Autophagy localization and cytoprotective role in cisplatin-induced acute kidney injury
전체 글
수치
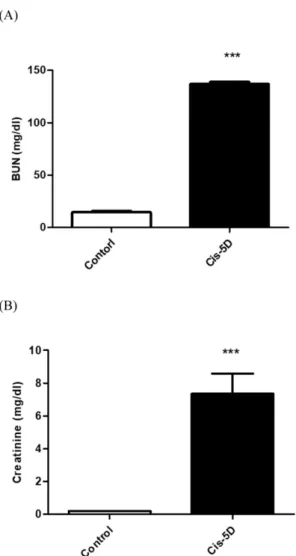
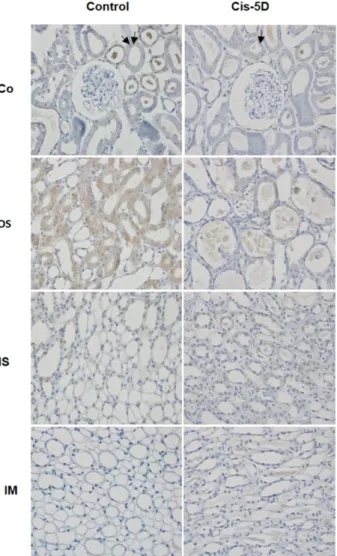

관련 문서
Results : The expression of p21 was increased in boderline serous tumor and serous cystadenocarcinoma in contrast to benign serous tumors. The expression of
The study was conducted to find out the relationship between teacher passion and intrinsic motivation and class attitude that was perceived by participants in physical
Effect of LRP6 on survivin expression in hypoxic cardiomyocytes (A) Expression of survivin in Ad-LRP6 and silencing LRP6 infected cardiomyocytes as determined by western
The combination treatment of PBA and 6-gingerol induced the cleavage of caspase-3, caspase-4, PARP, GRP78, p-eIF2α, and CHOP in DU145 cells and Western blot analysis for
Activation of Autophagy by Mangiferin Protects Auditory Hair Cells from Oxidative stress Induced Ototoxicity.. Gyeongmin
Taken together, these results suggested that latex containing the ficin inhibited the cell growth and induced apoptosis by caspase and Bcl-2 family signaling pathway in
This study aimed to evaluate the site and extent of injury, injury mechanism, player position, and the reinjury incidence in the hamstring by using magnetic
Based on numerical and experimental investigation results of the proposed H-S 3 HI strategy, the feasibility of harvesting the cooler-induced micro-vibration was demonstrated