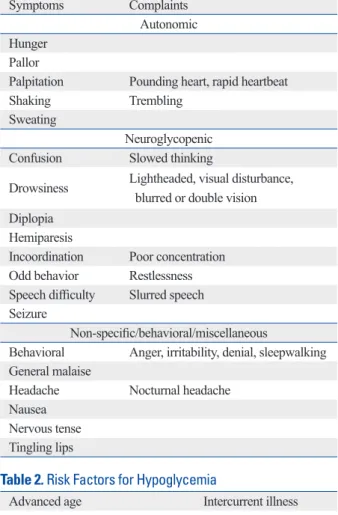
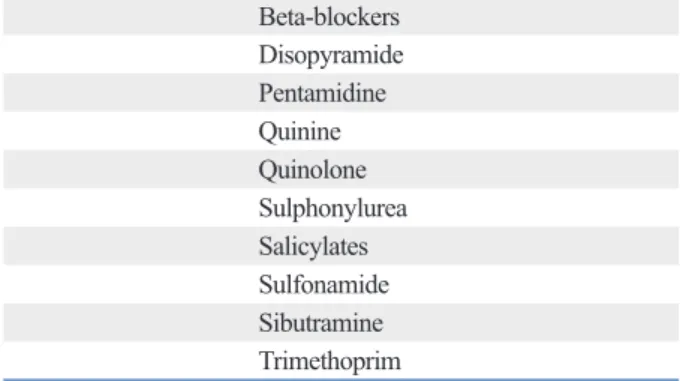
관련 문서
In the late nineteenth century, although the proportion of ether was increased and that of chloroform decreased due to the raised awareness of the high
(Background) Gallbladder wall thickening(GWT) and gallbladder contraction are often observed in patients with acute hepatitis.. The incidence of acute hepatitis A
In this randomized trial of patients receiving mechani- cal ventilation, the high-flow oxygen ventilation strategy during SBT did not reduce the risk of weaning failure on days
To identify causal variants among EGFR SNPs associated with the risk of glioma in a Korean population, a logistic regression analysis under an additive model adjusted for age
4,5 However, because of variations in the anatomy of the hard palate between patients, particular care must be taken during donor tissue harvesting not to damage structures
Factors associated with hypertension control in Korean adults: The fifth Korea National Health and Nutrition Examination Survey (KNHANES V-2).. Association of stress
activities in music education in an actual school setting, a systematic and specific music appreciation teaching method that is in accordance with the
The aim of this study was not only to compare the differences of ego-resilience between students with high experience and students with low experience in