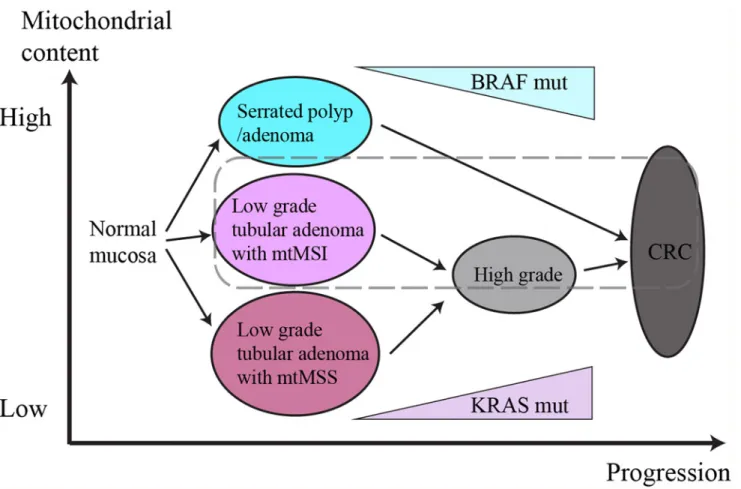
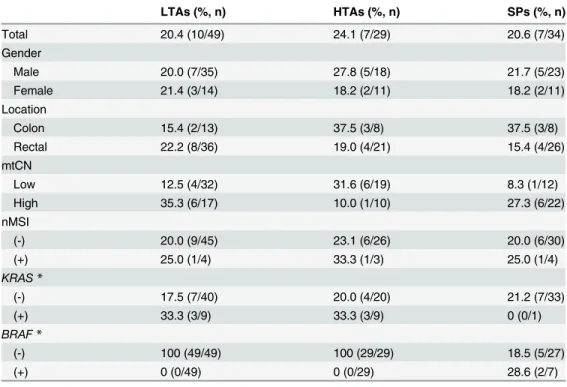
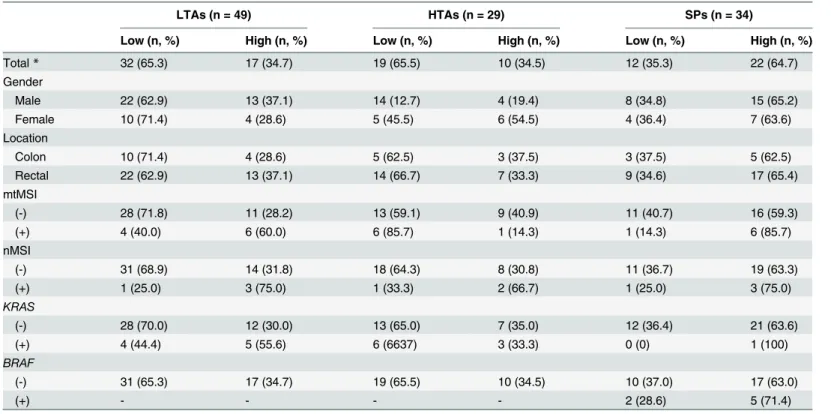
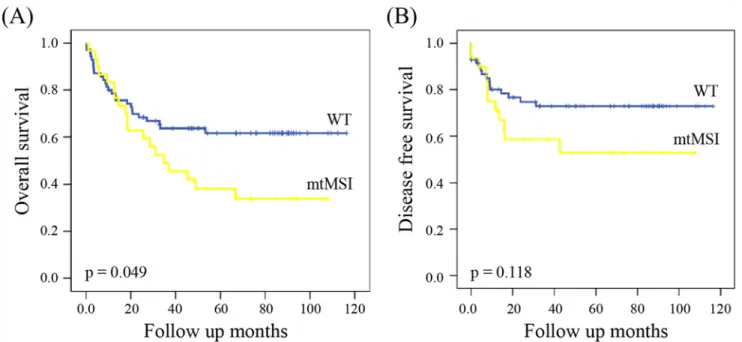
Genetic Characteristics of Mitochondrial DNA Was Associated with Colorectal Carcinogenesis and Its Prognosis
전체 글
수치

관련 문서
To examine the characteristics of its cutting edge, the material was trimmed to make a rectilinear cutting edge, and in addition to the cutting edge of the
In this study, water flow system of Danghang Bay, which has the narrow and long topographical characteristics with the narrow bay mouth and its flow
GDP impact of COVID-19 spread, public health response, and economic policies. Virus spread and public
Chapter 3 proposes the educational values and significance of chalk art through viewing its characteristics in public interest and comparing the example
We determined the nucleotide sequences of the mitochondrial DNA (mtDNA) control region using cloning and sequencing, and obtained the complete sequence from the cattle bones
Seed germination, cutting propagation and early growth characteristics were investigated in order to develop the method of cutting propagation and to find
In this study, we found that Pin1 plays a pivotal role in insulin- induced AP-1 activation through its interaction with p70S6K and prolongs activity of
The aim of this study was to determine the serum levels of the prohormone form of hepcidin, pro-hepcidin, and its relations with iron status and its