Enormous economic loss and casualties can occur as a result of the damages caused by material change, corrosion, degradation, and fatigue cracks in the welds and bolted
The corrosion behaviors of Al5454 alloy was investigated by potentiodynamic and potentiostatic polarization tests in 5 wt.% NaCl solution.. The pitting potential of the
The corrosion potential(E corr ), corrosion current density(I corr ), and corrosion rate of Ni-Ti files obtained from anodic polarization curves after electrochemical
In this study, in order to increase corrosion resistance and biocompatibility of Cp-Ti and Ti-6Al-4V alloy that surface of manufactured alloy was coated with TiN
Polymers containing amide, ester, or urethane may be attacked by hot acids or alkalies, and hydrolyzed..
Sources: metal plating, rechargeable batteries, smelting (제련), refining, mine drainage, industrial wastewater, corrosion of galvanized pipe in the water distribution
If less LiAlH 4 is used, still ester and alcohol is formed (no aldehyde), because the aldehyde reacts with the hydride as fast as it is formed Aldehyde is more. hydride as fast
Polymers are light, cheap, high strength/wt, tough, corrosion- resistant, insulating, low friction, ---.
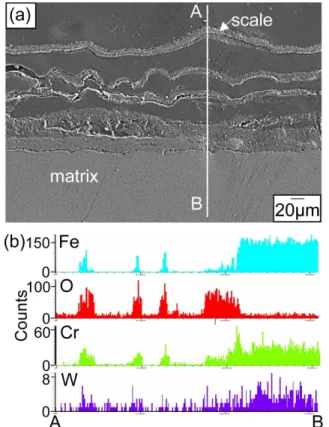
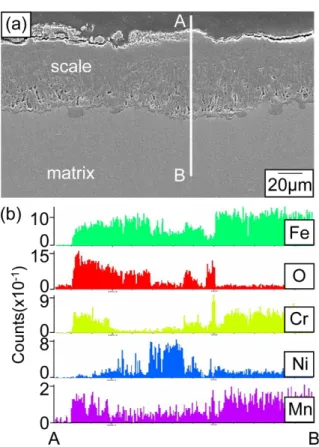
![Figure 1 shows corrosion kinetics of steels at 600- 600-800 o C for 50-200 h. Since chlorine in NaCl cracks and detaches protective Al 2 O 3 or Cr 2 O 3 oxide scales [8], weight gains displayed in Fig](https://thumb-ap.123doks.com/thumbv2/123dokinfo/5300633.378870/2.892.468.804.166.844/figure-corrosion-kinetics-steels-chlorine-detaches-protective-displayed.webp)