소아 급성림프모구백혈병에서의 비전신방사선조사 조혈모세포이식
임영태
1
ㆍ이규호1
ㆍ김세윤1
ㆍ박선영1
ㆍ하정옥2
ㆍ이재민1
1
영남대학교 의과대학 소아과학교실,
2대구파티마병원 소아청소년과
Myeloablative Hematopoietic Stem Cell Transplantation with a Non-total Body Irradiation Regimen for Treating Pediatric Acute Lymphoblastic Leukemia
Young Tae Lim, M.D.
1, Kyu Ho Lee, M.D.
1, Saeyoon Kim, M.D., Ph.D.
1, Sun Young Park, M.D.
1, Jeong Ok Hah, M.D., Ph.D.
2and Jae Min Lee, M.D.
11
Department of Pediatrics, College of Medicine, Yeungnam University,
2
Department of Pediatrics, Daegu Fatima Hospital, Daegu, Korea
Background: Total body irradiation (TBI) has been traditionally used as a conditioning regimen prior to hematopoietic stem cell transplantation (HSCT) in patients with pediatric leukemia. However, TBI can cause late sequelae such as growth impairment, cataract, hormone abnormalities, infertility, neurocognitive effects, and secondary malignancy in pediatric patients.
Methods: This single center retrospective study included 22 patients with acute lympho- blastic leukemia who were aged <18 years and underwent HSCT between May 1999 and December 2014; seven patients received a TBI-based regimen and 15 received a non-TBI regimen.
Results: The overall survival and event-free survival rates in the TBI group were not significantly different from those in the non-TBI group (overall survival rate 71% vs. 73%, respectively; P=0.906; event-free survival rate 71% vs. 73%, respectively P=0.923).
Conclusion: Our results indicate that non-TBI conditioning regimens can be an alter- native treatment option of the treatment of pediatric acute lymphoblastic leukemia under- going HSCT.
pISSN 2233-5250 / eISSN 2233-4580 https://doi.org/10.15264/cpho.2017.24.1.55 Clin Pediatr Hematol Oncol 2017;24:55∼63
Received on March 15, 2017 Revised on March 31, 2017 Accepted on April 19, 2017
Corresponding Author: Jae Min Lee Department of Pediatrics, Yeungnam University Hospital, 170, Hyeonchung-ro, Nam-gu, Daegu 42415, Korea
Tel: +82-53-620-3530 Fax: +82-53-629-2252 E-mail: mopic@yu.ac.kr
ORCID ID: orcid.org/0000-0001-6822-1051
Key Words: Hematopoietic stem cell transplantation, Total body irradiation, Acute lym- phoblastic leukemia, Children
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy, constituting one-third of all pediatric cancers, and is the most frequent cause of cancer-related death in patients aged less than 20 years. Because of recent
advancements in ALL therapy, the overall survival (OS) rate
of patients with ALL has increased from <10% in the 1960s
to over 90% at present [1]. Treatment of pediatric ALL varies
depending on clinical features such as the biologic features
of leukemia cells and response to treatment. Combined
chemotherapy is used for treatment of patients with stand-
ard risk, whereas hematopoietic stem cell transplantation
(HSCT) is performed in case of relapse or high-risk patients, including those with t(4;11)(q21;q23), t(9;22)(q34;q11.2), hypodiploidy, and hyperleukocytosis [2,3].
Since its introduction about 50 years ago, HSCT has been established as an important therapeutic modality for various diseases such as hematological malignancies, and diseases related to bone marrow failure and immunodeficiency [4].
An HSCT course mainly consists of a conditioning phase followed by stem cell infusion. The conditioning phase re- moves cancer cells and simultaneously induces immuno- suppression for successful engraftment of stem cells. A myeloablative conditioning regimen, which consists of total body irradiation (TBI) and cyclophosphamide, is commonly used as a conditioning method for HSCT in pediatric ALL [5]. TBI has unique advantages over chemotherapy, such as a therapeutic effect on sanctuary sites such as the testes and central nervous system, a homogenous dose regardless of blood supply, and no extrication- or detoxifica- tion-associated challenges [6]. However, TBI can cause vari- ous adverse effects such as growth impairment, cataract, hormone abnormalities, infertility, neurocognitive effects, and secondary malignancy in pediatric patients [7-10]. Therefore, non-TBI-based conditioning methods have been developed;
conditioning regimens combining busulfan and other an- ti-tumor agents are commonly used as representative non-TBI-based regimens. Busulfan-based regimens do not induce the adverse effects associated with TBI-based regi- mens, but have a higher rate of relapse after transplantation than TBI-based conditioning regimens do[11]. In addition, busulfan-based regimens have a weak anti-tumor effect on sanctuary sites, including the testes, compared to TBI-based regimens. For pediatric patients with ALL, a conditioning regimen should be carefully selected after considering the advantages and disadvantages of TBI.
Results of previous studies have indicated that TBI-based conditioning regimens are superior to non-TBI-based con- ditioning regimens in terms of therapeutic outcome [5,12].
However, most of these studies were performed in patient populations that included both adults and children with various kinds of cancers. Therefore, in this study, we retro- spectively compared survival outcomes and late complica- tions between a TBI group and non-TBI group.
Materials and Methods
1) Patient eligibility
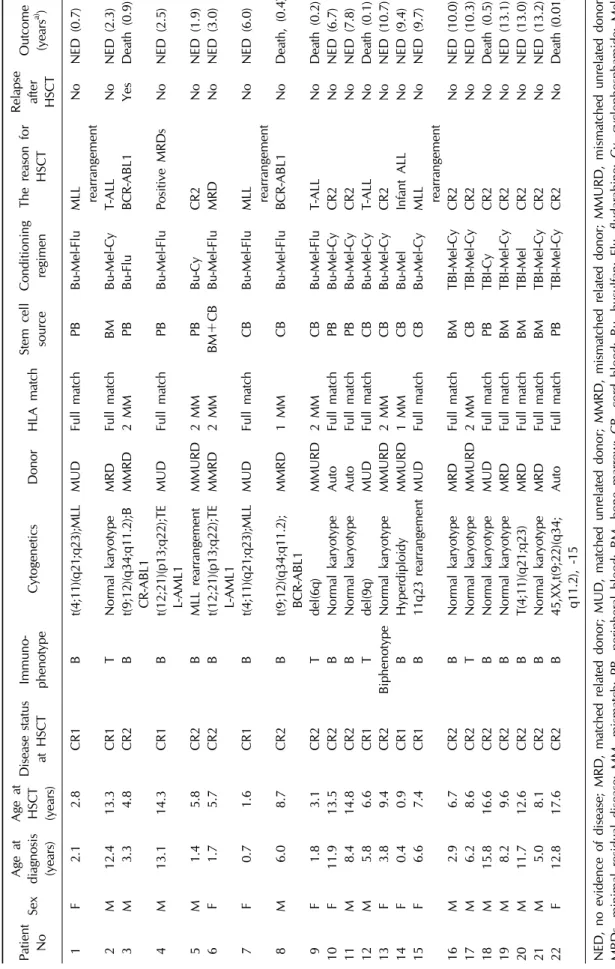
This retrospective study was conducted in 22 pediatric
patients with ALL who were aged <18 years and who un-
derwent HSCT at the Department of Pediatrics of
Yeungnam University Medical Center in Daegu, South
Korea between May 1999 and December 2014. The follow-
ing parameters were retrospectively compared between the
two groups and included in our analysis: age at trans-
plantation, time from diagnosis to transplantation, white
blood cell (WBC) count at transplantation, patient sex, dis-
ease status, donor status, stem cell source, time of neu-
trophil engraftment, time of platelet engraftment, frequen-
cies of acute graft-versus-host disease (GVHD) and chronic
GVHD, overall survival (OS), event free survival (EFS), re-
lapse after transplantation, and death. OS was defined as
the period from stem cell injection to death or last fol-
low-up observation, whereas EFS was defined as survival
with no event, i.e., relapse or death. The patients were div-
ided into a TBI and a non-TBI group: in the TBI group,
cyclophosphamide and melphalan were used as condition-
ing regimens with TBI, whereas, in the non-TBI group, bu-
sulfan was used as the primary agent in conjunction with
other agents such as melphalan, fludarabine, and cyclo-
phosphamide. The best available source of stem cells was
selected from among bone marrow, peripheral blood, and
umbilical cord blood. Regarding donor status, a related
full-matched donor was considered the top priority; if un-
available, an unrelated donor or mismatched donor was al-
so considered. If no donor was available, autologous stem
cell transplantation was considered. Cyclosporin and me-
thotrexate were mainly used for GVHD prophylaxis, and
anti-thymocyte globulin was added in cases with an un-
related donor. Neutrophil engraftment time was defined as
the number of days required after transplantation to achieve
an absolute neutrophil count of at least 0.5×10
9cells/L,
and platelet engraftment time was defined as the number
of days required to achieve a platelet count of at least
20.0×10
9cells/L without platelet transfusion. For GVHD,
acute GVHD was graded by degree of invasion into skin,
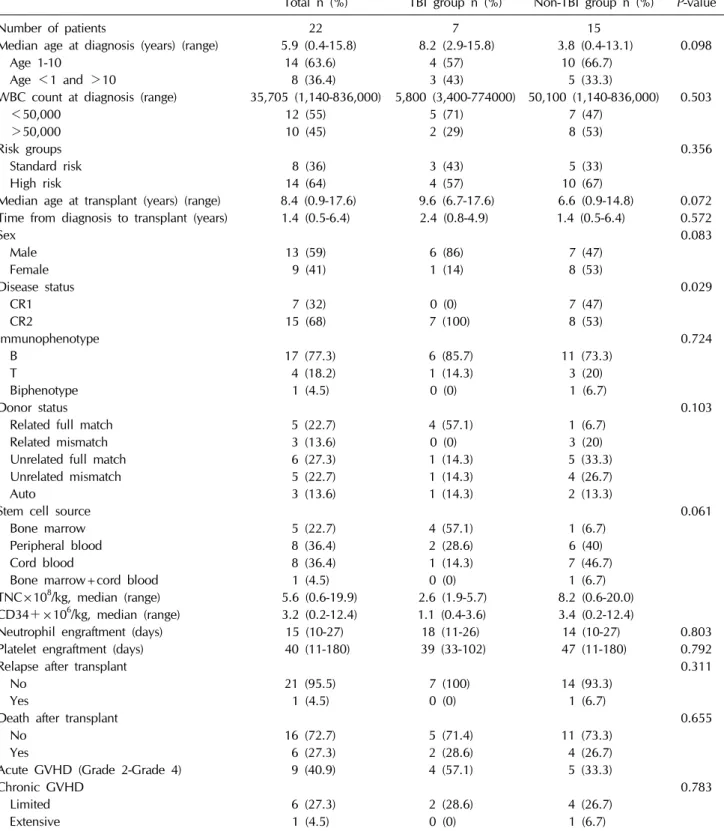
Table 1. Patient characteristics
Total n (%) TBI group n (%) Non-TBI group n (%)
P-value
Number of patients 22 7 15
Median age at diagnosis (years) (range) 5.9 (0.4-15.8) 8.2 (2.9-15.8) 3.8 (0.4-13.1) 0.098
Age 1-10 14 (63.6) 4 (57) 10 (66.7)
Age <1 and >10 8 (36.4) 3 (43) 5 (33.3)
WBC count at diagnosis (range) 35,705 (1,140-836,000) 5,800 (3,400-774000) 50,100 (1,140-836,000) 0.503
<50,000 12 (55) 5 (71) 7 (47)
>50,000 10 (45) 2 (29) 8 (53)
Risk groups 0.356
Standard risk 8 (36) 3 (43) 5 (33)
High risk 14 (64) 4 (57) 10 (67)
Median age at transplant (years) (range) 8.4 (0.9-17.6) 9.6 (6.7-17.6) 6.6 (0.9-14.8) 0.072 Time from diagnosis to transplant (years) 1.4 (0.5-6.4) 2.4 (0.8-4.9) 1.4 (0.5-6.4) 0.572
Sex 0.083
Male 13 (59) 6 (86) 7 (47)
Female 9 (41) 1 (14) 8 (53)
Disease status 0.029
CR1 7 (32) 0 (0) 7 (47)
CR2 15 (68) 7 (100) 8 (53)
Immunophenotype 0.724
B 17 (77.3) 6 (85.7) 11 (73.3)
T 4 (18.2) 1 (14.3) 3 (20)
Biphenotype 1 (4.5) 0 (0) 1 (6.7)
Donor status 0.103
Related full match 5 (22.7) 4 (57.1) 1 (6.7)
Related mismatch 3 (13.6) 0 (0) 3 (20)
Unrelated full match 6 (27.3) 1 (14.3) 5 (33.3)
Unrelated mismatch 5 (22.7) 1 (14.3) 4 (26.7)
Auto 3 (13.6) 1 (14.3) 2 (13.3)
Stem cell source 0.061
Bone marrow 5 (22.7) 4 (57.1) 1 (6.7)
Peripheral blood 8 (36.4) 2 (28.6) 6 (40)
Cord blood 8 (36.4) 1 (14.3) 7 (46.7)
Bone marrow+cord blood 1 (4.5) 0 (0) 1 (6.7)
TNC×108/kg, median (range) 5.6 (0.6-19.9) 2.6 (1.9-5.7) 8.2 (0.6-20.0) CD34+×106/kg, median (range) 3.2 (0.2-12.4) 1.1 (0.4-3.6) 3.4 (0.2-12.4)
Neutrophil engraftment (days) 15 (10-27) 18 (11-26) 14 (10-27) 0.803
Platelet engraftment (days) 40 (11-180) 39 (33-102) 47 (11-180) 0.792
Relapse after transplant 0.311
No 21 (95.5) 7 (100) 14 (93.3)
Yes 1 (4.5) 0 (0) 1 (6.7)
Death after transplant 0.655
No 16 (72.7) 5 (71.4) 11 (73.3)
Yes 6 (27.3) 2 (28.6) 4 (26.7)
Acute GVHD (Grade 2-Grade 4) 9 (40.9) 4 (57.1) 5 (33.3)
Chronic GVHD 0.783
Limited 6 (27.3) 2 (28.6) 4 (26.7)
Extensive 1 (4.5) 0 (0) 1 (6.7)
CR, complete remission; GVHD, graft-versus-host disease; TBI, total body irradiation; TNC, total neutrophil count; WBC, white blood cell.
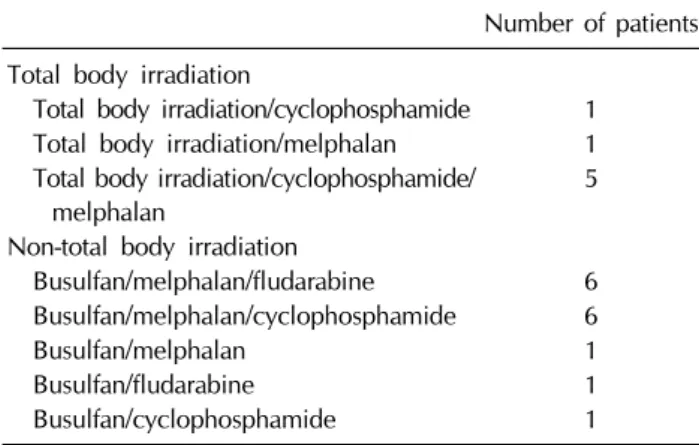
Table 2. Conditioning regimens
Number of patients Total body irradiation
Total body irradiation/cyclophosphamide 1 Total body irradiation/melphalan 1 Total body irradiation/cyclophosphamide/
melphalan
5 Non-total body irradiation
Busulfan/melphalan/fludarabine 6 Busulfan/melphalan/cyclophosphamide 6
Busulfan/melphalan 1
Busulfan/fludarabine 1
Busulfan/cyclophosphamide 1