pISSN 1598-642X eISSN 2234-7305
Establishment of Functional Cells for Vascular Defect Disease from Human Embryonic Stem Cell via Region Sorting Depending on
Cell Volume
Lee, Ji Hye 1† , Jumi Kim 2† , Hyung Min Chung 1,2 *, and Jung-Il Chae 3 *
1
Graduate School of Life Science, CHA Stem Cell Institute, College of Medicine, CHA University, Seoul 135-907, Korea
2
CHA Bio & Diostech Co., Ltd., Seoul 135-907, Korea
3
Department of Dental Pharmacology, School of Dentistry, Brain Korea 21 Project, Chonbuk National University, Jeonju 561-756, Korea
Received : November 8, 2011 / Revised : November 10, 2011 / Accepted : November 11, 2011
Human embryonic stem cells have been highlighted as a valuable cellular source in the regenerative medicine field, due to their pluripotency. However, there is the challenge of the establishment of specific functional cell type forms of undifferentiated human embryonic stem cells (hESC). To establish and purify functional cell types from hESCs, we differentiated undifferentiated hESCs into vascular lineage cells and sorted the specific cell population from the whole cell population, depending on their cell volume, and compared them with the non-sorted cell population. We observed that about 10% of the PECAM positive population existed in the VEGF induced differentiating human embryoid body (hEB), and differentiated hEBs were made into single cells for cell transplantation. After making single cells, we performed cell sorting using a fluorescence-acti- vated cell sorter (FACs), according to their cell volume on the basis of FSC region gating, and compared their therapeutic capacity with the non-sorted cell population through cell transplantation into hindlimb ischemic disease model mice. 4 Weeks after cell transplantation, the recovery rate of blood perfusion reached 54% and 17% in the FSC regions of sorted cells- and non-sorted cells, respectively. This result suggests that derivation of a functional cell population from hESCs can be performed through cell sorting on the basis of cell volume after preliminary differentiation induction. This approach may then greatly contribute to overcoming the limi- tations of marker sorting.
Keywords: human embryonic stem cell, flow cytometry, cell sorting, hind limb ischemia
Introduction
Several kinds of cardiovascular and neurodegenerative diseases originated from vascular degeneration have been considered as the most frequently cause of mortality and morbidity around the world [18].
In 1997, the cell transplantation therapeutic approach for
vascular defect disease was clinically applied after the discovery of circulating endothelial repopulating cells called endothelial progenitor cells (EPCs) in adult human peripheral blood,by Asahara and co-workers [3, 4].
After application of vascular lineage cell types obtained from adult cellular source to clinical trial, hESCs which are derived from the inner cell mass of blastocysts have been highlighted as novel valuable therapeutic cellular source in regenerative medicine field, due to their unique features including pluripotency, self-renewal and the capacity to differentiate into three germ layers both in vitro and in vivo [5, 14, 23].
Several previous studies were able to differentiate hESCs into functional cell type cells, including endothelial cell, pancreas cell, muscle cell, neuronal cell and blood cell,
*Corresponding author J.-I. C.
Tel: +82-63-270-4024, Fax: +82-63-270-4037 E-mail: jichae@chonbuk.ac.kr
H.-M. C.
Tel: +82-2-3468-3391, Fax: +82-2-3468-3373 E-mail: hmchung@cha.ac.kr
†
These authors contributed equally to this work.
through hEB formation, marker sorting, growth factor treatment, gene modification, enzyme treatment or a speci- fic isolation method [8, 14, 15, 17, 24-26].
In our previous study, we were able to acquire a high yield of endothelial like cells through isolation of the center region of attached hEBs through two steps enzyme treat- ment method and also confirmed the expression of the endothelial cell marker, PECAM (also known as CD31) [14]. In the next step, we tried to purify specific cell type which positive for specific cell surface marker using MACs or FACs sorting. However, the sorting of specific cell sur- face marker positive cell population, for example PECAM, was not too high enough for cell sorting, due to low cell number after allowing for low cell viability after laser sorting.
Therefore, we hypothesized that cell sorting on the basis of their cell size could be one option for purifying specific cell type, to overcome the limitation of low percentage of marker positive cell population, after preliminary differ- entiation induction, because cell size will be more similar after preliminary induction. Consequently, this study focuses on FSC region sorting depending on cell volume after preliminary differentiation induction using representative vascular lineage differentiation induction factor, VEGF [7], to enhance the differentiation efficiency of hESCs into vascular lineage cells.
Flow cytometry is microscopic particle counting technique, such as cells and chromosone, by suspending them in a fluid stream and suspending particles were detected by an electronic detection apparatus when passing them [13].
After development of flow cytometry technique, multi- parametric analysis of up to thousands of particles per second could be allowed at the physical and/or chemical point of view and routine diagnosis of health disorder was possible [20, 22].
FACs is a specialized type of flow cytometry to provide a method for sorting of specific cell type in heterogeneous mixture via light scattering and fluorescence characteristics of cells, and this is applied very usefully in scientific field due to their past, objective and quantitative recording of fluorescent signals from individual cells as well as physical separation of cells of particular interest. A beam of laser light of a single wavelength is used for detecting several parameters and a number of detectors are aimed at the point where the stream passes through the light beam, including one in line with the light beam (Forward Scatter or FSC)
and several perpendicular to it (Side Scatter or SSC), and fluorescence laser beam using one or more fluorescent detectors. FSC and SSC detect the each suspended particle (i.e., cells or nucleus) from 0.2 to 150 micrometers, and fluorescence detector perceive the excited fluorescent chemical, which labeled or attached in passing particle , through emitting light at a longer wavelength than the light source. Through these detection parameters, various types of information about the physical and chemical structure of each individual particle is collected and analyzed. FSC correlates with the cell volume and SSC depends on the inner complexity of the particle, such as shape of the nucleus, the amount and type of cytoplasmic granules or the membrane roughness [13, 20-22].
Despite the several usefulness of FACs, there is still mentioned about laser damage of cells because laser damage brings about low cell viability after laser sorting process.
To overcome the laser damage, we performed cell sort- ing procedure according to their cell volume on the basis of FSC region gating after preliminary differentiation induc- tion, because when cells were sorted by using only light source, cell viability could be enhanced.
Here, we investigated that the possibility of FSC region gating cell sorting method for generating functional cellular source from hESCs. We established the functional cellular source which positive for endothelial lineage cell charac- teristics, including tubule like structure formation on matri- gel and ac-LDL uptake ability, via FSC region gate sorting after preliminary differentiation induction using VEGF, and compared their therapeutic capacity with non-sorted cell population through cell transplantation into hindlimb ischemic disease model mice. Therapeutic efficacy was about 2.6 times higher in FSC region gate sorted cell population, which means that sorted cell population on the basis of their similar cell size after preliminary differenti- ation could be more effective for generating functional cell types for curing vascular defective disease.
Materials and Methods
hESC culture, EB formation and preliminary differ- entiation induction
Undifferentiated CHA-3 hESC were grown on mito-
tically inactivated STO cells (ATCC, Manassas, VA) in
DMEM/F12 (50:50; Gibco BRL, Gaithersberg, MD)
supplemented with 20% (v/v) serum replacement (Gibco)
and basic ES medium components including 1 mM L- glutamine (Gibco), 1% nonessential amino acids (Gibco), 100 mM beta mercaptoethanol (Gibco), and 4 ng/mL bFGF (Sigma, St. Louis, MO) [1]. The medium was changed every day, and the hESC were transferred to new feeder cells every 5~7 days with dissecting pipettes.
For EB formation, hESC were detached from feeder cells by Dispase (Gibco) and transferred to petri dishes and then suspended in bFGF-free hESC culture medium for 5 days.
For inducing preliminary differentiation induntion, hEBs were attached onto 0.2% gelatin (Sigma) coated dish for 5 days in 100 ng/ml VEGF treated medium condition. After 5 days, attached hEBs were made single cells through trypsinization for expansion and cultured in a defined medium (EGM-2;Clonetics, San Diego). Cell passages were performed with Trypsin-EDTA every 2-3 days.
Immunocytochemistry
The attached hEBs were cultured on 0.2% gelatin-coated glass cover slips. After culturing for 5 days on cover slips, the attached hEBs were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS for 5 minutes. After treatment with 1% normal goat serum for 30 minutes at room temperature, the cells were incubated with primary antibody for 24 hours at 4
oC against PECAM (BD Bioscience, San Diego, CA). Cells were washed with PBS and then incubated with a rhodamin-conjugated second antibody (Molecular Probes Inc., Eugene, OR) for 1 hour.
After washing with PBS, the stained slides were mounted with a glycerol-based mounting solution containing 2.5%
polyvinyl alcohol and 1,4-diazabicyclo octane (Sigma) with 4,6-diamidino-2-phenylindole (DAPI).
All images were analyzed with a LSM 510 META confocal microscope (Carl Zeiss Inc.; Oberkochen, Germany).
Flow cytometry
For FACS analysis, cells were dissociated with cell dissociation buffer (1X, Gibco) and then washed with PBS containing 2% (v/v) FBS. They were incubated with either isotype control or antigen-specific antibodies for 20 minu- tes: FITC-PECAM (BD Biosciences) and PE-conjugated goat anti-mouse IgG1 (Southern Biotech, Birmingham, AL).
FACS analysis were performed using a FACS Caliber Flow Cytometer (BD Bioscience). Data were collected from a minimum of 1 ×10
4PI-negative cells, and were
analyzed using the CELL Quest software (BD Bioscience).
Matrigel assays
0.2 ml of Matrigel (BD Bioscience) was solidified at 37
oC for 30 minutes in 4-well tissue culture plates. For induction of vascular formation, 0.2 mL of cell suspension containing 1 ×10
5differentiated hESC was plated on top of the Matrigel. Samples were incubated at 37
oC for 24 hours and then analyzed for formation of capillary-like structures.
Capillary formation was observed by phase-contrast micro- scopy.
Dill labeled ac-LDL uptake
To examine the uptake of Dill-labeled ac-LDL (Biomedi- cal Technologies, Stoughton, MA), cells were incubated with 10 µg/ml Dill-labeled ac-LDL for 4 hours at 37
oC.
Then,cells were washed three times with PBS and positive cells were analyzed by FACS analysis using a FACS Caliber Flow Cytometer (BD Bioscience). Data were col- lected from a minimum of 1 ×10
4PI-negative cells, and were analyzed using the CELL Quest software (BD Bio- science).
Mouse hindlimb ischemia model and Transplantation of CB- and PB-EPC
To develop hindlimb ischemia model, we followed the previously described procedure [8]. Mice at seven weeks of age were anesthetized with xylazine (20 mg/kg) and ketamine (100 mg/kg). The femoral artery and its branches were ligated through a skin incision with 5-0 silk (Ethicon).
The external iliac artery and all of the above arteries were then ligated. The femoral artery was excised from its proximal origin.
Immediately after arterial dissection, athymic mice were randomly assigned to one of three experimental groups.
The control group was injected with cell culture medium.
Sorted- (3.0 ×10
6cells) and non-sorted cells (3.0 ×10
6cells per mouse) were suspended in 200 µL of medium and transplanted intramuscularly into four sites of the gracilis muscle in the medial thigh using 29-gauge tuberculin syringes.
Laser Doppler imaging analysis
A laser Doppler perfusion imager (Moor Instruments)
was used for serial noninvasive physiological evaluation of
neovascularization. Mice were monitored by serial scann-
ing of surface blood flow in hindlimbs at several time points over a period of 4 weeks. The digital color-coded images were analyzed to quantify the blood flow in the region from the knee joint to the toe, and the mean values of perfusion were calculated.
Statistical analysis
Quantitative data are expressed as mean ± SD. Statistical analysis was performed with the t-test. A value of P<0.05 was considered statistically significant.
Results
Differentiation induction of hESCs into vascular lineage cells
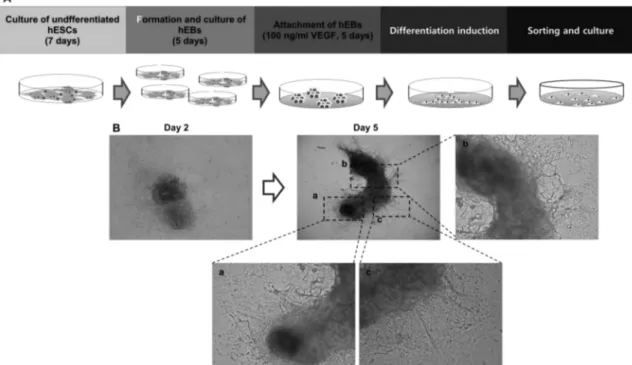
To establishment of functional cell types, undifferenti- ated hESCs were passed four different stage of differenti- ation. First, to induce spontaneous differentiation of hESCs, undifferentiated hESCs were detached for feeder cells and formed suspend cultured hEBs. hEBs were cultured for 5 days and attached on gelatin coated culture dished with VEGF for preliminary differentiation induction for 5 days.
After then, cells were made single cells for expansion and expanded single cells were sorted on the basis of their cell
volume (Fig. 1A).
To induction of hESCs into vascular lineage cells, hEBs were attached on 0.2 % gelatin coated culture dished in 100 ng/ml of VEGF treating medium condition. Through this preliminary differentiation induction using representative vascular lineage differentiation induction factor, VEGF, hEBs which cultured in normal culture condition were shown their morphological change as described in Fig. 1.
B. After VEGF treatment, cells were allowed to migrate out from the attached EB and differentiated cell were made network each other on culture dish (Fig. 1B)
Investigation of vascular lineage cell types in hEBs after preliminary differentiation
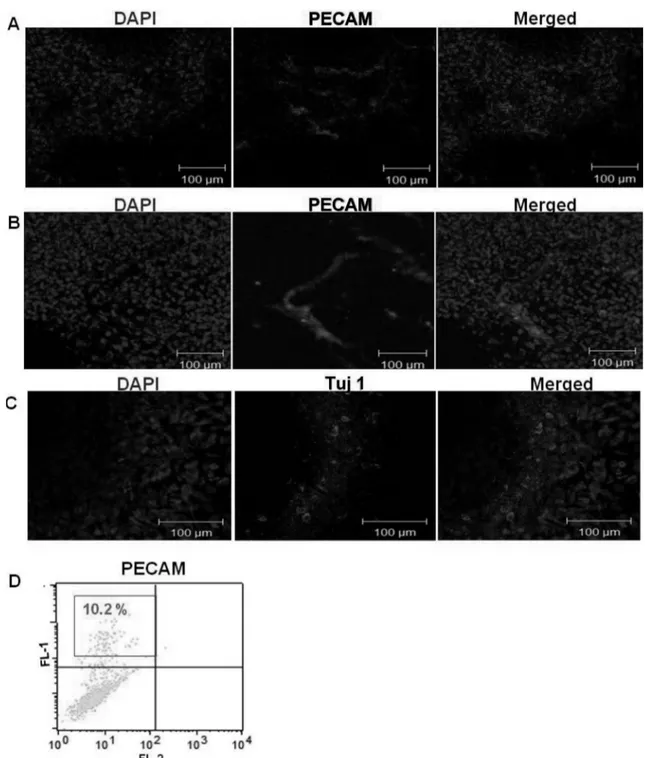
To confirm the vascular lineage cell population in hEBs after preliminary differentiation induction using VEGF treatment, we performed immunocytochemistry and FACs analysis against for representative endothelial cell marker, PECAM.
In immunocytochemical analysis using confocal micro- scope, endothelial-specific protein expression has been observed in several points of attached hEBs (Fig. 2A).
PECAM-positive cells were largely found in clusters arranged into vascular-like structures. Also, PECAM-positive cells
Fig. 1. Experimental scheme of this study. (A) Diagram of experimental scheme. Differentiation into vascular lineage cells from undif-
ferentiated hESCs was performed via differentiation induction stage and sorting stage, after preliminary differentiation of hEBs using
VEGF treatment (100 ng/ml). (B) Morphological change of attached hEBs with 100 ng/ml VEGF treatment for 5 days. Magnification is
40 × and 100×.
were observed as lining structure and did not existed solely in attached hEBs.
In FACs analysis using PECAM, we could detect about 10% of PECAM positive cell population in attached hEBs (Fig. 2B).
To examine the other lineage cells in attached hEBs, we
investigated the neuronal lineage cells using immunocyto- chemistry. Tuj-1 positive neuronal lineage cells (green) were detected in attached hEBs (Fig. 2C), which means that differentiated hEBs contained several kinds of cell types, not just vascular lineage cells, despite preliminary differentiation. Therefore, purifying step will be needed.
Fig. 2. Vasculogenic characterization of attached hEBs. (A and B) Immunocytochemistry analysis of day 5 attached hEBs against
PECAM (red, middle panel), and Tuj1 (green, middle panel) (C). Nucleus was detected with DAPI (blue, left panel) (D) FACs analysis
result of attached day 5 hEBs with PECAM.
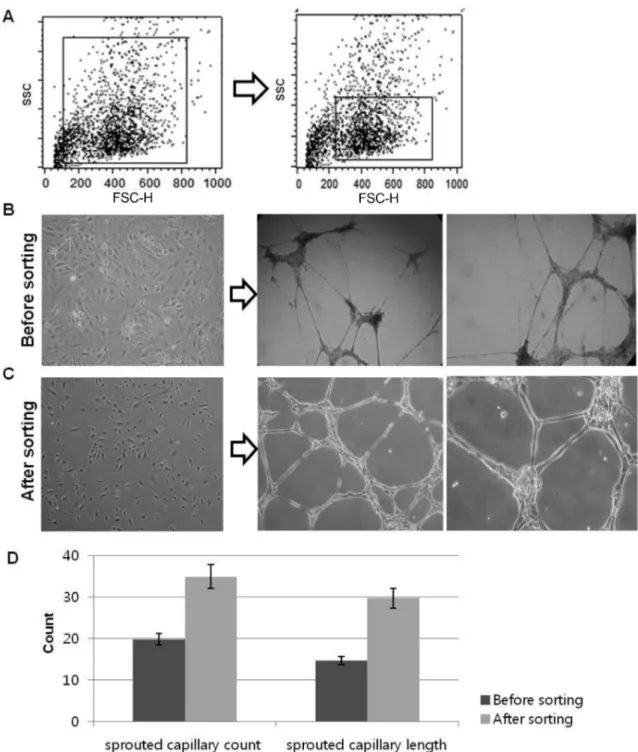
Comparison of sorted- and non-sorted cell population After preliminary differentiation induction through VEGF treatment, attached hEBs were made single cells for expan- sion and expanded single cells were sorted on the basis of their cell volume. As shown in Fig. 3 A, FSC region was scattered in broadly after preliminary differentiation induc- tion. To purify specific cell types, we sorted similar size of
cells via FSC region gate sorting method. We just focused on gated cells as shown in Fig. 3 A (right panel) in FSC region and sorted them. After sorting, we culture them in EGM-2 medium condition and compared their characteristics with non-sorted cells. In morphological analysis, non-sorted cells were shown mixed cell population; on the other hand, sorted cells were shown cobble stone like cell morphology
Fig. 3. Comparision of vasculogenic capacity of sorted- and non-sorted cell. (A) Comparison of FSC region gate on the basis of cell
size (B) Morphology and tubule forming assay on matrigel for 24 hr FSC region gate non-sorted cell (before sorting) (C) and FSC region
sorted cell (after sorting). (D) Quantification for comparing the vasculogenic ability of both cell populations.
(Fig. 3B and C, left panel). To compare the functional characteristics of both cell population as vascular cells, cells were cultured on matrigel and examined their ability of tubule-like structure formation (Fig. 3B and C, right panel). Although both cell population were formed tubule- like structure, however thinner and un-uniformed structure were observed in non-sorted cells (upper panel), on the other hand, sorted cell population spontaneously organized into vessel-like capillary structures on matrigel within 24 hours and maintained them (lower panel).
Comparison of the functional improvement after cell transplantation in a hindlimb ischemic disease mouse model
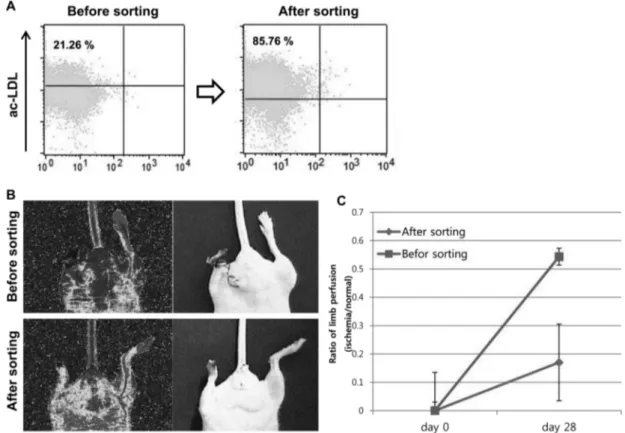
To verify the vascular lineage cell population before cell transplantation in both sorted- and non-sorted cells, the cells were incubated with Dill-labeled-ac-LDL and subse- quently examined by FACs analysis. The fluorescence of ac-LDL which detected by FACs were recorded as 21.26%
and 85.76% in non-sorted- and sorted cell population, respectively (Fig. 4A).
Then, to investigate the healing abilities, the cells were transplanted into an athymic mouse model of hindlimb ischemia. A total of 3.0 ×10
6sorted- and non-sorted cells were transplanted per mouse; mice injected with medium (EGM-2 MV) (n=3) were used as negative controls. The non-sorted cell transplanted group exhibited limb loss by auto-amputation (100%, limb loss/total animals = 2/3), as similar as medium injection group. By contrast, intramus- cular transplantation of the sorted cells reduced limb loss, and the mice that received the sorted cells exhibited limb salvage (limb salvage/total animals = 1/3) and mostly shown foot loss (foot loss/total animals = 2/3) as shown in Fig. 4B (lower panel).
The physiological function of the transplanted cells was evaluated by detecting blood perfusion in the ischemic limbs. We monitored blood flow in the ischemic hindlimb using a Laser Doppler perfusion imaging analyzer. The blood flow in the ischemic region of the hindlimb was im- mediately restricted after the surgical induction of ischemia.
In the cell transplantation group (3.0 ×10
6cells/mouse, n=3, P<0.05), the blood flow began to be restored and reached
Fig. 4. Comparision of therapeutic ability on hindlimb ischemic disease model mouse. (A) FACs analysis result of ac-LDL uptake
using non-sorted cells (Before sorting, left panel) and sorted cells (After sorting, right panel) (C) Investigation of therapeutic ability of both
sorted- (lower panel) and non-sorted cells (upper panel) (C) Measurement of blood perfusion rate for 4 weeks (28 days) after cell transplan-
tation. * P<0.05
at 0.17 ± 0.081 (ischemic to normal limb, n=3, P<0.05) and 0.54 ± 0.049 (ischemic to normal limb, n=3, P<0.05) in non- sorted and sorted cell transplantation group, respectively, after four weeks of cell transplantation. Which means that four weeks after cell transplantation, recovery rate of blood perfusion was reached at 54% and 17% in FSC region sorted cell- and non-sorted cell, respectively.
Discussion
Stem cells and stem cell derivate have been well known as critical source for clinical cell therapy [2, 9]. Due to their high self-renewability and viability, differentiated vascular cells from hESC are considered a potent resource for curing vascular diseases and repair injured vessel tissue. Therefore, differentiation of hESCs and purification of differentiated cell population is very important, as well as to expand them in large scale for cell therapy [10, 11, 27].
Furthermore, endothelial lineage cells which derived from stem cells have been focused for several kinds of car- diovascular and neurodegenerative diseases originated from vascular degeneration, because it is considered as the most frequently cause of mortality and morbidity around the world [6, 12, 19].
Several methods, including hEB formation, marker sorting, growth factor treatment, gene modification, enzyme treatment or a specific isolation method, were used to induce differentiation of hESC. However, purification of wanted cell population from various types of differentiated cells have been considered as important steps for establish- ment of functional cell types [5, 6, 14, 16, 25].
As these reasons, we aimed to differentiate hESC toward vascualr lineage spontaneously through EB formation and purified the specific cell population on the basis of their cell volume after preliminary differentiation induction using FACs sorting, and then evaluated the therapeutic efficacy in hindlimb ischemic disease model mouse.
In FACs sorting process, we used only light beam to overcome the laser damage, because FSC could be distinguished only using light beam, not using laser beam.
Due to this concept, cell viability could be enhanced after sorting process, in this study.
Following cell sorting process, we compared the phenotypic characteristics between sorted- and non-sorted cell population in aspect of vascular cell functionality using matrigel tubule forming assay and ac-LDL uptake analysis.
Morphologically, sorted cells were more uniformed than non-sorted cells, and cells were culture on matorigel to compare the functional characteristics of both cell popula- tion as vascular cells, thinner and un-uniformed structure were observed in non-sorted cells, on the other hand, sorted cell population spontaneously organized into vessel-like capillary structures on matrigel within 24 hours and main- tained them. Also, ac-LDL uptake analysis, the fluoresc- ence of ac-LDL which detected by FACs were recorded as 21.26% and 85.76% in non-sorted- and sorted cell popula- tion, respectively. In vitro analysis of functional aspect as vascular lineage cells of both cell types, the characteristics of sorted cells as vascular cells were more evidently closed.
Then, we investigated the therapeutic performance of both cell types in hindlimb ischemic disease model mouse.
The cells were transplanted into an athymic mouse model of hindlimb ischemia. A total of 3.0 ×10
6sorted- and non- sorted cells were transplanted per mouse.
The non-sorted cell transplanted group exhibited massive limb loss by auto-amputation, as similar as medium injec- tion group, by contrast, intramuscular transplantation of the sorted cells reduced limb loss, and the mice that received the sorted cells exhibited limb salvage and mostly shown foot loss.
The physiological function of the transplanted cells which evaluated by detecting blood perfusion in the ischemic limbs means that recovery rate of blood perfusion was reached at 54% and 17% in FSC region sorted cell- and non-sorted cell, respectively, four weeks after cell transplantation.
Here, we hypothesized that cell sorting on the basis of their cell size could be one option for purifying specific cell type, to overcome the limitation of low percentage of marker positive cell population and low cell viability after laser cell sorting. And resulted cell product shown the higher possibility as cellular source for cell therapy in vascular defective disease field.
Also, through this study, we could confirmed that the possibility of FSC region gating cell sorting method for generating functional cellular source from hESCs.
This result suggest that establishment of purified func-
tional cell population obtained from hESCs for cell thera-
peutic approach could be performed through cell sorting on
the basis of cell volume after preliminary differentiation
induction, without laser damage. And this approach will be
contributed in generation of functional cell type and also
beneficially applied to overcome the limitation of marker sorting.
Acknowledgments
This research was supported by a grant (SC3110) from the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education and Science, and by basic Science Research Program (2010- 0021532), and by the Next-Generation BioGreen 21 Pro- gram (PJ008116062011), Rural Development Administration, Republic of Korea. to J.I.C
R EFERENCES
1. Ahn, S. E., S. Kim, K. H. Park, S. H. Moon, H. J. Lee, G. J.
Kim et al. 2006. Primary bone-derived cells induce osteo- genic differentiation without exogenous factors in human embryonic stem cells. Biochem. Biophy. Res. Co. 340: 403- 408.
2. Amit, M., M. K. Carpenter, M. S. Inokuma, C. P. Chiu, C. P.
Harris, M. A. Waknitz et al. 2000. Clonally derived human embryonic stem cell lines maintain pluripotency and prolifer- ative potential for prolonged periods of culture. Dev. Biol.
227: 271-278.
3. Asahara, T., A. Kawamoto, and H. Masuda. Concise review:
circulating endothelial progenitor cells for vascular medi- cine. Stem Cells 29: 1650-1655.
4. Asahara, T., T. Murohara, A. Sullivan, M. Silver, R. van der Zee, T. Li et al. 1997. Isolation of putative progenitor endot- helial cells for angiogenesis. Science 275: 964-967.
5. Bai, H. and Z. Z. Wang. 2008. Directing human embryonic stem cells to generate vascular progenitor cells. Gene Ther.
15: 89-95.
6. Boyd, N. L., S. K. Dhara, R. Rekaya, E. A. Godbey, K. Has- neen, R. R. Rao et al. 2007. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell- derived embryoid bodies. Exp. Biol. Med. (Maywood) 232:
833-843.
7. Byrne, A. M., B.-H. D., Harmey J. H. 2005 Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell. Mol. Med. 9: 777-794.
8. Cho, S. W., S. H. Moon, S. H. Lee, S. W. Kang, J. Kim, J.
M. Lim et al. 2007. Improvement of postnatal neovascular- ization by human embryonic stem cell derived endothelial- like cell transplantation in a mouse model of hindlimb ischemia. Circulation 116: 2409-2419.
9. Deb, K. D. and K. Sarda. 2008. Human embryonic stem cells: preclinical perspectives. J. Transl. Med. 6: 7.
10. Drukker, M., H. Katchman, G. Katz, S.Even-Tov. Friedman, E. Shezen, E. Hornstein et al. 2006. Human embryonic stem cells and their differentiated derivatives are less susceptible
to immune rejection than adult cells. Stem Cells. 24: 221- 229.
11. Dvash, T., D. Ben-Yosef, and R. Eiges. 2006. Human embry- onic stem cells as a powerful tool for studying human embryogenesis. Pediatr. Res. 60: 111-117.
12. Ferreira, L. S., S. Gerecht, J. Fuller, H. F. Shieh, G. Vunjak- Novakovic, and R. Langer. 2007. Bioactive hydrogel scaf- folds for controllable vascular differentiation of human embryonic stem cells. Biomaterials 28: 2706-2717.
13. Fulwyler, M. J. 1965. Electronic separation of biological cells by volume. Science 150: 910-911.
14. Kim, J., S. H. Moon, S. H. Lee, D. R. Lee, G. Y. Koh, and H.
M. Chung. 2007. Effective isolation and culture of endothe- lial cells in embryoid body differentiated from human embryonic stem cells. Stem Cells Dev. 16: 269-280.
15. Klimanskaya, I., Y. Chung, S. Becker, S. J. Lu, and R.
Lanza. 2006. Human embryonic stem cell lines derived from single blastomeres. Nature 444: 481-485.
16. Layman, H., M. Sacasa, A. E. Murphy, A. M. Murphy, S. M.
Pham, and F. M. Andreopoulous. 2008 Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic ther- apy in a murine critical limb ischemic model.Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angio- genic therapy in a murine critical limb ischemic model. Acta Biomater. 5: 230-239.
17. Levenberg, S., J. S. Golub, M. Amit, J. Itskovitz-Eldor, and R. Langer. 2002. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U S A 99: 4391- 4396.
18. Loges, S., C. Roncal, and P. Carmeliet. 2009. Development of targeted angiogenic medicine. J. Thromb. Haemost. 7: 21- 33.
19. Lu, S. J., Q. Feng, S. Caballero, Y. Chen, M. A. Moore, M.
B. Grant et al. 2007. Generation of functional hemangio- blasts from human embryonic stem cells. Nat. Methods 4:
501-509.
20. Mr, L. 1999. Immunofluorescence Techniques in Flow Cytometry and Sorting Wiley: 341-353.
21. Ornatsky, O., D. Bandura, V. Baranov, M. Nitz, M. A. Win- nik, and S. Tanner. Highly multiparametric analysis by mass cytometry. J. Immunol. Methods 361: 1-20.
22. Qian, Y., C. Wei, E.-H. Lee, F. Campbell, J. Halliley, J. Lee et al. 2010. Elucidation of seventeen human peripheral blood B-cell subsets and quantification of the tetanus response using a density-based method for the automated identifica- tion of cell populations in multidimensional flow cytometry data. Cytometry Part B: Clinical Cytometry 78B.
23. Thomson, J. A., J. Itskovitz-Eldor, S. S. Shapiro, M. A.
Waknitz, J. J. Swiergiel, V. S. Marshall et al. 1998. Embry- onic stem cell lines derived from human blastocysts. Science 282: 1145-1147.
24. Wang, L. 2006. Endothelial and hematopoietic cell fate of human embryonic stem cells. Trends Cardiovasc. Med. 16:
89-94.
25. Wang, L., L. Li, F. Shojaei, K. Levac, C. Cerdan, P. Menen-
dez et al. 2004. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endot- helium with hemangioblastic properties. Immunity 21: 31-41.
26. Wang, Z. Z., P. Au, T. Chen, Y. Shao, L. M. Daheron, H. Bai et al. 2007. Endothelial cells derived from human embry- onic stem cells form durable blood vessels in vivo. Nat Bio-
technol. 25: 317-318.
27. Yamahara, K., M. Sone, H. Itoh, J. K. Yamashita, T. Yurugi- Kobayashi, K. Homma et al. 2008. Augmentation of neovas- cularizaiton in hindlimb ischemia by combined transplanta- tion of human embryonic stem cells-derived endothelial and mural cells. PLoS ONE 3: e1666.
국문초록
세포 크기 차이를 이용한 유세포 분석을 통한 인간배아줄기세포 유래 기능성 혈관세포의 확립 이지혜
1·김주미
2·정형민
1,2·채정일
3*
1
( 주)차바이오앤디오스텍,
2차의과학대학교 의생명과학대학원 분자발생학,
3