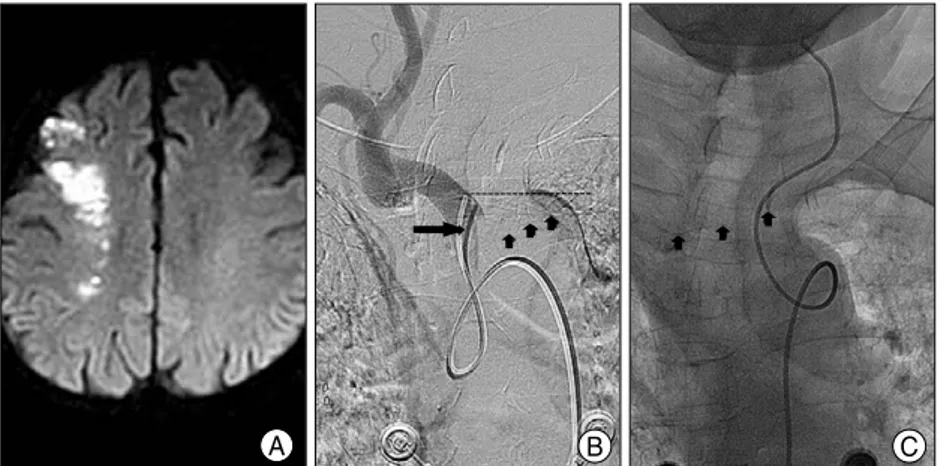
Contralateral Cerebral Infarction after Stent Placement in Carotid Artery : An Unexpected Complication
전체 글
수치
관련 문서
The locations of aneurysms were middle cerebral artery in 15 patients, cerebral artery in 15 patients, cerebral artery in 15 patients, cerebral artery in
Background : Janus tyrosine kinase 2 (JAK2) V617F mutation has been described in a high proportion of patients with Philadelphia
Recently, metal-assisted chemical etching (MACE) has been widely used for the synthesis of SiNWs, because this technique has advantages such as simplicity, low operating
In analyzing those textbooks, 3 main focuses have been observed; an aspect of external appearance, a musical aspect as a practice material, and an
Temperature sensor Angioplasty for canine common carotid artery. 0.6
• In an unbiased sampling scheme with total n samples for these two strata, nP(A) or na samples are used for stratum A and nP(R~A) or n(1-a) are used for stratum R~A..
- After selecting an element (vs. a nuclear species), the cross section for that element (vs. species) is used to determine which type of interaction has occurred.. -
• In an unbiased sampling scheme with total n samples for these two strata, nP(A) or na samples are used for stratum A and nP(R~A) or n(1-a) are used for