학 술 논 문
http://dx.doi.org/10.9718/JBER.2021.42.2.62
62
Hollow Poly(lactic acid) and Poly(lactic acid)/polyethylene glycol
Microspheres Prepared by A Simple Fluidic Device
Dayeon Jeong, Heeseok Jeong and Deuk Yong Lee*
Department of Biomedical Engineering, Daelim University
(Manuscript received 30 March 2021 ; revised 22 April 2021 ; accepted 23 April 2021)
Abstract: Hollow poly(lactic acid) (PLA) and PLA/polyethylene glycol (PLA/PEG) microspheres using 1 wt% poly-vinyl alcohol (PVA) emulsifier (continuous phase) are prepared by using tridecane (TD) as porogen in an oil-in-water emulsion using a simple fluidic device. PLA and PLA/PEG solutions in a mixture of DCM and TD were introduced into the fluidic device as a discontinuous phase. The average size of the PLA microspheres increased from 181±1.2 µm to 274±3.7 µm with increasing TD concentration from 0 to 8 wt%. PLA microspheres prepared without TD had few pores on the surface of the microspheres filled inside, while bimodal surface pores and hollow interior were observed for the PLA microspheres containing TD. As PEG was added to PLA, the size of microspheres and pores increased from 196±0.8µm to 297±12.6 µm and from 5.0±2.7 µm (unimodal distribution) to 4.3±2.0 µm to 36.8±13.9µm (bimodal distribution) with increasing TD concentration from 0% to 8%. The interior was changed from filled to hollow for PLA/PEG microspheres containing TD concentrations from 2% to 8%. PLA microspheres prepared with 6 wt% TD and PLA/PEG spheres with 4 wt% TD were determined as suitable candidates for fibroblast ingrowth. PLA and PLA/PEG microspheres with large void in the center of microsphere and bimodal surface pores can be effective for penetration and proliferation of host tissue.
Key words: Poly(lactic acid), Polyethylene glycol, Tridecane, Microsphere, Emulsion-solvent evaporation technique
I. Introduction
The drug-loaded microspheres can be injected into malfunctioned tissues and organs as stem cell carriers, or into small damaged and defective tissues as fillers to solve biomedical and cosmetic problems [1-6]. In addi-tion to biocompatibility, microsphere’s ability to encapsu-late and promote sustained release of drugs or growth factor makes them an ideal carrier for the transport of bioactive molecules for tissue regeneration and con-trolled drug delivery applications [2]. Large scaffold-centered cell necrosis and accumulated metabolic wastes due to oxygen and nutrient deficiency can be solved with the introduction of injectable porous microspheres.
Although porous microspheres are synthesized by var-ious methods, such as lyophilization [1], air incorpo-ration [3-5], emulsion-solvent evapoincorpo-ration [7,8], and thermally induced phase separation [9-11], they suffer from complex process and pore size control. The inter-connectivity and tailored porosity of the microspheres provide greater surface area, lower mass density, excel-lent cell attachment, cell proliferation, drug uptake and drug release kinetics [2]. Porous microspheres with a uniform size distribution and adjusted porosity are prepared by the oil-in-water emulsion-solvent evapo-ration technique (ESE) using a magnetic stirrer [12], a high-speed agitator [7,8], or a simple fluidic device [1,2,13,14]. Among the ESE methods, emulsification is achieved using a simple fluidic device to obtain uni-form microspheres with simple and easy pore size control. After the drug release through the pores or polymer matrix to the surroundings, the absorption of water causes swelling in the microspheres and the forma-tion of new pores, which increased the diffusion rate and chances of erosion [2].
*Corresponding Author : Deuk Yong Lee
Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
Tel: +82-31-467-4835
E-mail: duke1208@gmail.com
This work was supported by the Materials & Components Technology Development Program (Project No. 20003560), funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
63 properties such as good hydrophilicity, flexibility,
antiphago-cytosis against macrophage, resistance to immunological recognition, non-combination with proteins, and bio-compatibility [7,8,10]. Unlike copolymer synthesis, the blending technique is simple, easy, and inexpensive. Poly-mer blends offer the benefits of a wide range of mechan-ical properties and degradable rates by varying the PLA/PEG composition ratio. PLA and PEG are widely used as shell materials in microcapsules with proper composition ratio [8]. The microencapsulation of drug with PLA and PLA/PEG can be carried out by the oil-in-water (O/W) ESE technique using a high-speed agi-tator and a syringe pump [7,8]. The oil phase is formed by mixing PLA, PLA/PEG, nifedipine (NF), and dichloro-methane (DCM) and then dispersed in a solution of poly-vinyl alcohol (PVA) emulsifier to obtain microcapsules with controlled drug delivery. Our previous studies have shown that PCL, PLA, PCL/PEG, PCL/polyvinyl pyr-rolidone (PVP), and PLA/PEG capsules with a diam-eter of 1 to 5 µm with nifedipine (NF) can be obtained by ESE technique through proper adjustment of pro-cess parameters such as polymer concentration and stirring speed [7,8]. For fibroblast activity, pore size ranges of 5 to 15 µm and 40 to 150 µm are the best con-ditions for fibroblast ingrowth and binding [1]. The diameter of the porous microspheres need to be con-trolled for practical application because the diffusion of oxygen and nutrients is limited to several hundred micrometers. The microsphere size is controlled by cell type [2,6]. It is also reported that one large hollow pore in the center of the PLLA microsphere is created with the small pores when 6 wt% of alkane is applied [1]. The hollow void in the center of microsphere and its surface pores serve as pathways for cell migration and diffusion of oxygen and nutrient [6]. Alkane molecules
II. Experimental Details
PLA (Ingeo Biopolymer 4044D) was supplied by Nature-Works (USA). TD (CH3(CH2)HCH3) was purchased from
Sigma-Aldrich. PEG (Mw=20,000) and dichlorometh-ane (DCM, 99.5%, CH2Cl2) were purchased from Sam-chun Pure Chemical (Korea). All chemicals are used as received without further purification. The 2 wt% PLA solutions dissolved in DCM were prepared by stirring for 24 h at a speed of 300 rpm to obtain homogeneous solution. PLA and PLA/PEG (9/1) solutions were pre-pared. PVA dissolved in distilled water was refluxed for 2 h at 500 rpm and 80oC to obtain a viscous homo-geneous solution. The fluidic device consisted of a Tygon tube (1/16 in. inner diameter x 1/8 in. outer diameter), glass capillary (44.7 mL, ringcaps, Hirschmann, Germany), and 16 G and 30 G needles, as depicted in Fig. 1. The dis-continuous and dis-continuous phases were fed to a 30 G metal needle at a flow rate of 0.01 mL/min and the 16 G blunt-end metal needle at a flow rate of 1 to 20 ml/min, respectively. The discontinuous and the continuous phases placed in 5 and 50 mL B-D luer-lok syringes attached to a syringe pump (KDS-200, Stoelting Co., USA) were fed into a beaker containing the continuous phase. The collected droplets (yellow colored circle in Fig. 1(a)) were gently stirred at 50 rpm overnight using an overhead stirrer to evaporate the solvent. The droplets were then cleaned 3 times in ethanol solution and distilled water to remove the remnant solvent [1,7,8]. Then, they were lyophilized.
The as-dried microspheres were examined by using a scanning electron microscope (SEM, S-3000H, Hitachi, Japan) and an optical microscope (SV-55, Sometech, Korea) equipped with iSolution Lite image software to investigate the size and morphology [7,8,12]. For the
64
SEM observation, the microspheres were sputtered with Au/Pd to ensure higher conductivity. The chemical properties of the capsules are analyzed by using FT-IR (Spectrum Two, PerkinElmer, UK). All experiments are carried out in triplicate. Values in the text are stated as the mean±standard deviation, and p < 0.05 is weighed statistically significant [7,8].
Cytotoxicity was quantitatively evaluated by perform-ing an extraction test method on PLA and PLA/PEG microspheres according to the International Organiza-tion for StandardizaOrganiza-tion (ISO 10993-5) [9-12]. The PLA and PLA/PEG microcapsules were extracted aseptically in single strength Minimum Essential Medium with serum. The ratio of the microcapsules to extraction vehi-cle was 0.2 g/mL (ISO 10993-12). The test extracts were placed onto 3 separate confluent monolayers of NIH-3T3 fibroblasts (Korea Cell Line Bank, Korea) propagated in 5% CO2. EZ-cytox yielded a water-soluble formazan, which can provide different absorption spectra of the formed formazans. The absorbance of the colored solution was quantified by measuring at a wavelength of 415 nm with the microplate absorbance spectrophotometer (Bio-Rad, USA) [9-12].
III. Results and Discussion
A schematic diagram and a photograph of a simple fluidic device are shown in Fig. 1. The discontinuous (PLA, PAL/PEG) and continuous phases (PVA) placed in 5 and 50 mL B-D luer-lok syringes attached to syringe pumps were fed into the beaker containing the continuous
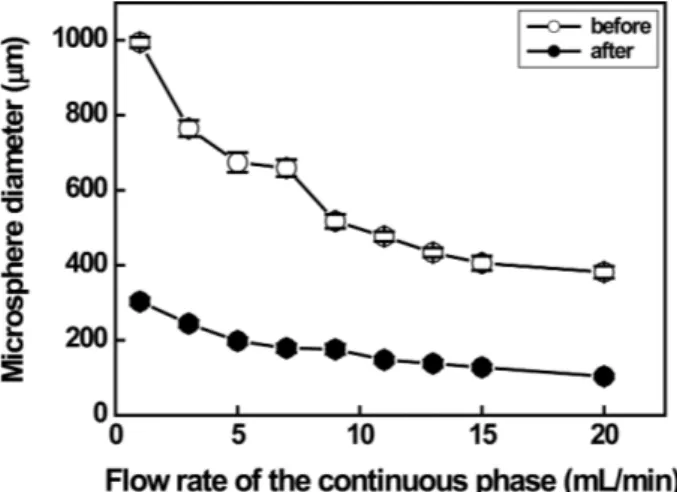
phase. The collected droplets were gently stirred at 50 rpm overnight using an overhead stirrer to evaporate the solvent. The droplets were washed in ethanol and distilled water. They were dried overnight in a vacuum oven to obtain microspheres. Flow rate of continuous phase as functions of PLA droplet and microsphere size is depicted in Fig. 2. The PLA droplet size can be adjusted by varying the polymer concentration and the flow rate of the continuous phase [1,6,13,16,17]. The size of drop-let before and after evaporation of solvent decreased gradually from 994µm to 381 µm and from 303 µm to 103µm, respectively, with increasing the flow rate of the continuous phase from 1 mL/min to 20 mL/min. The average diameters of PLA microspheres prepared at continuous flow rates of 1, 5, and 10 ml/min were 303,
Fig. 1. (a) A schematic diagram and (b) a photograph of a simple fluidic device based on a water-in-oil emulsion. Scale bar is 300μm
Fig. 2. The variation in size of 2 wt% PLA microspheres prepared in continuous phases at different flow rates before and after evaporation of the solvent. Note that the flow rate of the discontinuous phase was fixed at 0.01 mL/min
65
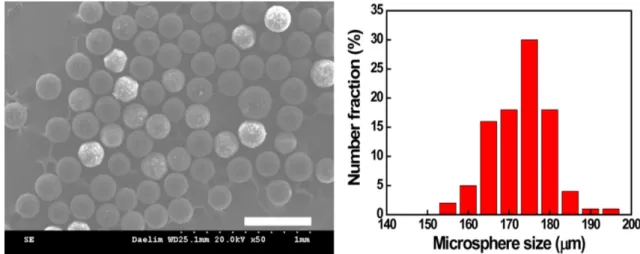
197, 169µm, respectively, as displayed in Fig. 3. When the flow rate was 10 mL/min, the coefficients of vari-ation (CVs) of droplets and microspheres, indicating uniformity in size, were 2.91% and 5.20%, respectively.
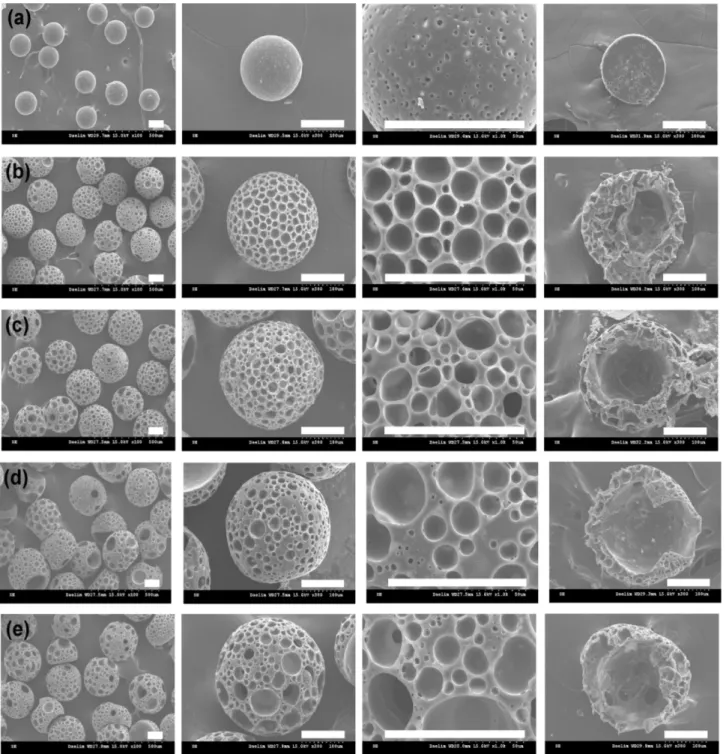
large hollow pore in the center and many small surface pores [1]. The bimodal porous structure of PLLA microbe-ads promoted penetration and proliferation of cells and host tissues. In this study, hollow PLA and PLA/PEG microspheres using 1 wt% PVA emulsifier (continuous phase) are prepared by using TD as porogen in a single oil-in-water emulsion using a simple fluidic device [6,13]. PLA and PLA/PEG solutions in a mixture of DCM and TD were introduced into the fluidic device as a discontin-uous phase. Surface and cross-sectional SEM images of PLA microspheres with a TD concentration of 0 to 8 wt% are shown in Fig. 5. The average size of PLA microspheres increased dramatically from 181±1.2µm to 216.8±13.4 µm, 247.2±9.3µm, 275.3±8.4 µm, and 297±13.4 µm as the TD concentration increased from 0 to 2, 4, 6, and 8 wt%. PLA microspheres prepared without TD had a few pores with a diameter of 3.0±1.1µm on the surface of the micro-spheres filled inside, whereas in PLA micromicro-spheres con-taining TD, bimodal surface pores and a hollow interior were observed [1]. Most fibroblasts have been reported to proliferate on the surface of microspheres with
uni-Fig. 3. Optical photographs of the PLA emulsion droplets and SEM images of the PLA microspheres prepared at different continuous flow rates of (a) 1 ml/min, (b) 5 ml/min, (c) 10 ml/min, and (d) 15 ml/min, respectively. Scale bar is 100μm
Fig. 4. (a) SEM images and (b) size distribution of porous PLA microsphere prepared at continuous flow rate of 10 ml/min. Scale bar is 500μm
66
modal porous structure. However, cells seeded into micro-spheres with a bimodal porous structure are known to grow prominently throughout the microspheres [1,6,13]. The bimodal surface pore size increased gradually from 4.0±1.3µm and 13.6±2.8 µm to 8.5±5.5 µm and 39.7± 11.3µm as the TD concentration rose from 2% to 8%. The bimodal pore sizes of PLA microspheres with 4 and 6 wt% TD were 4.5±1.7µm to 18.6±6.7 µm and 6.5±1.9
µm to 25.4±7.9 µm, respectively. The size of the hollow interior increased dramatically from 62µm to 204 µm with increasing TD concentration from 2% to 8%, as depicted in Fig. 5. Although microspheres with 8 wt% TD exhibited the largest surface and internal pores, they were easily crushed due to poor structural integ-rity. The PLA microspheres prepared with 6% TD were determined to be a suitable candidate for fibroblast
Fig. 5. SEM images of surface and cross-section of porous PLA microspheres as a function of tridecane concentration of (a) 0 wt%, (b) 2 wt%, (c) 4 wt%, (d) 6 wt%, and (e) 8 wt%, respectively. Scale bar is 100μm
67
Fig. 6. SEM images of surface and cross-section of porous PLA/PEG microspheres as a function of TD concentration of (a) 0 wt%, (b) 2 wt%, (c) 4 wt%, (d) 6 wt%, and (e) 8 wt%, respectively. Scale bar is 100μm
68
with TD concentrations increasing from 2% to 4%, 6%, and 8%, respectively, as illustrated in Fig. 6. The bimodal pore sizes of PLA/PEG microspheres with 4 and 6 wt% TD were 6.5±2.2µm to 19.1±6.1 µm and 7.9±5.0 µm to 22.3±8.2µm, respectively. The PLA/PEG microspheres prepared with 4% TD were determined as suitable can-didate for fibroblast behaviors. The CVs of the porous PLA (6wt% TD) and PLA/PEG (4wt% TD) microspheres were 3% and 3%, respectively, exhibiting high size uniformity. Considering the pore characteristics, PLA microspheres prepared with 6wt% TD and PLA/PEG microspheres with 4 wt% TD were determined as the best candidates for cell seeding and fibroblast ingrowth. PLA and PLA/PEG porous microbeads with a bimodal porous structure have been reported to promote penetration and proliferation of host tissue inside the microbeads, which has been demonstrated with the aid of cell culture assays using the spinner flask [1]. Large, well-connected bimodal pores are known to promote cell penetration and promote free diffusion of nutrients and metabolic wastes to improve cell proliferation into PLA and PLA/PEG microspheres [6,13]. Large void interiors in the center of PLA and PLA/PEG microspheres are attributed to the penetra-tion and proliferapenetra-tion of host tissue. However, PLA and PLA/PEG microspheres with a TD of 8 wt% were excluded due to poor mechanical property.
FT-IR spectra of PLA and PLA/PEG blends with dif-ferent concentration of TD are illustrated in Fig. 7. The peak intensity of C=O stretching located at 1752 cm-1, C-H bending at 1458 cm-1, and C-O-C stretching pres-ent at 1081 cm-1 increased dramatically compared to neat PLA due to the presence of TD, as depicted in Fig. 7(a). TD is an alkane with the chemical formula of C13H28. The PLA spectrum exhibited the main peak at 1752 cm-1 corresponding to C=O stretching for carboxylic acid (R-COOH) in PLA. Peaks at 1081 and 1182 cm-1 correspond-ing to C-O-C stretchcorrespond-ing were clearly visible. Peaks located at 1366 and 1458 cm-1 corresponded to CH2 bending. Peak at 2920 cm-1 related to -CH stretching was also observed. The hydroxyl groups (-OH) in PEG molecules react with the carboxyl groups (-COOH) in PLA molecules [8]. Char-acteristic PEG peaks of 1458, 1340, 1099, 957, and 840 cm-1 were clearly observed in both spectra of PLA/PEG and PEG, indicating that the presence of PEG struc-ture in the PLA/PEG blends [8,18-22]. The shift in the
peak of 1081 cm-1 towards lower frequency compared to peak in 1099 cm-1 for PEG was attributed to the binding of C-O-C groups [19]. The shift in the peak at 865 cm-1 towards higher frequency compared to 840 cm-1 was attributed to the ether bonding from PEG chains [18-22]. It is evident that PEG has very good miscibility with PLA because the terminal hydroxyl groups in PEG ecules can react with the carboxyl groups in PLA mol-ecules [20-22].
A cytotoxicity test of PLA and PLA/PEG capsules deter-mines whether a product or compound may have an adverse effect on tissues or cells [7-11]. The test extracts with PLA and PLA/PEG capsules showed no evidence of causing cytotoxicity regardless of the presence of TD. All exper-iments were performed in triplicate. PLA and PLA/PEG capsules exhibited quantitative cell viability of 87 to
Fig. 7. FT-IR spectra of (a) PLA and (b) PLA/PEG microcapsules containing different TD contents
69 108% compared to the negative control, as depicted in
Fig. 8. This was measured at a wavelength of 415 nm by using iMark microplate absorbance spectrophotom-eter [7-11]. The cell viability of PLA and PLA/PEG cap-sules containing different TD concentrations was higher than 87%. Therefore, PLA and PLA/PEG capsules with-out cytotoxicity were considered clinically safe.
IV. Conclusions
Hollow PLA and PLA/PEG microspheres using 1 wt% PVA emulsifier are prepared by ESE technique in an oil-in-water emulsion using a simple fluidic device. PLA and PLA/PEG solutions in a mixture of DCM and TD were used as a discontinuous phase. The size uniformity of emulsion droplets and PLA microspheres was optimized at a PVA flow rate of 10 ml/min. The average size of the PLA microspheres increased gradually with increasing TD concentration from 0 to 8 wt%. PLA microspheres prepared without TD had few pores (unimodal distri-bution) on the surface of the microspheres filled inside, while bimodal surface pores and hollow interiors were observed for the PLA microspheres containing TD. As PEG was added to PLA, the size of microspheres and pores increased from 196±0.8µm to 297±12.6 µm and from 5.0±2.7µm (unimodal distribution) to 4.3±2.0 µm to 36.8±13.9µm (bimodal distribution) with increasing TD concentration from 0% to 8%. The interiors were changed from filled to hollow for PLA/PEG microspheres containing TD concentrations from 2% to 8%. PLA micro-spheres prepared with 6 wt% TD and PLA/PEG micro-spheres
[1] Baek SW, Moon S, Kang RH, Ah Y, Kim H, Choi W. One-step fabrication of uniform biodegradable microbeads with unimodal and bimodal porous structures using spontaneous microphase separation. Macromol. Mater. Eng. 2018;1800139. [2] Jun Y, Oh H, Karpoormath R, Jha A, and Patel R. Role of
microsphere as drug carrier for ostegenic differentiation. Intl. J. Polym. Mater. Polym. Biomater. 2021;70;318-27. [3] Chun C, Lee DY, Kim J, Kwon M, Kim Y, Kim S. Effect of
molecular weight of hyaluronic acid (HA) on viscoelasticity and particle texturing feel of HA dermal biphasic fillers. Bio-mater. Res. 2016;20;275-81.
[4] Kim J, Lee DY, Kim E, Jang J, Cho N. Tissue response to implant of hyaluronic acid hydrogel prepared by microbeads. Tissue Eng. Reg. Med. 2014;11;32-8.
[5] Kim J, Lee DY, Kim T, Lee M, Cho N. Biocompatibility of hyaluronic acid hydrogel prepared by porous hyaluronic acid microbead. Met. Mater. Intl. 2014;20;555-63.
[6] Kim JH, Ryu T, Moon S, Lee J, Park K, Kim SE, Choi SW. Fabrication of poly(L-lactide) porous beads coated with hydroxy-apatite using a simple fluidic device for tissue engineering. Macromol. Res. 2015;23;501-4.
[7] Lim H, Shin J, Lee DY, Kim B, Song Y. Drug delivery behavior of PCL and PCL/PEG microcapsules prepared by high-speed agitator and syringe pump. Polym. (Korea) 2020;44;487-94. [8] Jeong H, Lim H, Lee DY, Song Y, Kim B. Preparation and drug release behavior of nifedipine-loaded poly(lactic acid)/ polyethylene glycol microcapsules. J. Nanosci. Nanotechnol. 2021;21(7);3735-41.
[9] Shin J, Jeong H, Lee DY. Synthesis of PVA/NaCMC hydro-gels crosslinked by cyclic freezing/thawing and subsequent gamma-ray irradiation and their properties. J. Biomed. Eng. Res. 2018;39(4);161-7.
[10] Shin J, Lee DY, Kim B, Yoon JI. Effect of polyethylene glycol molecular weight on cell growth behavior of polyvinyl alcohol/ carboxymethyl cellulose/polyethylene glycol hydrogel. J. Appl. Polym. Sci. 2020;137(48);49568.
[11] Shin J, Lee DY, Yoon JI, Song Y. Effect of CMC concentration on cell growth behavior of PVA/CMC hydrogel. Macromol. Res. 2020;28(9);813-9.
[12] Lee H, Lee DY, Song Y, Kim B. Poly(ε-caprolactone) micro-capsule with encapsulated nifedipine prepared by magnetic stir-rer. J. Biomed. Eng. Res. 2019;40;7-14.
[13] Choi S, Cheong IW, Kim J, Xia Y. Preparation of uniform microspheres using a simple fluidic device and their crystal-lization into close-packed lattices. Small. 2009;5;454-9. [14] Zhong F, Yang C, Wu Q, Wang S, Cheng L, Dwivedi P, Zhu Z, Fig. 8. Cell viabilities of PLA and PLA/PEG microcapsules
70
Si T, Xu RX. Preparation of pesticide-loaded microcapsules by liquid-driven coaxial flow focusing for controlled release. Intl. J. Polym. Mater. Polym. Biomater. 2020;69;840-7. [15] Jeong J, Rho J, Shin J, Lee DY, Hwang T, Kim KJ. Mechanical
properties and cytotoxicity of PLA/PCL scaffolds. Biomed. Eng. Lett. 2018;8(3);267-72.
[16] Choi S, Cheong I, Kim J, Xia Y. Preparation of uniform microspheres using a simple fluidic device and their crystal-lization into close–packed lattices. ACS Appl. Mater. Inter-faces. 2009;7;454-9.
[17] Ryu T, Jun D, Kim SE, Choi W. Sustained release of antibi-otics form uniform poly(e-caprolactone) microspheres prepared by a simple fluidic device with a tapered glass capillary. J. Bioact. Compatible Polym. 2014;29(4);318-29.
[18] Athanasoulia I, Tarantili PA. Preparation and characterization of polyethylene glycol/poly(L-lactic acid) blends. Pure Appl. Chem. 2017;89;141-52.
[19] Shameli K, Ahmad MB, Jazayeri SD, Sedaghat S, Shaban-zadeh P, Jahangirian H, Mahdavi M, Abdollahi Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Intl. J. Mol. Sci. 2012; 13;6639-50.
[20] Lee Y, Chang D. Fabrication, characterization, and biological evaluation of anti-HER2 indocyanine green-doxorubicin-encap-sulated PEG-b-PLGA copolymeric nanoparticles for targeted photochemotherapy of breast cancer cells. Sci. Rep. 2017;7; 46688.
[21] Zhou W, Qian H, Yan L, Luo D, Xu N, Wu J. Controlled release of clodronate from PLA/PCL complex microsphere. Mater. Lett. 2015;152;293-7.
[22] Matta AK, Rao RU, Suman KNS, Rambabu V. Preparation and characterization of biodegradable PLA/PCL polymeric blends. Procedia Mater. Sci. 2014;6;1266-70.


![Fig. 8. This was measured at a wavelength of 415 nm by using iMark microplate absorbance spectrophotom-eter [7-11]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/4888517.36496/8.892.102.412.147.386/fig-measured-wavelength-using-imark-microplate-absorbance-spectrophotom.webp)