Introduction
Introduction
Periodontal disease has characteristic of chronic inflamma-tion caused by oral bacterial infecinflamma-tions such as Porphyromo-nas gingivalis, which can lead to tooth loss due to periodontal destruction [1,2]. Lipopolysaccharide (LPS) permeates in the outer membrane of Gram-negative anaerobic bacteria such as
P. gingivalis which causes periodontitis. It stimulates the se-cretion of the inflammatory mediators such as interleukin (IL)-1, nitric oxide (NO), and prostaglandin E2 [3,4]. These mediators play an important role in connective tissue destruction and os-teoclastogenesis [5,6].
In the inflammatory diseases including chronic periodontitis, proteolytic enzymes such as matrix metalloproteinases (MMPs)
Int J Oral Biol 46:51-59, 2021 pISSN: 1226-7155 • eISSN: 2287-6618 https://doi.org/10.11620/IJOB.2021.46.1.51
Baicalin suppresses lipopolysaccharide-induced matrix
metalloproteinase expression: action via the
mitogen-activated protein kinase and nuclear factor κκB-related
protein signaling pathway
Seon-Yle Ko*
Department of Oral Biochemistry and Institute of Dental Science, Dankook University College of Dentistry, Cheonan 31116, Republic of Korea
Periodontal disease is an inflammatory disease that affects the destruction of the bone supporting the tooth and connective tissues surrounding it. Periodontal ligament fibroblasts (PDLFs) induce overexpression of matrix metalloproteinase (MMP) involved in periodontal diseaseʼs inflammatory destruction. Osteoclasts take part in physiological bone remodeling, but they are also involved in bone destruction in many kinds of bone diseases, including osteoporosis and periodontal disease. This study examined the effect of baicalin on proteolytic enzymesʼ production and secretion of inflammatory cytokines in PDLFs and RAW 264.7 cells under the lipopolysaccharide (LPS)-induced inflammatory conditions. Baicalin inhibited the expression of the protein, MMP-1 and MMP-2, without affecting PDLFs’ cell viability, suggesting its possibility because of the inhibition of phosphorylation activation of mitogen-activated protein kinase’s p38, and the signal transduction process of nuclear factor κB (NFκB)-related protein. Also, baicalin reduced the expression of MMP-8 and MMP-9 in RAW 264.7 cells. This reduction is thought to be due to the inhibition of the signal transduction process of NFκB-related proteins affected by inhibiting p65RelA phosphorylation. Also, baicalin inhibited the secretion of nitric oxide and interleukin-6 induced by LPS in RAW 264.7 cells. These results suggest that baicalin inhibits connective tissue destruction in periodontal disease. The inhibition of periodontal tissue destruction may be a therapeutic strategy for treating inflammatory periodontal-diseased patients.
Keywords: Baicalin, Scutellaria baicalensis, Matrix metalloproteinases, Cytokines
Received February 15, 2021; Revised March 18, 2021; Accepted March 19, 2021
*Correspondence to: Seon-Yle Ko, E-mail: seonyleko@dankook.ac.kr https://orcid.org/0000-0003-2541-9556
Copyright © The Korean Academy of Oral Biology
CC This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License
(http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
play an essential role in the process of connective tissue de-struction. MMPs degrade the extracellular matrix components including collagen, fibronectin, and core proteins of proteogly-can [7] and if it is excessively produced, it stimulates degrada-tion of the extracellular matrix of periodontal tissue, resulting in connective tissue destruction and alveolar bone loss, followed eventually by tooth loss.
These proteolytic enzymes are collagenase, gelatinase, and stromelysin, and depending on the substrate specificity, are classified, MMP-1, MMP-8, and MMP-13 as collagenase, MMP-2 and MMP-9 as gelatinase, and MMP-3, MMP-10, and MMP-11 as stromelysin [8,9]. In many earlier studies on periodontitis patients [10-12], MMP-1, MMP-2, MMP-8, and MMP-9 levels were observed to be increased in gingival cre-vicular fluid, periodontal tissues and periodontal ligament (PDL) cells.
It is a well-known fact that LPS stimulates macrophages, activates the signaling system for the mitogen-activated protein kinase (MAPK) as well as immunoreactive mediators such as NO. p38, one of the MAPK family, can be activated by phosphorylation of the subunit, which plays a vital role in the differentiation and proliferation of cell as well as inflamma-tion [13]. Besides, LPS plays a vital role in the activainflamma-tion of the nuclear factor κB (NFκB), a protein belonging to the Rel protein group, which controls the transcription of the target gene by the formation of a dimer. RelA/p50 dimer is the most common form of transcription factor NFκB. When the signal transfer to a cell, inhibitory κB (IκB) is phosphorylated by IκB kinase (IKK) and then degraded, and NFκB isolated from IκB regulates their transcription. RelA could also phosphorylate, and RelA phos-phorylation is known to give regulatory effects on transcrip-tional activation and protein stability [14,15].
The PDL is a cell-connective tissue that connects cementum to the surrounding bone. The periodontal ligament fibroblasts (PDLFs) are the primary cell types of PDLs and play a role in immune response and supporting cells for PDL. It suggests that the location of these cells in pathological conditions of periodontal disease plays an important role in regulating the inflammatory response and amplifying the inflammatory signal [16].
The osteoclast is a bone-resorbing cell derived from the monocyte/macrophage lineage, and the substances such as macrophage colony-stimulating factor and receptor activator of nuclear factor kappa-B ligand (RANKL) are essential for the os-teoclast survival and differentiation [17]. RANKL, if it is bound to RANK, attracts adapter molecules such as tumor necrosis
factor receptor-associated factor 6. This combination mediates a variety of signal transduction processes including NFκB, Jun N-terminal kinase (JNK), p38, extracellular signal-regulated kinase (ERK), and PI3K/AKT [18,19]. Bone destruction by os-teoclasts plays a vital role in pathological bone destruction in inflammatory diseases as well as physiological bone resorp-tion.
Recently, with the increasing interest in oriental herbal medi-cines, many studies are underway to treat various inflamma-tory diseases. Natural compounds such as flavonoids are found in many plants, including edible plants [20]. Of these, baicalin (7-glucuronic acid, 5,6-dihydroxyflavone) is a bioactive flavo-noid compound purified from the dried roots of the perennial plant, Scutellaria baicalensis. Baicalin, as a substance showing various biological features, is known to work on anti-inflam-matory, analgesic and antimicrobial effects [21]. It has also been reported that baicalin stimulates osteoblast differentiation through Wnt/b-catenin signal [22]. The purpose of this study is to induce the inflammation reaction using LPS in human PDL cells and mouse mononuclear cells and identify baicalin effect on the expression of MMP enzyme and its mechanism.
Materials and Methods
Materials and Methods
1. Reagents
LPS from Escherichia coli was purchased from Sigma-Aldrich (St. Louis, MO, USA). Baicalin (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (1,000×). Antibodies against MMP-1, MMP-2, JNK, p38, ERK, IKK, and actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antibod-ies against MMP-8 and MMP-9 were purchased from Abcam (Cambridge, UK). The antibodies against phospho-JNK, phos-pho-p38, ERK, IKK, p65RelA, phospho-p65RelA, AKT, and phospho-AKT were purchased from Cell Signaling (Danvers, MA, USA).
2. A culture of periodontal ligament fibroblasts and
RAW 264.7 cells
The human PDL tissue was taken from healthy teeth from three orthodontic patients. Dankook University Hospital Eth-ics Committee has approved it, and we had received consent before the extraction (IRB No. H-1009/006/002). After plac-ing in a culture dish, the PDL tissue, scraped in a mid-third of the tooth root surface with a surgical blade, we cultured small
dose of Dulbecco’s modified Eagle’s medium (DMEM) includ-ing 10% fetal bovine serum (FBS) and antibiotics (10,000 U/ mL penicillin G and supplemented with 10 µg/mL of strepto-mycin) (HyClone, South Logan, UT, USA). When we found out that the cells grew enough for the experiment while observing their growth from the outset, we removed the cells with 0.25% trypsin and 0.2% ethylenediaminetetraacetic acid and subcul-tured at a ratio of 1:5. Cells between the 5th and 9th genera-tions were used.
We cultured mouse monocyte/macrophage RAW 264.7 cells, obtained from the American Type Culture Collection (Manassas, VA, USA), in a cell incubator, maintained at the temperature of 37℃ and 5% CO2 atmosphere, with DMEM containing 10% FBS. Cell culture media were changed every 3 days.
3. Cell survival test using
methylthiazolyldiphenyl-tetrazolium bromide (MTT)
We seeded PDLFs or RAW 264.7 cells in 96-well plates in DMEM containing 10% FBS at a density of 104 cells/well. We treated them, after 24 hours, with 1 µg/mL LPS and serially di-luted baicalin. PDLFs were treated for 2 days, and RAW 264.7 cells were treated for 4 days. We tested cell survival rate with MTT analysis according to the manufacturer’s protocol (Sigma M5655) (Sigma-Aldrich). After the reaction, we removed the supernatant, dissolved the formazan granule in isopropyl alco-hol, and measured the absorbance using a microplate absor-bance reader.
4. Test by western blot
We plated PDLFs or RAW 264.7 cells in 6-well plates at a density of 5 × 105 cells/well. After 24 hours, we incubated the cells for 6 hours in serum-free medium, pretreated with serially diluted baicalin for 2 hours in medium containing 10% FBS, and then stimulated with 5 µg/mL LPS for 15 minutes or 2 hours. After incubation, we collected the cells and prepared protein samples using lysis solutions. Protein samples (30 µg/ lane) were electrophoresed using sodium dodecyl sulfate poly-acrylamide gel electrophoresis and transferred to a polyvinyli-dene fluoride membrane. We used as a block, Tris-buffered saline and Tween20, containing 5% bovine serum albumin, got reaction using such antibodies as MMP-1, MMP-2, MMP-8, MMP-9, phospho-ERK1/2 (Thr202/Tyr204), phospho-SAPK/ JNK (Thr183/Tyr185), p38, IKK, phospho-p65 RelA, and phospho-AKT, and then reprobed with
non-phospho antibodies corresponding to MAPK, IKK, p65RelA. The band was observed using X-ray film (Fuji film, Tokyo, Japan) exposure and chemiluminescence system (Amersham BioSci-ences, Buckinghamshire, UK).
5. IL-6 secretion test using ELISA
We plated RAW 264.7 cells in 96-well plates with 104 cells/ well and cultured them in α-minimum essential medium con-taining 10% FBS, to test the effect of baicalin on IL-6. One day later, we cultured the cells with 1 µg/mL of LPS and sequen-tially treated with diluted baicalin for 2 days. The amounts of IL-6 dissolved in the culture media were tested according to the manufacturer’s protocol, using an ELISA kit (R&D Systems, Minneapolis, MN, USA). Absorbance was detected at 450 nm, using a spectrophotometer.
6. Nitrite test
We evaluated the secretion of NO by measuring the level of nitrite in a stable oxidized form dissolved in culture media. To test the effect of baicalin on inducible nitric oxide synthase activation, we plated RAW 264.7 cells in 96-well plates at 104 cells/well and then cultured in α-MEM containing 10% FBS. After 24 hours, we cultured the cells with 1 µg/mL of LPS and diluted baicalin consecutively in the phenol red-free media for 72 hours. After incubation, we got a reaction from 100 µL of culture supernatant with 100 µL of Griess reagent (Sigma-Aldrich) in a 96-well reading plate and detected absorbance using a spectrophotometer at 550 nm.
7. Statistical analysis
For significance, Student’s t-test was used. All tests were conducted in triplicate. Each value represents the mean ± standard error. The difference was considered statistically sig-nificant at p < 0.05 and p < 0.01.
Results
Results
1. Expression results of MMP-1 and MMP-2 in
periodontal ligament cells
To examine the effect of baicalin on the expression of MMP, we observed the expression of MMP-1 and MMP-2 from the PDL cells by a western blot method. First, we confirmed the
cytotoxicity of baicalin. As a result, the cells treated with LPS and 1 and 10 µg/mL of baicalin showed no cytotoxicity (Fig. 1A). Therefore, we examined the expression of MMP-1 and MMP-2 in PDL cells and found that when treated with LPS, the protein expression of MMP-1 and MMP-2 increased, on the other hand, when treated with baicalin, was significantly reduced (Fig. 1B).
2. Baicalin’s effect on MAPK activation in periodontal
ligament cells
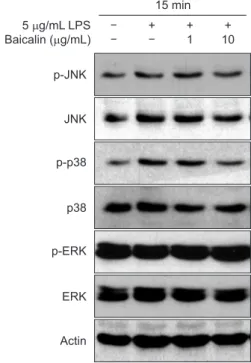
MAPKs activation was observed by western blot analysis to examine the effect of baicalin on the signal transduction pro-cess in PDL cells. For this, protein samples were prepared 15 minutes after treatment with 1 and 10 µg/mL baicalin, and the activation of ERK, JNK, and p38 was observed as the degree of phosphorylation. Activation of JNK and p38 was observed at 15 minutes after the treatment of LPS with PDL cells, but ERK did not show any distinct change. Pretreated baicalin before stimulation with LPS inhibited phosphorylation of p38 (Fig. 2).
3. An effect of baicalin on NFκB related activation in
periodontal ligament cells
Because it has been reported that NFκB could be a vital transcription factor in the expression of inflammatory cytokines by LPS, we investigated whether baicalin affects NFκB activa-tion in PDL cells. After pretreatment with baicalin for 2 hours,
stimulation with LPS was performed. We prepared protein samples and observed changes in phosphorylation of IKK. IKK phosphorylation was increased after 10 minutes of PDL cells
None 120 100 80 60 40 20 PDLF cell viability (% of vehicle) 0 10 1 Baicalin ( g/mL) Vehicle LPS A B 5 g/mL LPS Baicalin ( g/mL) MMP-1 MMP-2 Actin + + 1 + 10 2 hr
Fig. 1. (A) Effect of baicalin on the viability of periodontal ligament fibroblasts (PDLFs). PDLFs were treated with baicalin and 1 µg/mL of lipopolysaccharide (LPS) for 2 days. After the reaction, the formazan granules were solubilized, and the absorbance was measured using a microplate absorbance reader. Cell viability is expressed as the absorbance ratio. Values are expressed as means ± standard error (n = 3). (B) Effect of baicalin on LPS-induced protein
expres-5 g/mL LPS Baicalin ( g/mL) LPS JNK p-P38 P38 p-ERK ERK Actin + + 1 + 10 15 min 5 g/mL LPS Baicalin ( g/mL) p-JNK JNK p-p38 p38 p-ERK ERK Actin + + 1 + 10 15 min
Fig. 2. Effect of baicalin on lipopolysaccharide (LPS)-induced activation of mitogen-activated protein kinases. The periodontal ligament fibroblasts were pretreated with baicalin for 2 hours in a serum-starved state for 30 minutes, and stimulated with 1 µg/mL of LPS for 15 minutes. Protein levels of the indicated genes were determined using western blot analysis. JNK, Jun N-terminal kinase; ERK, extracellular signal-regulated kinase.
treated with LPS, and quantitative changes were observed up to 30 minutes after treatment. IKK phosphorylation was inhibited within 15 minutes in all cases treated with LPS and baicalin (Fig. 3).
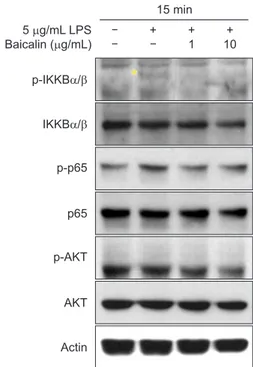
Therefore, the changes of phosphorylation of IKK, p65RelA, and AKT were observed 15 minutes after treatment with LPS. As a result, phosphorylation of IKK, p65RelA, and AKT was increased in LPS-treated cells, and the increased phosphory-lation was inhibited after pre-treatment with 10 µg/mL of ba-icalin (Fig. 4).
4. Expression result of MMP-8 and MMP-9 in RAW
264.7 cells
To examine the effect of baicalin on the expression of MMP,
5 g/mL LPS 10 g/mL Baicalin p-IKKB / IKKB / Actin
*
*
+ + + + + + + + + + 30 10 15 30 10 15 30 (min)Fig. 3. Effect of baicalin on lipopolysaccharide (LPS)-induced activation of inhibitory κB kinase (IKK). The periodontal ligament fibroblasts were pre-treated with baicalin for 2 hours in a serum-starved state for 30 minutes, and stimulated with 1 µg/mL of LPS for 10, 15, and 30 minutes. Protein levels of the indicated genes were determined using western blot analysis.
5 g/mL LPS Baicalin ( g/mL) p-IKKB / IKKB / p-p65 RelA p65 RelA p-AKT AKT Actin + + 1 + 10 15 min
Fig. 4. Effect of baicalin on lipopolysaccharide (LPS)-induced activation of inhibitory κB kinase (IKK), p65RelA, and AKT. The periodontal ligament fi-broblasts were pretreated with baicalin for 2 hours in a serum-starved state for 30 minutes, and stimulated with 1 µg/mL of LPS for 15 minutes. Protein levels of the indicated genes were determined using western blot analysis.
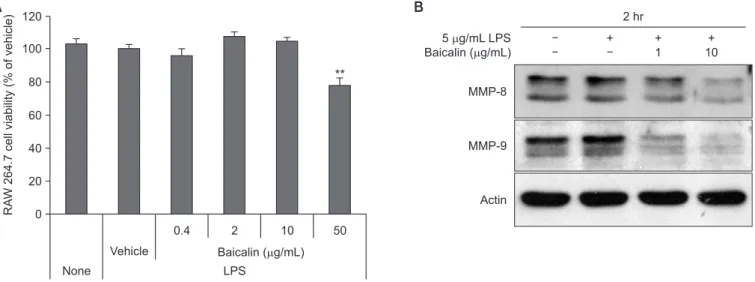
None 120 100 80 60 40 20 RA W 264.7 cell viability (% of vehicle) 0 0.4 Baicalin ( g/mL) Vehicle LPS A B 5 g/mL LPS Baicalin ( g/mL) MMP-8 MMP-9 Actin + + 1 + 10 2 hr 2 10 50 **
Fig. 5. (A) Effect of baicalin on RAW 264.7 cell viability. RAW 264.7 cells were treated with baicalin and 1 µg/mL of lipopolysaccharide (LPS) for 4 days. Cell viability was measured by an methylthiazolyldiphenyl-tetrazolium bromide assay and is expressed as the absorbance ratio. Values are expressed as means ± standard error (n = 3). (B) Effect of baicalin on LPS-induced protein expression. The RAW 264.7 cells were stimulated with 5 µg/mL of LPS for 2 hours. Protein levels of the indicated genes were determined using western blot analysis.
MMP, matrix metalloproteinase. **p < 0.01.
we used western blot method to observe the expression of MMP-8 and MMP-9 from RAW 264.7 cells. Treatment of LPS and 0.4 to 10 µg/mL baicalin did not show cytotoxicity in the culture of RAW 264.7 cells. At 50 µg/mL, baicalin showed a statistically significant 20% reduction in cell proliferation (Fig. 5A). Therefore, we found out that when treated with LPS, MMP-8 and MMP-9 protein expression increased and the increased MMP expression significantly reduced by pre-treat-ment with baicalin; from our examination of their expression in RAW 264.7 cells, treated with baicalin at a concentration of 10 µg/mL, which does not exhibit cytotoxicity (Fig. 5B).
5. An effect of baicalin on MAPK activation in RAW
264.7 cells
The effect of baicalin on the phosphorylation of MAPK was evaluated to examine which step of the signal transduction process is affected by baicalin in the osteoclast activation. RAW 264.7 cells were pretreated with baicalin for 2 hours and then induced with LPS for 15 minutes. The activation of MAPK molecules such as JNK, ERK1/2, and p38 was examined by western blot analysis. LPS induced phosphorylation of JNK,
ERK1/2 and p38, but no change by baicalin showed up (Fig. 6).
6. An effect of baicalin on NFκB-related activation in
RAW 264.7 cells
We investigated whether baicalin affects the activation of NFκB in RAW 264.7 cells. After pretreatment with baicalin for 2 hours, stimulation with LPS was performed. We prepared protein samples and observed changes in phosphorylation of IKK. The phosphorylation of IKK, p65RelA, and AKT was increased after 15 minutes treatment with LPS, and the increased phosphoryla-tion was inhibited after pre-treatment with baicalin (Fig. 7).
7. IL-6 and NO results
To observe the effect of baicalin on the secretion of inflam-matory mediators, we treated RAW 264.7 cells, in the culturing process, with LPS to promote dissolution of IL-6 and NO. As a result, we found out that the secreted amount of IL-6 and NO induced by LPS significantly decreased (Fig. 8).
5 g/mL LPS Baicalin ( g/mL) JNK p38 p-ERK ERK Actin + + 1 + 10 15 min p-JNK p-p38
Fig. 6. Effect of baicalin on lipopolysaccharide (LPS)-induced activation of mitogen-activated protein kinases. The RAW 264.7 cells were pretreated with baicalin for 2 hours in a serum-starved state for 30 minutes, and stim-ulated with 1 µg/mL of LPS for 15 minutes. Protein levels of the indicated genes were determined using western blot analysis.
5 g/mL LPS Baicalin ( g/mL) p65 p-AKT AKT Actin + + 1 + 10 15 min p-IKKB / IKKB / p-p65
*
*
Fig. 7. Effect of baicalin on lipopolysaccharide (LPS)-induced activation of inhibitory κB kinase (IKK), p65RelA, and AKT. The RAW 264.7 cells were pretreated with baicalin for 2 hours in a serum-starved state for 30 min-utes, and stimulated with 1 µg/mL of LPS for 15 minutes. Protein levels of
Discussion
Discussion
This study investigated effects of baicalin, one of the flavo-noids, on the regulation of LPS-induced MMP expression and activation in human PDLFs and mouse monocyte/macrophage RAW 264.7 cells. Even though baicalin is present in many plants and herbal extracts and many studies have shown ef-ficacy such as anti-inflammatory effects of baicalin under cer-tain conditions, no effect on PDL cells has been reported.
In chronic periodontitis patients, periodontal tissue destruc-tion is related to MMP-1 and MMP-2 transcript levels, and the amount of MMP-1 and MMP-2 protein has been observed to be higher in periodontal patients than in healthy periodontal tissues [23]. Thus, it can be assumed that the expression of MMP-1 and MMP-2 in PDLFs is related to the level of con-nective tissue destruction in periodontal tissue. The results of this study showed that baicalin significantly decreased the expression of MMP-1 and MMP-2 in PDLFs compared to the control group treated with LPS alone.
It has recently been reported that MMP-8 is produced not only in polymorphonuclear leukocytes [24] but also in non-ep-ithelial karyotype cells such as gingival epnon-ep-ithelial cells, gingival fibroblasts, and PDL cells [25,26]. Guan et al. [27] reported that Prevotella intermedia infection stimulates MMP-8 expression and activates this protein in PDL cells. Therefore, MMP-8 sig-nificantly increase, in case of patients with chronic periodonti-tis, in the gingival fluid, gingival tissue, and PDL cells, but it is remarkably inhibited after their surgical periodontal treatment,
which indicates that MMP-8 is involved in pathological deg-radation of periodontal tissue. The results of this study show that baicalin inhibits the degradation of connective tissue by inhibiting the expression of MMP-8 and MMP-9 in RAW 264.7 cells. Thus, inhibition of MMP-8 and MMP-9 expression could be thought of as helpful in managing the progression of peri-odontal disease.
In this study, we confirmed that baicalin not only inhib-its the expression of MMP-1 and MMP-2 in PDLFs but also decreases the expression of MMP-8 and MMP-9 in mouse monocyte/macrophage. Furthermore, we examined the effect on intracellular signaling to determine which molecular mecha-nism was involved. We have found that baicalin inhibited the phosphorylation of p38 increased by LPS in PDLFs, while ob-serving changes in the MAPKs activation after inducing an in-flammatory response by LPS treatment. In addition, the effect of baicalin was observed concerning the NFκB-related signal transduction system. Regulation of phosphorylation of RelA is known to play an important role in activating NFκB pathway. It is also known that kinases such as casein kinase II, AKT, and IKK could induce phosphorylation of RelA [28]. This study showed that LPS treatment increased phosphorylation of IKK and AKT as well as RelA phosphorylation. When treated with baicalin in PDLFs and RAW 264.7 cells, the increased phorylation was suppressed. Since inhibition of RelA phos-phorylation causes a decrease in DNA binding capacity, we can deduce that baicalin will decrease NFκB transcriptional activity. When periodontal tissue is exposed to bacteria,
inflamma-None 120 100 80 60 40 20 IL-6 (% of vehicle) 0 0.4 Baicalin ( g/mL) Vehicle LPS A 2 10 None 120 100 80 60 40 20 NO (% of vehicle) 0 0.4 Baicalin ( g/mL) Vehicle LPS B 2 10 * * ** ** ** **
Fig. 8. Effects of baicalin on lipopolysaccharide (LPS)-induced interleukin -6 (IL-6) (A), and nitric oxide (NO) secretion (B). RAW 264.7 cells were cultured with 1 µg/mL of LPS for 2 days. After incubation, the cytokines were measured using an ELISA kit and Griess reagent. Values are expressed as means ± standard error (n = 3).
tion occurs, and cytokine secretion is promoted. Therefore, we observed changes in cytokine secretion from monocytes after treatment with baicalin. IL-6 is an activator of bone destruc-tion, and excessive production of this cytokine is involved in periodontal tissue destruction. In this study, baicalin inhibited LPS-activated IL-6 release in monocytes. NO, another in-flammatory factor, is involved in bone loss and is produced in the salivary glands of the mouth and secreted into saliva. Mi-croorganisms that induce inflammatory infections are known to stimulate immune cells such as macrophages to further promote NO production by oral tissues [29]. Recently, NO has been known to be a target for the development of therapeutic agents in periodontal disease patients because NO plays an important role in disease-related tissue degradation. In this study, baicalin reduced LPS-activated NO secretion in mono-cytes. Therefore, it is thought that baicalin may help to control the progression of periodontal disease by inhibiting the
ex-pression of the proteolytic enzyme as well as the production of an inflammatory cytokine.
In conclusion, the results of this study show that baicalin has an inhibitory effect on connective tissue destruction of peri-odontal tissue, which indicates that it is shown by inhibition of MAPK and NFκB related proteins. The inhibition of periodontal tissue destruction may be recommended as a therapeutic strategy for the treatment of inflammatory periodontal disease patients. However, the results of this study suggest that it is necessary to study animal models of periodontitis to verify whether baicalin can benefit the clinical environment.
Conflicts of Interest
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
References
References
1. Feng Z, Weinberg A. Role of bacteria in health and disease ofperiodontal tissues. Periodontol 2000 2006;40:50-76. doi: 10. 1111/j.1600-0757.2005.00148.x.
2. Mysak J, Podzimek S, Sommerova P, Lyuya-Mi Y, Bartova J, Janatova T, Prochazkova J, Duskova J. Porphyromonas gingi-valis: major periodontopathic pathogen overview. J Immunol Res 2014;2014:476068. doi: 10.1155/2014/476068.
3. Zhang D, Chen L, Li S, Gu Z, Yan J. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immun 2008;14:99-107. doi: 10. 1177/1753425907088244.
4. Souza PP, Palmqvist P, Lundgren I, Lie A, Costa-Neto CM, Lundberg P, Lerner UH. Stimulation of IL-6 cytokines in fi-broblasts by toll-like receptors 2. J Dent Res 2010;89:802-7. doi: 10.1177/0022034510366898.
5. Quinn JM, Gillespie MT. Modulation of osteoclast formation. Biochem Biophys Res Commun 2005;328:739-45. doi: 10. 1016/j.bbrc.2004.11.076.
6. Islam S, Hassan F, Tumurkhuu G, Ito H, Koide N, Mori I, Yo-shida T, Yokochi T. 5-Fluorouracil prevents lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells by inhibiting Akt-dependent nuclear factor-kappaB ac-tivation. Cancer Chemother Pharmacol 2007;59:227-33. doi: 10.1007/s00280-006-0261-2.
7. Birkedal-Hansen H. Role of matrix metalloproteinases in
human periodontal diseases. J Periodontol 1993;64 Suppl 5S:474-84. doi: 10.1902/jop.1993.64.5s.474.
8. Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology 1998;139:1338-45. doi: 10.1210/ endo.139.3.5818.
9. Mizutani A, Sugiyama I, Kuno E, Matsunaga S, Tsukagoshi N. Expression of matrix metalloproteinases during ascorbate-in-duced differentiation of osteoblastic MC3T3-E1 cells. J Bone Miner Res 2001;16:2043-9. doi: 10.1359/jbmr.2001.16.11. 2043.
10. Yuan C, Liu X, Zheng S. Matrix metalloproteinase-8 levels in oral samples as a biomarker for periodontitis in the Chinese population: an observational study. BMC Oral Health 2018; 18:51. doi: 10.1186/s12903-018-0512-8.
11. Mauramo M, Ramseier AM, Mauramo E, Buser A, Terva-hartiala T, Sorsa T, Waltimo T. Associations of oral fluid MMP-8 with periodontitis in Swiss adult subjects. Oral Dis 2018;24:449-55. doi: 10.1111/odi.12769.
12. Ateia IM, Sutthiboonyapan P, Kamarajan P, Jin T, Godovikova V, Kapila YL, Fenno JC. Treponema denticola increases MMP-2 expression and activation in the periodontium via reversible DNA and histone modifications. Cell Microbiol 2018;20:e12815. doi: 10.1111/cmi.12815.
13. Travan S, Li F, D’Silva NJ, Slate EH, Kirkwood KL. Differential expression of mitogen activating protein kinases in periodon-titis. J Clin Periodontol 2013;40:757-64. doi: 10.1111/jcpe. 12123.
14. Moynagh PN. The NF-kappaB pathway. J Cell Sci 2005;118(Pt 20):4589-92. doi: 10.1242/jcs.02579.
15. Lewander A, Gao J, Carstensen J, Arbman G, Zhang H, Sun XF. NF-κB p65 phosphorylated at serine-536 is an indepen-dent prognostic factor in Swedish colorectal cancer patients. Int J Colorectal Dis 2012;27:447-52. doi: 10.1007/s00384-011-1356-8.
16. Jönsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. J Periodontal Res 2011;46:153-7. doi: 10.1111/ j.1600-0765.2010.01331.x.
17. Suda T, Nakamura I, Jimi E, Takahashi N. Regulation of os-teoclast function. J Bone Miner Res 1997;12:869-79. doi: 10. 1359/jbmr.1997.12.6.869.
18. Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. Char-acterization of the intracellular domain of receptor activator of NF-kappaB (RANK). Interaction with tumor necrosis factor receptor-associated factors and activation of NF-kappab and c-Jun N-terminal kinase. J Biol Chem 1998;273:20551-5. doi: 10.1074/jbc.273.32.20551.
19. Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone 2002;30:71-7. doi: 10.1016/s8756-3282(01)00657-3.
20. Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res 1997;57:2916-21.
21. Wang GF, Wu ZF, Wan L, Wang QT, Chen FM. Influence of baicalin on the expression of receptor activator of nuclear factor-kappaB ligand in cultured human periodontal ligament cells. Pharmacology 2006;77:71-7. doi: 10.1159/000092853. 22. Guo AJ, Choi RC, Cheung AW, Chen VP, Xu SL, Dong TT,
Chen JJ, Tsim KW. Baicalin, a flavone, induces the
differen-tiation of cultured osteoblasts: an action via the Wnt/beta-catenin signaling pathway. J Biol Chem 2011;286:27882-93. doi: 10.1074/jbc.M111.236281.
23. Pietruska M, Pietruski J, Skurska A, Bernaczyk A, Zak J, Zelazowska B, Dolińska E, Paniczko-Drezek A, Wysocka J. Assessment of aprotinin influence on periodontal clinical sta-tus and matrix metalloproteinases 1, 2 and their tissue inhibi-tors saliva concentrations in patients with chronic periodonti-tis. Adv Med Sci 2009;54:239-46. doi: 10.2478/v10039-009-0027-2.
24. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and bio-chemistry. Circ Res 2003;92:827-39. doi: 10.1161/01.RES. 0000070112.80711.3D.
25. Tervahartiala T, Pirilä E, Ceponis A, Maisi P, Salo T, Tuter G, Kallio P, Törnwall J, Srinivas R, Konttinen YT, Sorsa T. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J Dent Res 2000;79:1969-77. doi: 10.1177/00220345000790120801.
26. Zhou J, Windsor LJ. Porphyromonas gingivalis affects host collagen degradation by affecting expression, activation, and inhibition of matrix metalloproteinases. J Periodontal Res 2006;41:47-54. doi: 10.1111/j.1600-0765.2005.00835.x. 27. Guan SM, Shu L, Fu SM, Liu B, Xu XL, Wu JZ. Prevotella
intermedia upregulates MMP-1 and MMP-8 expression in human periodontal ligament cells. FEMS Microbiol Lett 2009; 299:214-22. doi: 10.1111/j.1574-6968.2009.01748.x. 28. Ruiz PA, Kim SC, Sartor RB, Haller D.
15-deoxy-delta12,14-prostaglandin J2-mediated ERK signaling inhibits gram-neg-ative bacteria-induced RelA phosphorylation and interleukin-6 gene expression in intestinal epithelial cells through modula-tion of protein phosphatase 2A activity. J Biol Chem 2004; 279:36103-11. doi: 10.1074/jbc.M405032200.
29. Wattamwar PP, Kolte RA, Kolte AP, Shah KK. Influence of interventional nonsurgical periodontal treatment on levels of salivary and serum nitric oxide in smokers and nonsmokers with chronic periodontitis. J Indian Soc Periodontol 2016;20: 592-6. doi: 10.4103/jisp.jisp_106_16.