although SAMMPRIS with the Wingspan stent failed to show a benefit for intracranial stenting over intensive medical management in the secondary prevention of stroke
The ‘Investigation of outcomes after GOLimumab Discon- tinuation,’ or GOLD, was an observational study involving 10 centers nationwide that analyzed clinical data
Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR