J Bacteriol Virol. Vol 48. No 4. December 2018; 48(4): 137-146 http://dx.doi.org/10.4167/jbv.2018.48.4.137
eISSN 2093-0249
JBV
Cysteine-Added Mutants of Turnip Yellow Mosaic Virus
In-Sun Shin, Doyeong Kim and Tae-Ju Cho*
School of Life Sciences, Chungbuk National University, Cheongju, Korea
Corresponding Tae-Ju Cho
School of Life Sciences, Chungbuk National University, Cheongju 28644, Korea.
Phone : +82-43-261-2309 Fax : +82-43-267-2306 E-mail : tjcho@chungbuk.ac.kr
Received : August 07, 2018 Revised : October 16, 2018 Accepted : October 29, 2018
No potential conflict of interest relevant to this article was reported.
Copyright © 2018 Journal of Bacteriology and Virology
©This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/
license/by-nc/3.0/).
Native turnip yellow mosaic virus (TYMV) is relatively unreactive to maleimide agents, indicating few reactive thiol groups on TYMV. In the present study, we aimed to construct TYMV mutants that have reactive cysteine residues on the surface. To this end, we prepared a library of TYMV mutants where the Thr residue at the C-terminus of coat protein (CP) was replaced by a random sequence of six amino acids that included one cysteine. This library was introduced into Nicotiana benthamiana by agroinfiltration. The CP sequence of the TYMV RNA isolated from inoculated leaves was amplified by reverse transcription-PCR and then used to construct a second library. This process was repeated one more time, and the CP sequences of the TYMV RNA in the inoculated leaves were analyzed. Based on the analysis of over 11,000 CP sequences, the Cys mutants representing most abundant TYMV RNAs were constructed. Analysis of the mutants showed that four Cys mutants were nearly comparable to wildtype with respect to CP and viral RNA levels in N.
benthamiana. All these mutants were highly reactive to fluoresceine-5-maleimide.
This demonstrates that TYMV can be modified to have additional functional groups on the surface that would be useful for drug delivery.
Key Words: TYMV, Cys-added mutant, Reactive cysteine
INTRODUCTION
Turnip yellow mosaic virus (TYMV) is a nonenveloped icosahedral plant virus which has a single (+)-strand RNA as a genome. TYMV capsid is ca. 30 nm in diameter and is composed of 180 identical 20 kDa coat proteins (1). By now, there have been efforts to use plant viruses for medical applications (2, 3). The advantages of using plant viruses as drug delivery vehicles include their safety in terms of handling and their abundance in infected tissue (> 1 mg/g tissue). For these reasons, plant viruses are considered to be ideal building blocks for nanocarriers. Many plant viruses, including tobacco mosaic virus (TMV), can be purified following simple isolation protocols. Furthermore, in vitro assembly protocols using purified coat proteins have been established for many viruses (2).
In viruses, coat proteins are regularly arranged and many functional groups exist on the surface. Therefore, viral capsids can be decorated with diverse molecules, such as drugs, peptides, fluorescent dyes, MRI contrast agents, and gold nanoparticles. In this respect, Wang et al. (4) showed that a variety of molecules-ranging from small molecules, such as fluorescent dyes, to large structures, such as quantum dots-could be conjugated to cowpea mosaic virus (CPMV). Other plant viruses including TMV
JBV
Bacteriology and Virology VOL 48. NO 4. DECEMBER 2018 have been investigated for this purpose (5).Similarly to many other plant viruses, TYMV is robust under a variety of conditions. TYMV is stable from 4℃ to RT for months and 60℃ for several hours. It is stable in a wide pH range (4~10), up to 50% organic solvent, and in a variety of reaction conditions (6). Furthermore, TYMV has unique properties that can be exploited for ideal drug delivery. Specifically, empty particles can be easily prepared by simple physical and chemical treatments, such as high pressure treatment, a freeze-thaw process and treatment with alkaline solution (7). The freeze-thaw process is known to result in a hole of 6~9 nm (8). Recently, it was demonstrated that the empty particles prepared by the freeze-thaw process were suitable to load fluorescent dyes. In addition, it was observed that TYMV efficiently entered mammalian cells when the particle was conjugated with cell penetrating peptide, such as Tat (9). Therefore, TYMV has a great potential as a drug delivery vehicle.
Commonly used functional groups in surface modification of viral nanoparticles are primary amines and carboxyl moieties (10).
Barnhill et al. (6) reported that TYMV has 60 amine groups reacting with N-hydroxysuccinimide ester and 90~120 carboxyl groups reacting with amines per virus particle. These functional groups can be used to conjugate fluorescent dyes and other molecules using simple chemistry. Another popular functional group for conjugation is a sulfhydryl group of solvent-exposed cysteine residue. The reactive thiols provide coupling sites for molecules with maleimides or halogen-substituted acetamides (11). Thiol groups also react with gold nanoparticles. Unfortunately, however, TYMV does not have reactive cysteine residues on the surface.
In the present study, we sought to construct Cys mutants that have reactive thiol groups. To this end, a random sequence of amino acids was introduced into the C-terminus of coat protein (CP) using the genetic engineering approach. One of the inserted residues was cysteine. After three rounds of propagation in Nicotiana benthamiana, the TYMV RNA sequence was analyzed using high-throughput sequencing strategy. Based on the sequence data, Cys mutants were constructed and then examined for their reactivity to fluorescein-5-maleimide. The results showed that several Cys mutants hold promise for further examination.
MATERIALS AND METHODS
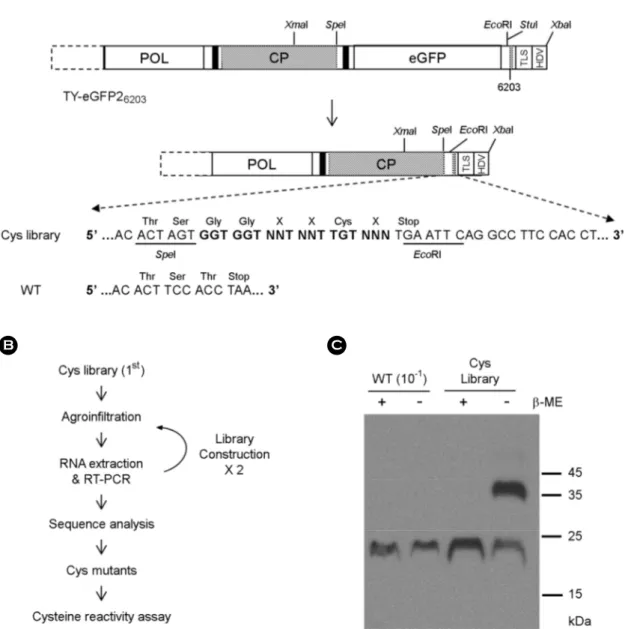
Construction of Cys library
To prepare a TYMV Cys mutant library, the following two primers were used to amplify a part of CP as well as to introduce a random sequence of amino acids including a cysteine at the C-terminus of CP: 5'-TCGATTCCCGGGTCAAAGATT-3' (upstream primer; the XmaI site is underlined), 5'-CCTGAATTCANNNACAANNANNACCACCACTAGTGTCCGTGATGAGC-3' (downstream primer; the EcoRI site is underlined. N represents any nucleotides). The PCR product was digested with XmaI and EcoRI, and inserted into a TYMV replicon TY-eGFP26203 (12). The TYMV replicon DNA was introduced to E. coli to prepare the 1st Cys mutant library. Thereafter, the library DNA was used to transform Agrobacterium tumefaciens. Then, A. tumefaciens bacteria were introduced to Nicotiana benthamiana leaves by agroinfiltration. After seven days, TYMV RNA was extracted from the agroinfiltrated leaves. The CP sequence of the viral RNA was amplified by reverse transcription-PCR (RT-PCR) and used to create the 2nd library. The upstream primer used was the same as the primer used in the 1st library construction. The downstream primer used was 5'-GGAGAATTCTCTAGATGGCTCTCCCTTAG-3' (the XbaI recognition site is underlined). The PCR product was digested with XmaI and XbaI, and inserted into TY-eGFP26203. For the 3rd library construction, encapsidated viral RNA was used.
Construction of Cys mutants
Cys mutants were prepared by replacing the sequence between XmaI and EcoRI sites of TY-eGFP26203 with PCR-amplified DNA. The upstream primer used in the PCR amplification was as follows: 5'-TCGATTCCCGGGTCAAAGATT-3' (the XmaI site
was as follows: 5'-GCTGAATTCATTAACAAGGACGACC-3' (the EcoRI site is underlined). For the construction of Cys 1A, the following downstream primer was used: 5'-CTGAATTCAAAGACAAGGACGACCACCACTAGTGGCCGTGATG-3' (the EcoRI and SpeI sites are underlined).
Isolation and purification of TYMV
Agroinfiltration was performed following the procedure described in Cho and Dreher (13). Seven days after agroinfiltration, the infiltrated leaves were collected and homogenized in 4 ml of 0.1 M sodium phosphate buffer (pH 7.0) containing 20 mM β-mercaptoethanol (β-ME) per g tissue. The mixture was extracted with 0.2 ml chloroform per g tissue. After centrifugation at 7,000 rpm in a microfuge for 10 min, virus particles were precipitated by adding 0.5 volume of 30% polyethylene glycol (PEG) 8000. After overnight incubation at 4℃, the sample was centrifuged at 5,000 g for 10 min, and the pellet was dissolved in distilled water containing 10 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP). The suspension was again extracted with 0.4 volume of chloroform. TYMV in the aqueous phase was precipitated by adding 0.5 volume of 30% PEG and 0.05 volume of 5 M NaCl. The pellet obtained after centrifugation was dissolved in distilled water containing 10 mM TCEP. For TYMV RNA and protein extraction, the leaf samples were frozen in liquid nitrogen immediately after collection, and then stored at -80℃.
Analysis of RNA
Total RNA was isolated from frozen N. benthamiana leaf samples using Easy-Red (iNtRON Biotechnology, Sungnam-si, Korea).
The ribonuclease protection assay for encapsidated RNA in TYMV virions and the Northern blot analysis were performed using a DIG-labeled DNA probe representing the TYMV CP ORF as previously described (13). For the RNA sequence analysis, nucleotide sequence corresponding to the C-terminal part of TYMV CP was amplified by RT-PCR using the same primers as used in the 2nd and 3rd library construction. The DNA sequence was analyzed by a high-throughput sequencing method at ebiogen (Seoul, Korea). Briefly, sample preparation for sequencing was performed using NEBNext® UltraTM DNA Library Prep Kit for Illumina (New England Biolabs, Beverly, MA, USA) according to the manufacturer's instructions, and high-throughput sequencing was performed as single-end 100 sequencing using NextSeq 500 (Illumina, San Diego, CA, USA).
Analysis of coat protein
To examine the TYMV coat protein expression, leaf samples (0.1 g) were ground in 400 μl of 0.1 M sodium phosphate buffer (pH 7.0) containing 20 mM β-ME. Protein concentration of the leaf extract was determined by Bradford assay using bovine serum albumin as the standard. In the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), an equal volume of 2X SDS sample buffer was added to the leaf extract, which was boiled for 10 min and then loaded into 12.5% SDS- polyacrylamide gel. The gel was run at 30 mA for 80 min, and the proteins in the gel were examined by staining with Coomassie.
Alternatively, TYMV CP was detected by a chemiluminescent method using LuminataTM Forte, employing anti-TYMV rabbit antiserum and HRP-conjugated goat anti-rabbit IgG.
Fluorescein labeling
Before the conjugation reaction, 100-fold excess of TCEP was added to the TYMV samples. The TYMV particles were made react with a 25-fold molar excess of fluorescein-5-maleimide (Sigma, USA) in 20 mM sodium phosphate buffer (pH 7.2), 150 mM NaCl at room temperature (RT) for 2 h. After the reaction, the unreacted fluorescein-5-maleimide was removed using a 7 K Zeba spin desalting column (Thermo Fisher Scientific, Waltham, MA, USA).
JBV
Bacteriology and Virology VOL 48. NO 4. DECEMBER 2018RESULTS
Preparation of Cys-added TYMV mutant library
According to an X-ray crystallographic study of TYMV, C-terminus of the coat protein is exposed to solvent environment (14).
Accordingly, the C-terminal region could be an appropriate site for the introduction of a reactive cysteine residue. Previously, we examined whether the N- or C-terminus of TYMV CP could accommodate an extension of 15 amino acids representing a c-Myc epitope peptide (15). The results showed that N-terminus could be modified without compromising the CP assembly.
However, C-terminal extension turned out to be detrimental to the CP assembly and replication. In another study of a TYMV mutant with a five amino acid (Tyr-Val-Leu-Asp-Arg) extension at the C-terminus, Bransom et al. (16) reported that the mutant replicated normally and showed systemic symptom, although the yield of the mutant was slightly lower than wildtype.
In a preliminary experiment, we constructed two mutants that had five additional amino acid residues at the C-terminus. The C-terminal extension included a cysteine. One of the two mutants did not replicate at all. The other replicated, but the viral products were about 10~20% of wildtype (data not shown). Therefore, in the hope to obtain cysteine-added mutants that would efficiently replicate as wildtype, we decided to make a library in which a random sequence of amino acid residues was introduced at the C-terminus of TYMV CP (see Fig. 1A). These mutants have a cysteine residue near the C-terminus and three random amino acid residues around cysteine. The DNA containing a random sequence of C-terminal extension was prepared by PCR and used to replace the C-terminal part of CP in a TYMV replicon TY-eGFP26203 (12), which was constructed using a Ti plasmid binary vector pCB302-3.
The Cys mutant library DNA was introduced to E. coli, resulting in over 100,000 bacterial colonies. Plasmid DNA from all the transformed bacteria was then used to transform A. tumefaciens. The bacterial colonies produced were again at least 100,000 in number. Transformed A. tumefaciens grown on agar plates were collected by scraping, and the mixture was used to inoculate N. benthamiana leaves by agroinfiltration.
To screen for the efficiently replicating TYMV Cys mutants, total RNA was extracted from the inoculated leaves at seven days post-inoculation and used to prepare a next library (the 2nd library) by amplifying the sequence corresponding to the C-terminal CP by RT-PCR and inserting into the TYMV replicon (see Fig. 1B). The library was re-introduced to N. benthamiana. In order to enrich TYMV variants which were good at viral RNA packaging and at replication, encapsidated viral RNA was used to prepare the 3rd library. The leaves were collected after 7 days of inoculation, and the leaf extract was examined by SDS-PAGE and Western blot analysis using anti-TYMV CP antibody (see Fig. 1C). The results showed that an additional band of about 40 kDa appeared in the case of the Cys mutant library. The '40 kDa' band was not intense in wildtype. Since the '40 kDa' band corresponds to the size of a TYMV CP dimer and disappears in the presence of β-ME, the '40 kDa' band was assumed to be generated by cross- linking between the CP subunits. This could be indicative of the reactivity of the newly introduced cysteine residue. Cysteine residues exposed on the surface can cause cross-linking of viral particles, leading to aggregation of viral particles. However, in our samples, heavy precipitation was not observed. Therefore, the '40 kD' band was assumed to be mostly ascribed to cross- linking between neighboring CP subunits within viral particles.
Preparation and analysis of Cys mutants
A. tumefaciens harboring the 3rd library was introduced into N. benthamiana leaves by agroinfiltration, and the encapsidated TYMV RNA extracted from the agroinfiltrated leaves was analyzed using high-throughput sequencing. Of the 11,584 sequences analyzed, occurrences of all sequences in the sequencing data were counted. Based on the frequency, we initially selected 10 variant CP sequences whose frequencies were above 20. Considering that the chance of occurrence of a random sequence is 1/16,384, the frequency of each of the selected sequences was thus more than 30 times compared to random chance. Among the 10 Cys mutants, four mutants (Cys mutants 1A, 2, 3 and 5) were chosen for further study primarily based on the CP expression level. The nucleotide and amino acid sequences of the mutants are shown in Fig. 2A. As for Cys 1A, the sequence upstream
of the ACTAGT sequence (SpeI recognition site) was found to be changed from GAC to GCC. This resulted in a change in amino acid from Asp to Ala. This substitution was not observed in other Cys mutants. Therefore, the change was not derived from the primer used in the mutant library construction. Rather, it would indicate natural variation (mutation) and selection.
Figure 1. TYMV Cys mutant library. (A) Library construction. A random sequence of three amino acids and one cysteine was introduced to the C-terminal part of TYMV CP by PCR-amplification and insertion of the PCR product into XmaI/EcoRI sites of TY-eGFP26203 (for further details, see MATERIALS AND METHODS). The C-terminal extension is highlighted in bold face. (B) Screening of a Cys mutant library. A. tumefaciens containing the library was introduced to N. benthamiana. In a week, RNA was extracted from the inoculated leaf and the TYMV CP sequence was amplified by RT-PCR. The amplified DNA was used to generate the 2nd library, which was introduced again to N. benthamiana. After this process was repeated, the TYMV CP sequence was examined by high-throughput sequencing analysis using encapsidated TYMV RNA. (C) Examination of CP. Leaf extract was prepared in the presence (+) or absence (-) of 20 mM β-mercaptoethanol (β-ME) from the leaf inoculated with the 3rd library or wild-type TYMV. 2 μl of each leaf extract sample (1:10 diluted sample for wildtype) were loaded onto 12.5% SDS- polyacrylamide gel. After electrophoresis, the proteins were examined by Western blot analysis using TYMV CP antibody.
JBV
Bacteriology and Virology VOL 48. NO 4. DECEMBER 2018Number Nucleotide Sequence Amino acid sequence Occurrence*
1A GCCACTAGTGGTGGTCGTCCTTGTCTTTGAATTCAG A T S G G R P C L Stop 182
2 GACACTAGTGGTGGTCGTCCTTGTTAATGAATTCAG D T S G G R P C Stop 77
3 GACACTAGTGGTGGTCATCCTTGTACCTGAATTCAG D T S G G H P C T Stop 48 5 GACACTAGTGGTGGTCGTGCTTGTTCATGAATTCAG D T S G G R A C S Stop 47
*Occurrence among the 11,584 CP sequences analyzed.
Figure 2. Cys mutants. (A) Sequence of the Cys mutants. Nucleotide and amino acid sequences of Cys mutants are shown. In Cys1A, the nucleotide sequence upstream of the ACTAGT is GCC, whereas, in other mutants, the sequence is GAC. (B) Western blot analysis of CP in leaf extracts. 1 μg of total protein for each sample was loaded onto 12.5% SDS-polyacrylamide gel. After electrophoresis, the proteins were examined by Western blot analysis using TYMV CP antibody. (C) Northern blot analysis of TYMV RNA. 500 ng of total RNA were size-fractionated in a 1% agarose gel and examined by Northern blot analysis using the DIG-labeled TYMV CP DNA as a probe. The blots were developed by chemiluminescent immunodetection of DIG. Upper and lower arrowheads indicate genomic and subgenomic RNAs of TYMV, respectively. The panel below the Northern blot shows 25S rRNA stained with EtBr. (D) Systemic infectivity of the Cys mutants. Chinese cabbage was inoculated with the TYMV constructs containing wildtype or the Cys mutants, and young systemically infected leaf (5th leaf from the inoculated leaf) was collected two weeks post-inoculation. Total protein (1 μg) in the leaf extract prepared in the presence of β-ME was examined for its CP levels by Western analysis as described in Fig. 2B.
after 7 days using the Western blot analysis of CP expression (Fig. 2B). Here, protein concentration was determined by Bradford assay for each leaf extract, and equal amounts in terms of protein were loaded into the SDS-polyacrylamide gel. The results showed that the Cys mutants produced nearly as much CP as wildtype. Cys mutants 1A and 2 showed a strong '40 kDa' band in the absence of β-ME in the extraction buffer. The intensity of the '40 kDa' band decreased in the presence of β-ME. In the cases of Cys 3 and Cys 5, the '40 kDa' bands were about the same, irrespective of the presence or absence of β-ME; however, an increased amount of β-ME or TCEP was found to lower the level of the upper band (data not shown). The analysis of leaf RNA by Northern blot hybridization using DIG-labeled TYMV CP DNA showed that the Cys mutants replicated nearly as efficiently as wildtype (see Fig. 2C).
Systemic infectivity and cysteine reactivity of Cys mutants
We tested whether the Cys mutants were able to infect systemically. To this end, we inoculated Chinese cabbage, a natural
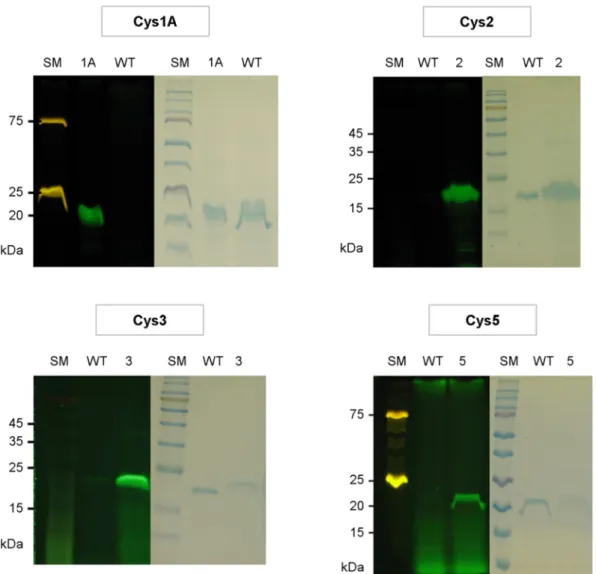
Figure 3. Cysteine reactivity. Wildtype (WT) and Cys mutants were reacted with fluorescein-5-maleimide, and analyzed by SDS- PAGE. After viewing under a UV lamp (left panels), the gel was stained with Coomassie (right panels). Two different size markers were used, and some components (75 kDa and 25 kDa) of the marker used with Cys 1A and 5 were fluorescent.
JBV
Bacteriology and Virology VOL 48. NO 4. DECEMBER 2018 host of TYMV, with the Cys mutants and examined young systemic leaves. Systemic mosaic symptom was observed in all the Chinese cabbage plants inoculated with Cys mutants at a similar timing as the one observed with wildtype (about 10 days after inoculation), although a slightly delayed symptom development was observed with Cys 1A mutant. The 5th leaf from the inoculated leaf was then analyzed for the presence of TYMV CP (see Fig. 2D). The results showed the presence of viral products in all cases, indicating that the CPs of all Cys mutants can assemble into intact virions and move systemically. However, CP levels of the Cys mutants were generally lower than those of the wildtype, especially in the case of Cys 1A mutant. This finding is not consistent with the result obtained in N. benthamiana (see Fig. 2B). Possible explanations for this discrepancy will be discussed in the Discussion section.We then examined the cysteine reactivity of the Cys mutants. To this end, conjugation reaction with fluorescein-5-maleimide was performed. The Cys mutant viral particles were purified by two rounds of PEG precipitation. After purification, the viral particles were kept in 10~20 mM TCEP until conjugation reaction, since, as demonstrated by Wang et al. (4), TCEP provides stabilization of cysteine-exposed virus particles and does not inhibit the reaction with maleimides. The TYMV particles were made react with a 25-fold molar excess of fluorescein-5-maleimide, and the unreacted dyes were removed using a 7 K Zeba spin desalting column. Thereafter, the reaction products were examined by SDS-PAGE. UV light irradiation of the Cys mutant samples showed a bright green fluorescence, whereas a very weak fluorescence was observed in the case of wildtype (Fig. 3, left panels).
The same gel stained with Coomassie showed that the fluorescent band corresponds to 20 kDa TYMV coat protein (Fig. 3, right panels). This suggests that the newly introduced cysteine residue was highly reactive to fluorescein-5-maleimide in all Cys mutants.
DISCUSSION
Native TYMV has four Cys residues, all of which are not very reactive. Since some cysteine residues are present inside the virus particles (14), they are not reactive. One cysteine residue is predicted to be on the surface. Obviously, however, this one too is nearly unreactive, probably because it is buried in the valley on the surface. The results obtained in the present study show that all tested Cys mutants are very reactive to fluorescein-5-maleimide. This clearly shows that the C-terminus of the CP subunit is exposed to solvent environment and can be freely accessed. It also suggests that the amino acid residues around the newly added cysteine are not of great importance in terms of the reactivity of the cysteine. In general, the C-terminal extension does not seem to negatively affect the CP assembly. Although Bransom et al. (16) reported that attempts to purify the mutant particles with five amino acid C-terminal extension by PEG precipitation were unsuccessful, the PEG precipitation worked well in our experiments, indicating that surface property of our mutants was not significantly changed with the extension.
The low CP level in Chinese cabbage systemic leaf and delayed symptom development observed with Cys 1A suggest that Cys 1A virions might be unstable, as in the case of the mutant reported by Bransom et al. (16) who showed that the C-terminal extension of five amino acids resulted in less stable virions and that the CP level in young systemically infected leaf was ca. 30%
compared to that of wildtype. The overall decrease of the CP levels might also indicate a defect in the systemic spread of some Cys mutants. This could be due to the presence of reactive thiol groups on virus surface. Long-distance movement of plant viruses involves translocation from mesophyll cells to sieve elements via successive crossings of small openings like plasmodesmata (17). TYMV seems to move systemically in the form of virus particles, since, as demonstrated by Bransom et al. (16), systemic spread does not occur without CP synthesis. Therefore, the aggregation of viral particles by interviral cross-linking through disulfide linkages, even though it is not extensive, could hamper systemic spread. With regard to this hypothesis, it is noteworthy that Cys 1A sample showed a distinct 'slowly moving' band in agarose gel, indicating the presence of some cross-linked viral particles.
Alternatively, the low level of CP in Chinese cabbage could be ascribed to innate immunity elicited by the mutants. Previous research has demonstrated that insertion of a foreign sequence into TYMV genome severely restricts viral replication, and co-expression of an RNAi suppressor p19 relieves the inhibition (18). Although, in this study, p19 construct was co-introduced
of Chinese cabbage. This difference could have resulted in the low level of CP in Chinese cabbage.
The Cys mutants obtained in the present study would be very useful in drug delivery. Drugs or fluorescent dyes have been conjugated to or loaded into nanoparticles derived from plant viruses in several different ways. For example, an anticancer drug doxorubicin was encapsulated into hibiscus chlorotic ringspot virus (19) by in vitro reconstitution in the presence of the drug or covalently bound to external surface carboxylates of the CPMV viral nanoparticle (20). The drug-loaded or drug-conjugated viral nanoparticles would be further modified for efficient drug delivery. Cancer-specific ligand would also be used for targeting.
Finally, plant viral nanoparticles need to be decorated with cell penetrating peptide to make them enter mammalian cells. This requires the presence of as many diverse functional groups as possible on the surface of nanoparticles. The contribution of our results is that thiol groups are now available, which adds high-utility functional groups to TYMV delivery vectors.
ACKNOWLEDGMENT
The present study was financially supported by the Research Year of Chungbuk National University in 2016.
REFERENCES
1) Dreher TW. Turnip yellow mosaic virus: transfer RNA mimicry, chloroplasts and a C-rich genome. Mol Plant Pathol 2004;
5:367-75.
2) Steinmetz NF, Evans DJ. Utilization of plant viruses in bionanotechnology. Org Biomol Chem 2007;5:2891-902.
3) Yildiz I, Shukla S, Steinmetz NF. Applications of viral nanoparticles in medicine. Curr Opin Biotechnol 2011;22:901-8.
4) Wang Q, Lin T, Tang L, Johnson JE, Finn MG. Icosahedral virus particles as addressable nanoscale building blocks. Angew Chem Int Endl 2002;41:459-62.
5) Steinmetz NF. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomedicine 2010;
6:634-41.
6) Barnhill HN, Reuther R, Ferguson PL, Dreher T, Wang Q. Turnip yellow mosaic virus as a chemoaddressable bionanoparticles.
Bioconjug Chem 2007;18:852-9.
7) Michels B, Leimkühler M, Lechner MD, Adrian M, Lorber B, Witz J. Polymorphism of turnip yellow mosaic virus empty shells and evidence for conformational changes occurring after release of the viral RNA. A differential scanning calorimetric study. Eur J Biochem 1999;264:965-72.
8) Katouzian-Safadi M, Berthet-Colominas C. Evidence for the presence of a hole in the capsid of turnip yellow mosaic virus after RNA release by freezing and thawing. Decapsidation of turnip yellow mosaic virus in vitro. Eur J Biochem 1983;137:
47-53.
9) Kim D, Lee Y, Dreher TW, Cho TJ. Empty Turnip yellow mosaic virus capsids as delivery vehicles to mammalian cells. Virus Res 2018;252:13-21.
10) Rohovie MJ, Nagasawa M, Swartz JR. Virus-like particles: Next-generation particles for targeted therapeutic delivery. Bioeng
JBV
Bacteriology and Virology VOL 48. NO 4. DECEMBER 2018 Transl Med 2017;2:43-57.11) Schoonen L, van Hest JC. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale 2014;6:
7124-41.
12) Shin HI, Cho TJ. A sequence in coat protein open reading frame is required for Turnip yellow mosaic virus replication. J Bacteriol Virol 2011;41:109-16.
13) Cho TJ, Dreher TW. Encapsidation of genomic but not subgenomic Turnip yellow mosaic virus RNA by coat protein provided in trans. Virology 2006;356:126-35.
14) Canady MA, Larson SB, Day J, McPherson A. Crystal structure of turnip yellow mosaic virus. Nat Struct Biol 1996;3:771-81.
15) Shin HI, Chae KH, Cho TJ. Modification of Turnip yellow mosaic virus coat protein and its effect on virion assembly. BMB Rep 2014;46:495-500.
16)Bransom KL, Weiland JJ, Tsai CH, Dreher TW. Coding density of the turnip yellow mosaic virus genome: roles of the overlapping coat protein and p206-readthrough coding regions. Virology 1995;206:403-12.
17) Hipper C, Brault V, Ziegler-Graff V, Revers F. Viral and cellular factors involved in phloem transport of plant viruses. Front Plant Sci 2013;4:154.
18) Shin HI, Kim IC, Cho TJ. Replication and encapsidation of recombinant Turnip yellow mosaic virus RNA. BMB Rep 2008;
41:739-44.
19) Ren Y, Wong SM, Lim LY. Folic acid-conjugated protein cages of a plant virus: a novel delivery platform for doxorubicin.
Bioconjug Chem 2007;18:836-43.
20) Aljabali AA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ. CPMV-DOX delivers. Mol Pharm 2013;10:3-10.