Cancer is one of the main causes of the death in the world and results from indiscriminate cell proliferation caused by the accumulation of mu- tations in genes that control cell proliferation and apoptosis. Lung cancer has the highest in- cidence rate and mortality exemplified by less than a 15% 5-year survival rate. This high mortal- ity is because most patients are diagnosed at a stage with difficult surgical options and the effects of anti-cancer agents that were used were not
good. Chemotherapy has been playing a major role in treating patients with lung cancer for the last 30 years, but there are still many limitations in the available therapeutic agents.1,2 However, entering the 2000s, as a therapeutic agent that targets the epidermal growth factor receptor (EGFR) in lung cancer emerged, the situation has changed. Gefitinib and erlotinib, small molecule inhibitors of EGFR tyrosine kinase (EGFR TKI), can be taken orally, thus greatly reducing the
Original Article
Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
Eun Jin Lim, Jeunghoon Heo, Young-Ho Kim
Department of Molecular Biology and Immunology, College of Medicine, Kosin University, Busan, Korea
Objectives: The aim of this study was whether quercetin induces cell death by caspase and MAPK signaling pathway in EGFR mutant lung cancer cells
Methods: PC-9 cells, EGFR mutant lung cancer cells, were treated various times and concentrations of quercetin and harvested and measured using MTT assay, DNA fragmentation, Western blotting, and FACS analysis.
Results: Treatment with quercetin in PC-9 cells resulted in inhibition of cell growth through apoptosis.
Quercetin-induced apoptosis was associated with caspase-dependent manner. Quercetin also significantly increased levels of phosphor-p38 and decreased levels of phosphor-ERK, indicating that quercetin induces p38 MAPK signaling pathway in PC-9 cells. Quecetin treatment also generated the release of cytochrome c in PC-9 cells; however, pretreatment with rotenone or z-LEHD-fmk, significantly attenuated quercetin-induced apoptosis.
Conclusions: Our data indicate that quercetin exhibits EGFR mutant lung cancer effects through apoptosis by caspase dependent and mitochondrial pathway.
Key Words: EGFR mutant, Lung cancer, MAPK, Mitochondria, Quercetin
Corresponding Author: Young-Ho Kim, Department of Molecular Biology and Immunology, College of Medicine, Kosin University, 262, Gamchen-ro, Seo-gu, Busan, 49267, Korea
Tel: +82-51-990-6505 FAX: +82-51-990-3081 E-mail: kimyh@kosin.ac.kr
Received:
Revised:
Accepted:
Oct. 21. 2014 Nov. 18, 2014 Dec. 16, 2014
inconvenience of treatment. However, although some EGFR mutant lung cancer cells have a good initial response to EGFR TKI inhibitors, the drug tolerance eventually develops.
Quercetin is a compound found in various plants including fruits, vegetables and green tea.
Quercetin can play a role as an antioxidant or a prooxidant depending on the concentration.3,4 Quercetin had been studied for its pharmacological effects on inflammation, atherosclerosis, hyper- tension and neurodegeneration.5-9 Quercetin has been reported to have a role as a preventive and therapeutic agent in various tumors.10-16 Quercetin is known to induce apoptosis in cancer cells and inhibit cell growth by suppressing activities of vari- ous enzymes.17,18
In order to find the biochemical mechanism for the anti-cancer effect of quercetin we inves- tigated changes in protein expression related to cell proliferation inhibition and apoptosis in PC-9 human lung cancer cells and found that quercetin induced apoptosis through a caspase-dependent and p38 MAPK pathway.
MATERIALS AND METHODS
1. MATERIALS
PC-9 cells from the American Type Culture Collection (Rockville, MD, USA) were aliquoted
and incubated under at 37℃ in 5% CO2 using RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) including 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. To measure the cell proliferation during quercetin treatment, 2×105 of PC-9cells were aliquoted in each well of a 6well plate and incubated by differing the treatment time of quercetin at the titer. Quercetin used in these experiments was purchased from LKT Laboratories (St, Paul, MN, USA) and used by dissolving in dimethyl sulfoxide (DMSO) at 100 mM concentration.
2. Study Method 1) Cell MTT Assay
To measure the degree of cell proliferation in- hibition during quercetin treatment, PC-9 cells were aliquoted into 96 well plates and incubated at differing treatment times of quercetin at the titer. After incubating with MTT reagent at 0.5 mg/mL concentration for 2 hours, the medium was removed and DMSO was aliquoted by 0.1 mL. After dissolving all formazan formed in the well, the absorbance was measured at 540 nm using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
2) Flow cytometry analysis for cell apoptosis measurement
To analyze the degree of apoptosis, cells were
washed twice with PBS, 1×binding buffer was added and the cells were treated with annexin V-FITC. Cells were stained at room temperature in the dark for 20 minutes and analyzed using FACS.
3) DNA Fragmentation Analysis
In order to observe apoptosis induced DNA fragmentation, after treating the cells with quer- cetin by concentration, the cells were collected and treated at 4℃ for 30 min in the lysis buffer [5mM Tris-Hcl (pH 7.5), 5 mM EDTA, 0.5% Triton
X-100]. After collecting the supernatant by cen- trifugation, it was treated with 0.5 mg/mL protei- nase K (Sigma-Aldirch) at 50℃ for 3 hours. After that, a mixed solution of phenol : chloroform : isoamyl alcohol (25:24:1) was added and inverted for 30 minutes, the supernatant was separated by centrifugation, and then isopropanol and 5 M NaCl were added to this and it was incubated overnight at 4℃. The pellet was separated using centrifugation and was washed with 70% ethanol.
The pellet was dissolved in distilled water with an appropriate amount of RNase A, mixed with
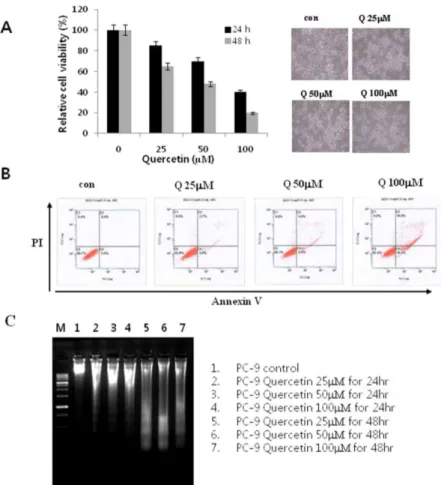
Fig. 1. Effects of quercetin on cell growth and cell death in PC-9 cells. A. The growth inhibition was measured by MTT assay. B. The cell death distribution was analyzed by flow cytometry. C. After incubation with indicated concentrations of quercetin for 24 h, cells were collected and DNA was isolated. Con: control, Q: quercetin, PI: propidium iodide.
gel loading dye and electrophoresed on a 1.5%
agarose gel at 50 V for 1 hour and stained with ethidium bromide (EtBr) and observed under the ultraviolet (UV).
4) Protein Separation, Electrophoresis and Western Blot Analysis
The prepared cells were collected and washed with PBS. An appropriate amount of lysis buffer [25 mM Tri-Cl (pH 7.5), 250 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM phenylmethlsulfony fluo- ride (PMSF), 5 mM dithiothreitol (DTT)] was added and reacted for 1 hour at 4℃ and the supernatant
was collected after centrifugation for 30 minutes at 14,000 rpm. The protein concentration of the supernatant was quantified using Bio-Rad pro- tein-quantifying reagent, and a sample solution was made by mixing the equivalent amount of Laemmli sample buffer. With the equivalent amount of protein prepared by the above proce- dure, the protein was separated by electro- phoresis using sodium dodecyl sulphate (SDS) – polyacrylamide gel. After transferring the acryl- amide gel containing the separated protein to a nitrocellulose membrane, it was treated with PBS-T (0.1% Tween 20 in PBS) containing 5% skim
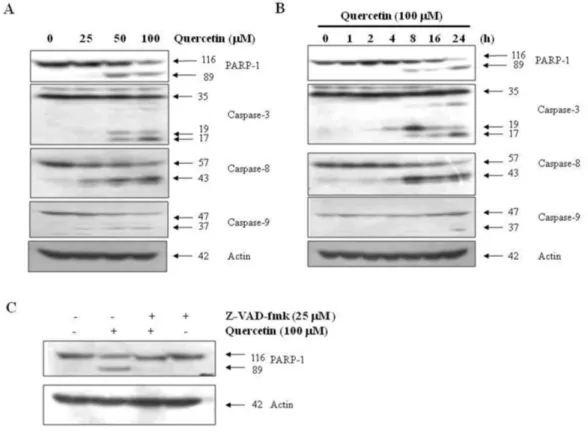
Fig. 2. Effects of quercetin on caspase activation. A. PC-9 cells were treated for 24 h with the indicated concentrations of quercetin. B. PC-9 cells were treated 100 uM quercentin with the indicated various time. C. PC-9 cells were treated with 100 uM quercetin in the presence or absence of pan-caspase inhibitor (z-VAD-fmk) for 24 hr.
milk and blocked for non-specific protein. The first antibody was added to the prepared mem- brane and incubated overnight at 4℃. After wash- ing with PBS-T, it was reacted for 1 hour at room temperature using the second antibody. It was washed again using PBS-T, and treated with the enhanced chemiluminescence solution and the specific protein amount was analyzed.
5) Statistical analysis
Data was represented as the mean ± standard deviation. In order to find out whether there is any difference in the survival rate in the cell differ- ences between control cells and cells treated with various concentrations of quercetin, they were analyzed using Student’s t-test.
RESULTS
1. PC-9 Cell Proliferation and Apoptosis by Quercetin Treatment
To determine the effect of quercetin on PC-9 lung cancer cell proliferation, cells were in- cubated with quercetin at various titers (25 uM
~ 100 uM) for 24hr and 48hrs and an MTT assay was performed. Figure 1A shows the results of the MTT assay and the inhibition of PC-9 cell proliferation under the quercetin treatment con- centrations for these time periods. 100 uM quer-
cetin treatment resulted in more than 60% in- hibition of proliferation after 24 hours and this inhibition was confirmed by microscopy (Fig. 1A).
To find out if quercetin treatment is related to inhibition of cell proliferation and cell death, protein interactions on the cell surface were ob- served using the flow cytometer. When apoptosis occurs, the phosphatidyl serine located in the inner side of the phospholipid bilayer of the cell membrane translocates to the outside. The dye reagent Annexin V can monitor this movement of phosphatidyl serine and was used to quantify cell apoptosis. It was observed that the frequency of cells undergoing apoptosis was quercetin de- pendent and increased in a concentration de- pendent manner (Fig. 1B). In order to re-confirm whether there is any association between apopto- sis induction and proliferation inhibition in PC-9 cells by quercetin, we tested for DNA fragmenta- tion, which is a phenomenon specific to apoptosis. For this, PC-9 cells were treated with various concentrations of quercetin and, in- cubated for 24 hours or 48 hours and DNA frag- mentation assays were performed by DNA electrophoesis. The results of DNA fragmentation assays, shown in Fig. 1C, exhibit a quercetin con- centration and time dependent DNA fragmentation. These results demonstrate that the inhibition of PC-9 cell proliferation by qeur- cetin treatment is closely related to apoptosis
induction.
2. Quercetin treatment induces apoptosis caused by caspase-dependent activity To find out which cellular pathway mediates quercetin-dependent apoptosis, PC-9 cells were treated with quercetin at various concentrations and times, and caspase activities were measured using Western blot. As a result, it was confirmed that quercetin induced caspase-3, caspase-8 and caspase-9 mediated fragmentation all in a con- centration and time-dependent manner.
Additionally, fragmentation of PARP-1, a sub- strate of caspase-3, was observed (Fig. 2A and 2B)., In order to find out if the activation of cas- pase plays an important role in apoptosis induced by quercetin, apoptosis was examined by treating cell with the pan-caspase inhibitor Z-VAD-fmk.
The results showed that the apoptosis was in- hibited by Z-VAD-fmk (Fig. 2C). These results demonstrate that the quercetin-mediated apop- tosis in PC-9 cells was dependent on caspase-3.
3. Effect of the MAPK Pathway By Quercetin To investigate whether the MAPK pathway was involved in quercitin-induced apoptosis, PC-9 cells were treated with quercetin at various con- centrations and times and the activation of c-Jun N-terminal kinase (JNK), extracellular responsive kinase (ERK) and p38 MAPK involved in MAPK signaling were observed. Fig. 3 shows that querce- tin increased p38 phosphorylation in a concen- tration and time-dependent manner, and the phosphorylation of ERK decreased depending on the concentration and time. A significant differ- ence was not observed in JNK phosphorylation Fig. 3. Effects of quercetin on the activation of MAPK in PC-9 cells. PC-9 cells were treated with 100 uM quercetin for the indicated concetrations (A) and times (B). The cell extracts were prepared for Western blot analysis of p-p38, p-JNK, p-ERK. Actin was used as an internal control
by quercetin. Therefore, the increase of p38 MAPK phosphorylation and decrease of ERK were likely associated with apoptosis induction by quercetin.
4. Effect on Mitochondrial Damage by Quercetin
The pathways to activate the caspase that is important in apoptosis include an extrinsic path- way through activation of apoptosis receptor and an intrinsic pathway through mitochondrial damage. Apoptosis by quercetin was determined
to operate through a caspase-dependent pathway as shown in Figure 2. And, among reactions by p38 MAPK activation, it is known that ROS pro- duction is activated by mediating mitochondria.
To examine the effect of quercetin on mitochon- dria, cytoplasmic levels of cytochrome cwere measured in cells treated with quercetin. A con- centration-dependent increase in cytochrome cexpression was observed in the cytoplasm of cells treated quercetin (Fig. 4A). The mitochon- drial damage caused by quercetin releases cyto- chrome cinto the cytoplasm where it induces for-
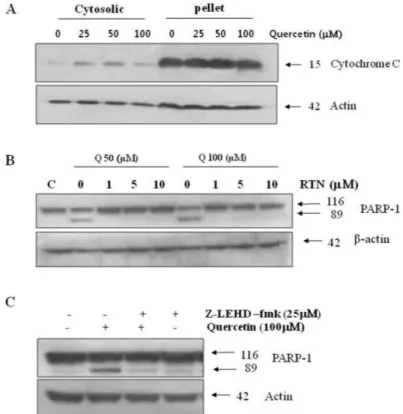
Fig. 4. Effects of quercetin on mitochondrial dependent signaling pathway. A. PC-9 cells were treated with indicated concentrations quercetin for 24 h, while mitochondrial and cytoplasmic extracts were prepared as described in “Materials and methods”section. B. Cells were incubated with 50 uM and 100 uM quercetin for 24 h, in the absence or presence of various concentrations rotenone (RTN). C. Cell were treated with 100 uM quercetin for 24 h, in the absence or presence of caspase-9 inhibitor (z-LEHD-fmk, 25 uM). Q: quercetin.
mation of the caspase-9 and Apaf (apoptotic pro- tease activating factor) complex and leads to apoptosis. After pre-treating with Z-LEHD-fmk, a selective Caspase-9 inhibitor, the cells treated with quercetin were collected, and checked for the progress of apoptosis. The results showed that PARP-1 fragmentation, a marker of apopto- sis, was inhibited (Fig. 4C). Apoptosis induced by quercetin is likely caused by release of cyto- chrome cfrom mitochondria and activation of procaspase-9. To inhibit activation of mitochon- dria, cells were incubated with the complex I inhibitor, rotenone, before quercetin treatment.
The apoptosis induced from quercetin was ob- served to be inhibited dependent on the rotenone concentration in cells (Fig. 4B). Based on these results, apoptosis by quercetin was confirmed to be mediated by the intrinsic mitochondrial dependent signal, which is one of the known apoptosis mechanisms.
Discussion
Quercetin induced cell proliferation and cell death of lung cancer cells in a concentration and time dependent manner. Cell death occurred through a caspase-3 dependent pathway, and en- hanced p38 MAPK phosphorylation. Quercetin induced apoptosis through a caspase-9 depend-
ent pathway which mediated mitochondrial re- lease of cytochrome c.
Cell death is largely classified as apoptosis or necrosis and these are distinguished by the cell morphology and biochemical characteristics.
Unlike necrosis, which causes inflammation by releasing the intracellular contents to the outside of the cell after destruction of the cell membrane as the cell is inflated, apoptosis causes cell death actively controlled by the genes. Apoptosis in- cludes phenomena such as cell membrane de- struction, cytoplasm and chromatin con- densation, and regular DNA cleavage. Apoptosis also acts in body development and maintaining homeostasis importantly.19,20
By using PC-9 cells, which are EGFR mutant lung cancer cells, this study was conducted to analyze cell proliferation and apoptosis by treat- ing PC-9 cells with quercetin, which has been found to have major anti-cancer effects among natural ingredients. According to results of Figure 1, we observed through MTT assay and FACS anal- ysis experiments that quercetin inhibited cell proliferation and induced apoptosis in PC-9 lung cancer cells. Using a DNA fragmentation assay to identify apoptosis by quercetin, it was con- firmed that fragmentation was produced depend- ent on time and concentration of quercetin treatment. Since caspase activity is known to be a major factor in apoptosis, the increases of vari-
ous caspase-8, caspase-9 and caspase-3 activ- ities were observed. Also, pan-caspase inhibitor (Z-VAD-fmk) was used to find out whether this is caspase dependent (Fig. 2). We observed how the expression of the MRPK family, a major signal transduction pathway in cells, affects cells treated with quercetin. p38 MAPK phosphorylation in- creased in a time and concentration dependent manner, indicating that it was involved in intra- cellular signaling by quercetin. However, p-ERK phosphorylation decreased in a time dependent manner, and there were no changes in p-JNK phosphorylation (Fig. 3). The decrease of p-ERK phosphorylation is involved in the inhibition of cell proliferation and the increase of p38 MAPK phosphorylation is known to be due to the intra- cellular ROS signal.21,22
External damage and stress causes a dysfunc- tion of mitochondria and consequently, it is known to activate caspase proteins and cause cell death by apoptosis.23 Cellular stress increases the permeability of the mitochondrial membrane and releases factors such as cytochrome c that induce the activation of caspase-9 proteins, acti- vating the intrinsic apoptosis pathway.24 Therefore, the apoptosis induced by quercetin increases intracellular ROS production by p38 MAPK signaling, which is caused by mitochon- drial damage, and caspase-9 activity is induced by the release of cytochrome c from the mi-
tochondria and this pathway enhances apoptosis (Fig. 4).
Based on the above results, the inhibition of PC-9 cell proliferation induced by quercetin showed that direct cell death caused by apoptosis and mitochondria mediated apoptosis occurred simultaneously. As a result of this study, p38 MAPK signaling by quercetin acts crucially as an initial factor of intracellular apoptosis in PC-9 cells.
REFERENCES
1. Socinski MA. Addressing the optimal duration of therapy in advanced, metastatic non-small-cell lung cancer. In: Perry MC, eds. American Society of Clinical Oncology Education Book. Alexanderia:
Lisa Greaves; 2003. p.144-52.
2. Nocholosn RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001;37:S9-15.
3. Wätjen W, Michels G, Steffan B, Niering P, Chovolou Y, Kampkötter A, et al. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. J Nutr 2005;135:525–31.
4. Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev 2010;68:418–28.
5. Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function.
Inflamm. Allergy Drug Targets 2010;9:263–85.
6. Ishizawa K, Yoshizumi M, Kawai Y, Terao J, Kihira Y, Ikeda Y, et al. Pharmacology in health food:
metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J Pharmacol Sci 2011;115:466–70.
7. Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep 2009;61:67–75.
8. Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf 2009;8:397–409.
9. Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents.
Molecules 2010;15:7792–814.
10. Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett 2008;269:315–25.
11. Seufi AM, Ibrahim SS, Elmaghraby TK, Hafez EE.
Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant ac- tivity: molecular and histological evidences. J Exp Clin Cancer Res 2009;28:80–7.
12. Priyadarsini RV, Vinothini G, Murugan RS, Manikandan P, Nagini S. The flavonoid quercetin modulates the hallmark capabilities of hamster buccal pouch tumors. Nutr Cancer 2011;63:218–
26.
13. Camargo CA, da Silva ME, da Silva RA, Justo GZ, Gomes-Marcondes MC, Aoyama H. Inhibition of
tumor growth by quercetin with increase of surviv- al and prevention of cachexia in Walker 256 tu- mor-bearing rats. Biochem Biophys Res Commun 2011;406:638–42.
14. Mendoza EE, Burd R. Quercetin as a systemic che- mopreventative agent: structural and functional mechanisms. Mini Rev Med Chem 2011;11:1216–
21.
15. Djuric Z, Severson RK, Kato I. Association of dietary quercetin with reduced risk of proximal colon cancer. Nutr Cancer 2012;64:351–60.
16. Cincin ZB, Unlu M, Kiran B, Bireller ES, Baran Y, Cakmakoglu B. Molecular mechanisms of quer- citrin-induced apoptosis in non-small cell lung cancer. Arch Med Res 2014;45:445-54.
17. Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ.
Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol 2008;75:1946-58.
18. Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem 2007;100:998-1009.
19. Elimore s. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495-516.
20. Wang J, Yu J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther 2008;7:1875-84.
21. Galluzzo P, Martini C, Bulzomi P, Leone S, Bolli A, Pallottini V, et al. Quercetin-induced apoptotic cascade in cancer cells: antioxidant versus estro-
gen receptor alpha-dependent mechanisms. Mol Nutr Food Res 2009;53:699-708.
22. Lee YK, Hwang JT, Kwon DY, Surh YJ, Park OJ.
Induction of apoptosis by quercetin is mediated through AMPKalpha1/ASK1/p38 pathway. Cancer Lett 2010;292:228-36.
23. Wang J, Yu Y, Hashimoto F, Sakata Y, Fujii M, Hou DX. Baicalein induces apoptosis through
ROS-mediated mitochondrial dysfunction path- way in HL60 cells. Int J Mol Med 2004;14:627-32.
24. Yang J, Xiao YL, He XR, Qiu GF, Hu XM.
Aesculetin-induced apoptosis through a ROS-mediated mitochondrial dysfunction path- way in human cervical cancer cells. J Asian Nat Prod Res 2010;12:185-93.