Characterization of cathepsin B cysteine
protease from Naegleria fowleri
excretory-secretory proteins
exerting immunomodulatory effects
by
Jinyoung Lee
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
Characterization of cathepsin B cysteine
protease from Naegleria fowleri
excretory-secretory proteins
exerting immunomodulatory effects
by
Jinyoung Lee
A Dissertation Submitted to The Graduate School of Ajou University in Partial Fulfillment of the
Requirements for the Degree of
Doctor of Philosophy
Supervised by
Ho-Joon Shin, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Jinyoung Lee is approved.
SUPERVISORY COMMITTEE
Sun Park Ho-Joon Shin Kyongmin Kim Myung-Hee Kwon Byoung-Kuk NaThe Graduate School, Ajou University
December, 19th, 2014
i
PART I
-ABSTRACT-
Effect of Naegleria fowleri excretory-secretory proteins
on BV2 microglial cells
Naegleria fowleri, a free-living amoeba that is found in diverse environmental habitats, can cause an acute fulminating hemorrhagic meningoencephalitis, called primary amoebic meningoencephalitis (PAM) in experimental animals and in humans. Pathogenicity of N. fowleri may be induced by contact-dependent and contact-independent mechanisms, and both mechanisms lead to death of host cells. Several proteins secreted from N. fowleri are likely to be associated with contact-independent pathogenic mechanism of the amoeba. The excretory and secretory proteins of N. fowleri (Nf-ESPs) include phospholipase, proteases, peroxiredoxins and thrombin receptor. However, the precise mechanism induced by Nf-ESPs in PAM is not fully understood yet. In this study, the cytopathic changes and inflammatory responses induced by Nf-ESPs were analyzed in BV2 microglial cells. Several cytokines, particularly IL-1α and TNF- α were highly up-regulated by Nf-ESPs. Also, activation of P38 and JNK was observed in BV2 cells treated with Nf-ESPs. The results collectively suggest that Nf-ESPs may play an important role in N. fowleri mediated-PAM through induction of inflammatory response in microglial cells and activation of MAPKs signal pathway.
ii
Keywords: Naegleria fowleri, excretory/secretory proteins, cytokine, MAPK, microglial cell
iii
PART II
-ABSTRACT-
A novel cathepsin B and cathepsin B-like cysteine protease
from Naegleria fowleri excretory-secretory proteins
and their biochemical properties
Naegleria fowleri causes a lethal primary amebic meningoencephalitis (PAM) in human and experimental animals. Cysteine proteases of parasites play key roles in nutrient uptake, excystment/encystment, host tissue invasion, and immune evasion. In this study, we cloned N. fowleri cathepsin B (nfcpb) and cathepsin B-like (nfcpb-L) genes from our N. fowleri cDNA library. The full-length sequence of genes consisted of 1,038 bp and 939 bp (encoded 345 and 313 amino acid), and molecular weight were 38.4 kDa and 34 kDa, respectively. The identities of nfcbp and nfcpb-L were 56% and 46% with Naegleria gruberi, respectively. Recombinant proteins of nfcpb and nfcpb-L were produced in Escherichia coli BL21 with molecular weight of 38.4 kDa and 34 kDa, respectively. Refolded rNfCPB and rNfCPB-L showed maximum proteolytic activity at pH 4.5, and most effective substrate was Z-LR-MCA. The rNfCPB and rNfCPB-L cleaved effectively several proteins including IgA, IgG, IgM, collagen, fibronection, hemoglobin and albumin. These results suggest that NfCPB and NfCPB-L are may
iv
play important roles in host tissue invasion and immune evasion as pathogens on PAM development due to N. fowleri.
Keywords: Naegleria fowleri, cysteine protease, excretory/secretory proteins, Cathepsin B, Cathepsin B-like
v
TABLE OF CONTENTS
ABSTRACT ... i
TABLE OF CONTENTS ... v
LIST OF TABLE ... viii
LIST OF FIGURES ... ix
ABBREVIATION ... xi
PART I I. Introduction... 2
II. Materials and Methods ... 13
A. N. fowleri culture and production of excretory-secretory proteins ... 13
B. Culture of mouse microglial BV2 cell ... 13
C. MTT assay ... 14
D. Cytokine array assay ... 14
E. RNA isolation ... 15
F. Reverse transcription polymerase chain reaction (RT-PCR) ... 15
G. Preparation of cell lysate ... 17
vi
III. Results ... 19
A. SDS-PAGE patterns of Nf-ESPs ... 19
B. Nf-ESPs induces cell death in BV2 cells ... 20
C. Cytokine expression in BV2 cells treated with Nf-ESPs ... 22
D. mRNA expression of cytokines genes ... 24
E. Nf-ESPs induced MAPKs activation in BV2 cells ... 26
IV. Discussion ... 28 V. Conclusion ... 34 References ... 35 국문요약 ... 48 PART II I. Introduction... 51
II. Materials and Methods ... 58
A. N. fowleri cultures and preparation of Nf-ESPs ... 58
B. Gene cloning ... 58
C. Expression, purification and refolding of recombinant NfCPB and NfCPB-L ... 59
vii
E. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and immunoblotting ... 61
F. Semi-quantitative revers transcription PCR ... 62
G. Enzyme activity assay ... 63
H. Biochemical properties of rNfCPB and rNfCPB-L protein ... 64
I. Determination of cysteine protease in rNfCPB and rNfCPB-L protein ... 64
J. Degradation of host proteins by rNfCPB and rNfCPB-L protein ... 65
K. MTT assay ... 66
L. Statistical analysis... 66
III. Results ... 67
A. Cloning and sequence analysis of nfcpb and nfcpb-L ... 71
B. Differential expression of NfCPB and NfCPB-L in N. fowleri ... 77
C. Expression, purification and refolding of rNfCPB and rNfCPB-L protein 79 D. Biochemical characterization of rNfCPB and rNfCPB-L protein ... 81
E. Degradation of host proteins ... 86
F. rNfCPB and rNfCPB-L induces cell death of BV2 cells ... 89
IV. Discussion ... 91
V. Conclusion ... 96
References ... 97
viii
LIST OF TABLES
PART Ⅰ
Table 1. Genebank accession numbers and primer sequence for RT-PCR ... 16
PART Ⅱ
Table 1. Classification of the annotated clusters of N. fowleri ESTs... 67 Table 2. Kinetic parameters for substrate hydrolysis
ix
LIST OF FIGURES
PART I
Fig. 1. Three stages of Naegleria fowleri ... 3
Fig. 2. SDS-PAGE analysis pattern of Nf-ESPs ... 19
Fig. 3. Viability of BV2 cells treated with different concentration of Nf-ESPs ... 21
Fig. 4. Profiles of cytokines and chemokines induced by Nf-ESPs ... 23
Fig. 5. IL-1α and TNF-α mRNA expression in BV2 cells by RT-PCR ... 25
Fig. 6. Activation of MAPKs (P38, JNK and ERK) in BV2 cells treated with Nf-ESPs ... 27
PART II Fig. 1. Full-length cDNA sequence of nfcpb and nfcpb-L ... 72
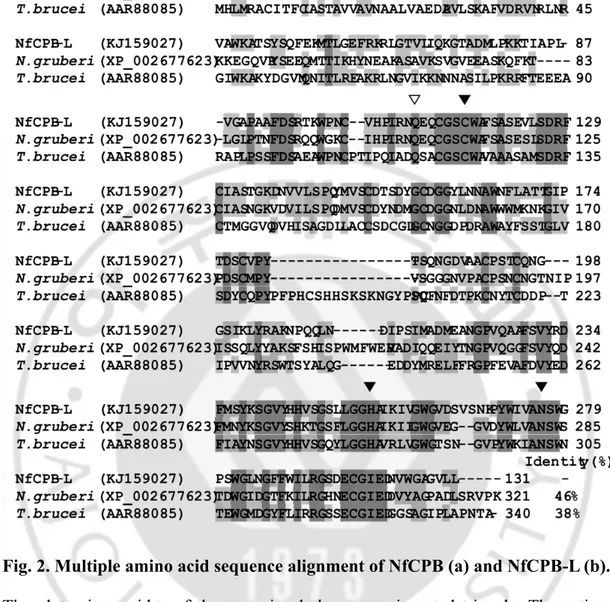
Fig. 2. Multiple amino acid sequence alignment of NfCPB and NfCPB-L ... 74
Fig. 3. Phylogenetic analysis of amino acid sequences of nfcpb and nfcpb-L with various organisms ... 76
Fig. 4. Semi-quantitative RT-PCR for detection of nfcpb and nfcpb-L ... 78
Fig. 5. Expression, purification and refolding of rNfCPB and rNfCPB-L protein . 80 Fig. 6. The pH profiles of enzyme activity of rNfCPB and rNfCPB-L protein ... 82
x
Fig. 7. Enzyme stability of rNfCPB and rNfCPB-L protein. ... 83 Fig. 8. Proteolytic activity of rNfCPB and rNfCPB-L as cysteine protease
by incubating with various protease inhibitors ... 84 Fig. 9. The proteolytic ability of rNfCPB and rNfCPB-L on various
human immunoglobulins. ... 87 Fig. 10. Degradation of various human proteins by proteolytic activity
of rNfCPB and rNfCPB-L... 88 Fig. 11. Viability of BV2 cells treated with different concentration of
xi
ABBREVIATION
Blood-brain barrier (BBB) c-Jun N-terminal kinases (JNK) Cathepsin B-Like (CPB-L) Cathepsin B (CPB)
Cell division cycle (CDC)-like kinase (CLK) Center for Disease Control and Prevention (CDC) Central nervous system (CNS)
Cerebrospinal fluid (CSF) Chinese hamster ovary (CHO) Cyclin-dependent kinase (CDK) Excretory-secretory proteins (ESPs)
Extracellular signal-regulated kinases (ERK) Glycogen synthase kinase (GSK)
Granulocyte coolly-stimulating factor (G-CSF) Heat shock protein 70 (HSP70)
xii Inflammatory bowel disease (IBD)
Interferon gamma-induced protein 10 (IP10) Interferon gamma (IFN-γ)
Interleukin-1 (IL-1)
Interleukin-1 alpha (IL-1α) Interleukin-6 (IL-6)
Interleukin 1 receptor antagonist (IL-1ra) Intracellular adhesion molecule I (ICAM-I) Macrophage colony-stimulating factor (M-CSF) Macrophage inflammatory protein 1 alpha (MIP-1α) Macrophage inflammatory protein 1 beta (MIP-1β) Macrophage inflammatory protein 2 (MIP2)
Major histocompatibility complex class II (MHC class II)
Matrix assisted laser desorption/ionization time of flight mass spectrophotometer (MALDI-TOF)
Mitogen activated protein kinase (MAPKs)
xiii Polymerase chain reaction (PCR)
Primary amoebic meningoencephalitis (PAM)
Regulated on activation normal T cell expressed and secreted (RANTES) Soluble intracellular cell adhesion molecules 1 (SICAM1)
1
PART I
Effect of Naegleria fowleri excretory-secretory proteins
on BV2 microglial cells
2
I. Introduction
A. Naegleria fowleri
Free-living amoebae are ubiquitous protozoa including the genera Naegleria, Acanthamoeba, Balamuthia and Sappinia. Within the genus Naeglera, N. fowleri, N. gruberi, N. jardini, N. lovaniensis, N. andersoni and N. australiensis have been described (De Joncheere et al., 1982; Derr-Harf and De Jonckheere, 1984). Pathogenic N. fowleri was firstly isolated by Carter in 1970. N. fowleri is a thermophilic amoeba with the ideal growth temperatures between 35 - 46ºC and can tolerate up to 45ºC. Meanwhile, non-pathogenic N. gruberi has an optimum growth temperature of 22 - 35ºC.
N. fowleri is found in various areas such as moist soil, water, and sediment. It is a pathogenic amoeba causing fatal primary amoebic meningoencephalitis (PAM), an acute and severe central nervous system (CNS) disease in experimental animal and humans (Cater, 1970; Culberson, 1971; De Jonckheere, 1987). When water containing amoeba gets into the nasal cavity, the organism subsequently migrates to the CNS via the olfactory bulb (Carter, 1968; Carter, 1970; Carter, 1972).
N. fowleri has three life stages, cyst, trophozoite, and flagellate, in its life cycle depending on environmental conditions (Fig. 1).
3 Fig. 1. Three stages of Naegleria fowleri.
4
The persistence of certain stage depends on temperature, nutrients, and salt concentration in the environment. When the amoeba encounters harsh environmental conditions, it turns into cyst, which can be transported by air or by animals that carry wet or dry mud, slime, etc. (Sleigh, 1989). The cyst of N. fowleri is generally round shaped form, sizing from 7 to 15 μm in diameter. It has a thick and smooth double-layered cyst wall, which contains two or three flat, mucus-plugged pores. The cyst can survive much lower temperatures and can be protected from desiccation and food deprivation (John, 1982).
The trophozoite of N. fowleri, is an infective and reproductive stage. The trophozoite has characteristic morphological features such as a clear nucleus with a large and dense karyosome surrounded by a halo and no peripheral nuclear chromatin. It has granular cytoplasm containing many vacuoles. In this stage, the amoeba migrates or eats by pseudopodia. The trophozoite is the vegetative or feeding stage and has characteristic subcellular structure called food cups or amoebastomes, which are cytoplasmic extensions of the surface and are responsible of ingestion of bacteria, yeast and cellular debris, and also may serve as attachment organelles. The size and number of food cup depend on the species and strain of Naegleria (Marciano-Cabral and John, 1983; John et al., 1984; John et al., 1985; Lattyak et al., 1985; Marciano-Cabral and Fulford, 1986).
The flagellate stage is a transient form and can be induced by incubation in nutrient buffers. Differentiation of feeding dividing organism into
non-5
dividing flagellates involves changes in cell shape, decrease of cytoplasmic vacuoles, and synthesis of all organelles of the flagella apparatus (Marciano-Cabral, 1988). The mature flagella apparatus has two flagella, basal bodies, microtubules, and a single striated rootlet or rhizoplast (Dingle and Fulton, 1966; Fulton, 1977). This transformation is reversible within 24 hours (Marciano-Cabral, 1988).
B. Primary amoebic meningoencephalitis (PAM)
PAM is an acute fatal central nervous system (CNS) disease caused by infection with N. fowleri. The infection occurs when water containing the amoeba enters the nasal route. The amoebic infection is usually associated with water activity such as swimming or diving in lakes and rivers during summer. In general, the amoebic infections occur after people put their head under contaminated water. Sometimes, infection can be happened when people cleanse their nose during religious practices or use a device such as neti-pot to rinse their sinuses through the nose (Schuster and Visvesvara, 2004).
Central for Disease Control and Prevention (CDC) reported that a total of 111 persons infected with N. fowleri has been dead from 1962 to 2008 in USA In Pakistan, 13 patients infected with N. fowleri was died by PAM in 2011.(Yoder et al., 2010; Shakoor et al., 2011)
6
After N. fowleri trophozoites attach to the nasal mucosa, they invade the mucosal epithelial layer and migrate along the olfactory nerve tracts. The amoebae cross the cribriform plate and reach to the brain. The cribriform plate is more porous in children than adults, which can be possible reason for the higher incidence of PAM in children and young adults.
The initial targets of amoebic destruction are the olfactory lobes of the brain, since their proximity to the point of entrance of amoeba into the CNS. Other areas can be affected by the amoebae are the base of the brain, the brainstem, and the cerebellum (Martinez, 1985). In infected persons, a large number of amoebae are predominantly found in the perivascular regions, and hemorrhagic necrosis is usually observed in olfactory bulbs and brain. The amoebae can be also existed in the meninges, perivascular spaces, and sanguineopurulent exudates (Carter, 1968; Carter, 1970; Carter, 1972).
Major clinical symptoms of PAM are very similar to bacterial meningitis, which hampers accurate initial diagnosis of PAM. The initial PAM symptoms include headache, fever, nausea or vomiting followed by severe clinical manifestations such as stiff neck, confusion, lacked of attention to people and surrounding, loss of balance, seizures, and hallucinations. If the infected person is not properly treated, the patient usually died within 10 days (Ma et al., 1990).
7 C. Pathogenicity of N. fowleri
The pathogenicity of N. fowleri has been largely recognized in three possible pathogenic mechanisms, a contact-dependent, a contact-independent and an autophagy-related mechanism. Acanthamoeba spp., another free living amoeba, produce many pathogenic factors such as protease (Hadas and Mazur, 1993; Mitro et al., 1994; Mitra et al., 1995; Yang et al., 1997; Khan et al., 2000; Shin et al., 2001), elastase (Ferrant and Bates, 1988), mannose-mediated adhesion proteins (Lether et al., 1998). N. fowleri trophozoites can destroy nerve cells as well as other cell types by trogocytosis using food-cup structure (Brown, 1979; Marciano-Cabral et al., 1982; Marciano-Marciano-Cabral and Fulford, 1986). Heat shock protein 70 (HSP70) and Nf-actin strongly expressed in pseudopodia or phagocytic food-cup of N. fowleri (Song et al., Song et al., 2008; Sohn et al., 2010). Previously, Nfa1 gene was cloned from N. fowleri, which is strongly expressed in food-cup structure (pseudophodia) (Shin et al., 2001). This protein is located on the food-cup structure which is responsible for ingestion of cell during feeding (Song et al., 2006). According to above studies, Nfa1 has very important roles in contact-dependent mechanism of N. fowleri (Jung et al., 2008). In general, adhesion is one of the crucial steps for the pathogenicity of N. fowleri, which is significant increased the binding to target cells. Pathogenicity of N. fowleri would be a complex process which involves contact-dependent as well as contact-independent
8 pathways in order to kill target cells.
D. Diagnosis and therapy
The PAM can be diagnosed by several laboratory methods: (1) Direct microscopic test, which detects rapidly moving motile N. fowleri in the fresh sample of cerebrospinal fluid (CSF) by microscope (Visvesvara, 2010), (2) immunohistochemistry (IHC) test, which stains N. fowleri in tissue by using a specific antibody against the amoeba (Visvesvara, 2010; Rocha-Azevedo, 2009), (3) Polymerase chain reaction (PCR) method, which amplifies N. fowleri-specific genes in CSF or tissue samples (Marciano-Cabral et al., 2003; Qvarnstrom et al., 2006; Robinson et al., 2006), (4) Direct cultivation, which cultivates N. fowleri in the patient’s specimens in artificial culture medium (Visvesvara, 2010).
Many anti-parasitic and anti-microbial drugs have been tested for in vitro and in vivo cidal activity for N. fowleri. But, only an amphotericin B has been identified to be effective for treatment of the amoeba, even though the drug can cause effect in the kidney and other organs. Treatment of amphotericine B to the amoeba resulted in membrane distortion, including the nuclear envelope, rough and smooth endoplasmic reticula, and plasma membrane blebbing (Schuster and Rechthand, 1975). Although PAM caused by N. fowleri infection has been fatal,
9
only a few patients have recovered after receiving intravenous or intrathecal injection of amphotericin B alone or combination with miconazole (Schuster and Visvesvara, 2004). Bauman et al. (2009) has reported that treatment combining miconazole, sulfadiazine, and tetracycline shows limited success only when administered early. Recently, several studies suggested that miltefosin, anti-Leshmania drug, shows some therapeutic activity in N. fowleri infection (Kaminsky, 2002; Schuster et al., 2006; Kim et al., 2008). Miltefosine also showed successful therapeutic effect in patients infected with other free-living amoebae such as Balamuthia and Acanthamoeba (Deetz et al., 2003; Aichelburg et al., 2008, Deetz). In addition, other therapeutic agents, such as clotrimazole, itraconazole, fluconazole, and ketoconazole, have been tested in vitro for N. fowleri infection with varying degrees of efficacy.
E. Microglia and cytokines
The central nervous system (CNS) is consisted of the two major structures, the brain and spinal cord. Several types of structures protect the delicate neurons and support cells of the nervous system. The skeletal system, meninges, CSF and the blood-brain barrier (BBB) work together to protect the CNS by maintaining chemical and physical environments in stable. Despite such extensive protection
10
systems, the CNS can be invaded and damaged by a various microbes (Scheld, 1991; Townsend and Scheld, 1998). The neuroglia protecting, surrounding and nourishing the neurons there are several types of glia, including astrocytes, microglia and oligodendroglia.
The phagocytic microglia gave many thin projection and wander around the CNS clearing debris including dead nervous tissue cells and pathogens. Microglia not only responds to pathological conditions involving immune activation, but also become activated in neurodegenerative conditions that are not considered immune mediated. Resting microglia form a neuron-glia network with potential macrophage-like function (Nakajima and Kohshka, 1993), including expression of major histocompatibility complex class II (MHC class II) (Sedgwick et al., 1993), intercellular adhesion molecule I (ICAM-I) (Zielasek et al., 1993), phagocytosis of microorganisms (Chao et al., 1992; Nakajima and Kohsaka, 1993), and production of free radicals such as nitric oxide (NO) and reactive oxygen intermediates (ROI) (Chao et al., 1993; Chao et al., 1996; Hu et al., 1995). Moreover, microglia release many kinds of inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) (Chao et al., 1992; Suzumura et al., 1993). In CNS, microglia play protective immune response as inflammatory or immunoregulatory cells (Suzumura et al., 1993).
Cytokines are extracellular signaling molecules which mediate cell to cell communication. They have important roles in diverse biological processes such as
11
cellular growth, differentiation, gene expression, migration, immunity and inflammation. TNF and IL-1 are two of the most well studied pro-inflammatory cytokines (Dinarello et al., 1989). TNF is an adipokine, which is involved in systemic inflammation, and is a member of a group of cytokines that simulate the acute phase inflammatory reaction. TNF regulates the immune cells, and induce fever, apoptotic cell death, and inflammation. IL-1 induces production of a complex network of pro-inflammatory cytokines and initiates inflammatory responses (Dinarello, 2011). IL-1 increases the expression of adhesion factors on endothelial cells to enable transmigration of immunocompetent cells. IL-1 is important part in the inflammatory response of the body against infection.
F. Aims of this study
With pathogenesis of N. fowleri causing an acute fulminating hemorrhagic PAM, contact-dependent and contact-independent mechanisms are major pathogenic mechanisms. Recent study reported that Nfa1, Nf-actin and HSP70 are cloned and characterized as contact-dependent mechanism related genes (Shin et al., 2001; Song et al., 2008; Sohn et al, 2010). Furthermore, the lysate of N. fowleri induces strongly cytopathic effect and inflammatory response in primary rat microglial and astrocytes (Oh et al., 2008; Kim et al., 2009). As a contact-independent mechanism of N. fowleri, it was suggested that amoebic ESPs
12
containing many virulence factor such as cathepsin protease, secretory lipase, peroxiredoxins and thrombin receptor will be involved (Kim et al., 2009). But, their detailed role in pathogenicity of N. fowleri has not fully studied. In this study, to understand the pathogenic mechanisms involved in the initiation of N. fowleri infection, inflammatory response of mouse BV2 microglial cells induced by N. fowleri ESPs was evaluated.
13
II. Materials and Methods
A. N. fowleri culture and production of ESPs
N. fowleri (Carter NF69 strain, ATCC No. 30215) was incubated at 37°C and maintained in Nelson’s media supplemented with 5% fetal bovine serum (FBS; Gibco, Grand Island, New York, USA) and antibiotics. N. fowleri trophozoites obtained from culture were pelleted and washed three times with phosphate-buffered saline (PBS, pH 7.4). The trophozoites were resuspended with PBS and then incubated at 37°C for 1 h in order to obtain ESPs. The supernatant were collected after centrifugation at 800 x g for 15 min at 4°C.
B. Culture of mouse microglial BV2 cells
Mouse BV2 microglial cells, were axenic cultured with Dulbecco’s Modified Eagle Medium (DMEM; Welgene, Daegu, Korea) containing 5% FBS (Gibco), 1% penicillin/streptomycin (Gibco) and 1% L-glutamine (Welgene) at 37°C, 5% CO2
14 C. MTT Assay
BV2 cells were cultured as monolayer in DMEM containing 5% FBS (Gibco), 1% penicillin/streptomycin (Gibco) and 1% L-glutamine (Welgene) at 37°C, 5% CO2 using 96 well cell culture plate (Nunc A/S, Roskilde, Denmark). Brief
procedures were as follows: 2ⅹ104 cells were treated with different concentrations of Nf-ESPs (6.25, 12.5, 25, 50 and 100 μg/ml) at 37℃ for 24 h. The supernatant was discarded from plate and then added 100 μl of MTT solution (1 mg/ml of concentration) (Sigma Aldrich, St. Louis, MO, USA). The plate was incubated for 3 h at 37°C and then 100 μl of dimethyl sulfoxide (DMSO; Sigma) was added. The reactant was practiced at 595 nm with ELISA reader.
D. Cytokine array assay
For the parallel determination of the relative levels of selected mouse cytokine and chemokines, mouse BV2 microglial cells were untreated or treated with Nf-ESPs 100 μg/ml, incubated for 12 or 24 h, and then harvested the culture supernatant. The supernatant was collected by centrifugation at 1,000 x g for 5 min at 4℃. The supernatant was quantified using Proteome ProfilerTM Mouse Cytokine
Array Panel A (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instruction.
15 E. RNA isolation
To determine the effect of Nf-ESPs treatment on mRNA expression, BV2 microglial cells were treated with Nf-ESPs (100 μg/ml), incubated for 0, 1, 3, 6, 9, 12 and 24 (h), and harvested, respectively. Total RNA was isolated using RNAiso (TAKARA, Japan) according to instruction of manufacturer. The isolated RNA was quantified by spectrophotometry and equalized, and purity was checked on 1% agarose gel.
F. Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was reverse transcribed into cDNA using oligo dT primer, The reverse transcription (RT) reaction using 1 μg of total RNA were performed at 52°C for 50 min, and then subsequently for 5 min at 85°C and at 4°C, respectively, according to the instructions of the manufacturer. RT-PCR was performed using primers specific for each cytokine gene (table 1). The amplified product was separated on 1.5% agarose gel, stained with ethidium bromide (EtBr), and observed.
16
17 G. Preparation of cell lysate
Mouse BV2 microglial cells cultured with Nf-ESPs for 0, 1, 3, 6 and 9 h, were pelleted, suspended in lysis buffer (Intron Biotechnology, Seongnam, Korea), and then rocked gently at 4°C for 30 min. The supernatant was collected by centrifugation at 13,000 rpm for 5 min at 4°C. Protein concentration was estimated by Bradford assay (Intron Biotechnology) using bovine serum albumin (BSA) as a standard.
H. Western blotting
To observe the Mitogen-activated protein kinase (MAPK) activation in mouse BV2 cells treated with Nf-ESPs, the protein samples were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using reducing sample buffer (62.2 mM Tris, pH 6.8; 10% glycerol; 10% 2-mercaptoethanol; 3% SDS; and 0.1% bromophenol blue), boiled for 5 min prior to loading onto the gel, and then separated by electrophoresis. For western blotting, proteins were transferred onto polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA) for 40 min at 20 V on the Trans-blot® semi-dry transfer cell (Bio-Rad, Hercules, CA, USA). Following transfer, the membranes was blocked with 5% BSA for overnight at 4°C and reacted with rabbit polyclonal anti-ERK
18
(extracellular signal-regulatory kinase), rabbit polyclonal anti-phosphor-ERK, rabbit-polyclonal anti-P38, rabbit polyclonal anti-phosphor-p38, rabbit polyclonal anti-JNK (c-Jun N-terminal kinase) and rabbit polyclonal anti-phosphor-JNK antibodies (1:1000, Cell Signaling, Beverly, MA) in PBS, 5% BSA overnight at 4°C. The membrane was washed with PBS containing 0.05% Tween-20 (PBST) three times for 10 min and reacted with secondary antibody of a horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:5,000, Cell Signaling) for 2 h at room temperature. After washing with PBST three times and anti-reactive bands were revealed by enhanced chemical luminescence (ECL) (Amersham Biosciences, Buckinghamshire, England).
19
III. Results
A. SDS-PAGE patterns of Nf-ESPs
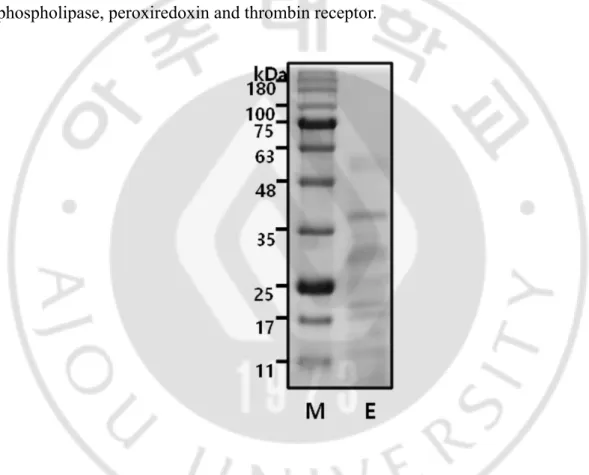
SDS-PAGE analysis of Nf-ESPs revealed 7 major protein bands ranging 13 – 55 kDa (Fig. 2). Nf-ESPs have been known to include proteins such as proteases, phospholipase, peroxiredoxin and thrombin receptor.
Fig. 2. SDS-PAGE analysis pattern of Nf-ESPs. Nf-ESPs were separated on 12% SDS-PAGE gel and the proteins were stained with coomassie blue. M, protein size marker; E, Nf-ESPs
20 B. Nf-ESPs induces cell death in BV2 cell
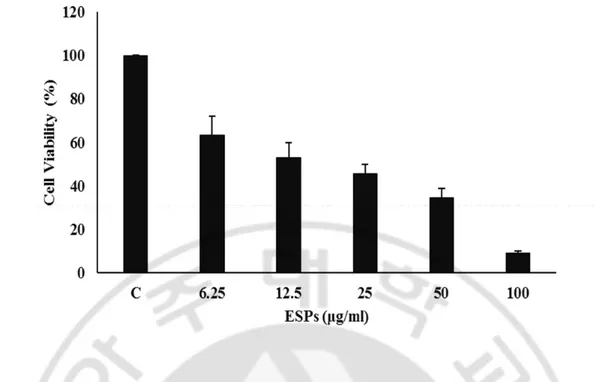
To investigate the cell damage and inhibition of microglial proliferation by Nf-ESPs, BV2 cells were treated with different concentrations of Nf-ESPs, and the cell viability was assessed by MTT assay. BV2 cells treated with Nf-ESPs showed markedly reduced cell viability in a dose dependent manner (none, 6.25, 12.5, 25, 50 and 100 μg/ml).
21
Fig. 3. Viability of BV2 cells treated with different concentration of Nf-ESPs. N. fowleri induced cell death in BV2 cells. The cells were treated with different concentrations (none, 6.25, 12.5, 25, 50 and 100 μg) of Nf-ESPs for 24 h and then cell viability of BV2 cells were measured by MTT assay. Cell viability was calculated by the percent of control.
22
C. Cytokine expression in BV2 cells treated with Nf-ESPs
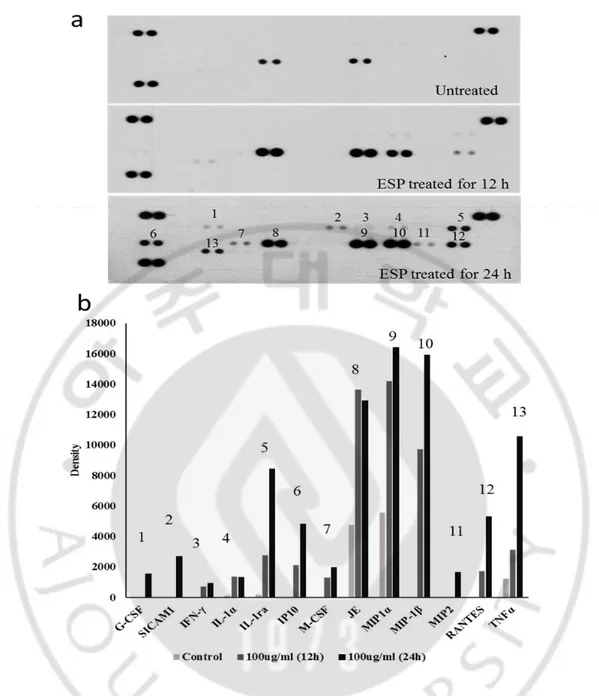
To observe the cytokine expression patterns in BV2 cells induced by N. fowleri ESPs, BV2 cell were treated with N. fowleri ESPs (100 μg/ml) for 12 h and 24 h, and culture supernatant was analyzed by using mouse cytokine array kit. The BV2 cells secreted various cytokines and chemokines (Fig. 4.) The cytokines and chemokines expression were increased time-dependent manners (untreated, ESP treated for 12 and 24 h) (Fig. 4a and 4b). The chemotactic cytokine JE and MIP1- α were secreted from untreated BV2 cells. However, several cytokine such as IFN-γ, IL-1α, IL-1ra and TNF-α secretion were increased from BV2 cell after treated with N. fowleri ESPs for 12 h. In particularly, pro-inflammatory cytokine IL-1α and TNF-α were significantly increased in Nf-ESPs treated BV2 cells for 24 h
23
Fig. 4. Profiles of cytokines and chemokines induced by Nf-ESPs. BV2 cells were either untreated or treated with 100 μg/ml of N. fowleri ESP for 12 and 24 h, respectively. Mouse cytokine array (a) and the graph shows densitometric analysis of mean density (b).
24 D. mRNA expression of cytokine genes
Mouse cytokine array showed that various cytokine and chemokine expressed in BV2 cells after Nf-ESPs treatment. Especially, pro-inflammatory cytokine IL-lα and TNF-α important cytokines in initiation of inflammatory response were detected. To confirm the cytokine expression in the analysis by cytokine array, RT-PCR was performed. RT-PCR analysis suggested that Nf-ESPs strongly induced mRNA expression of IL-1α and TNF-α in BV2 microglial cells (Fig. 5). The highest expression levels of IL-1α and TNF-α mRNA were identified at 6 h and 3 h after Nf-ESPs treatment, respectively.
25
Fig. 5. IL-1α and TNF-α mRNA expression in BV2 cells by RT-PCR. BV2 cells were treated with Nf-ESPs (100 μg/ml) for 0, 1, 3, 6, 9 (0-9 h). β-actin was used as a control for equal cDNA loading. PCR product were analyzed on 1.5% agarose gel and stained with EtBr (a). The graphs indicate densitometric analysis of IL-1α and TNF-α relative to β-actin, respectively (B) and (c).
26
E. Nf-ESPs induced MAPKs activation in BV2 cells
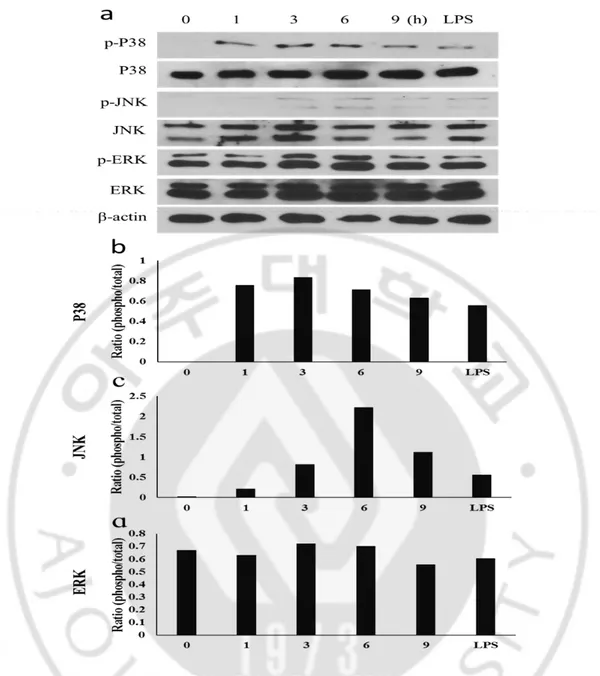
Mitogen activated protein kinase (MAPKs) are intracellular signal transduction factor, and they mediate pro-inflammatory process. Furthermore, MAPKs regulate cell function such as proliferation, gene expression, mitosis and apoptosis. To analyze the MAPKs signal transduction pathway induced by Nf-ESPs stimulation, phosphorylation of several mitogen-activated protein kinase P38, JNK and ERK were analyzed in BV2 cells treated with Nf-ESPs (100 μg/ml) in a time-dependent manner (Fig. 6a). P38 phosphorylation was increased by treatment of Nf-ESPs for 1 h and the highest level was identified at 3 h (Fig. 6b). The JNK phosphorylation was slightly increased when BV2 cells were treated with Nf-ESPs for 1 h and maximum level of phosphorylation was observed 6 h (Fig. 6c). Meanwhile, ERK phosphorylation was not clearly identified (Fig. 6d).
27
Fig. 6. Activation of MAPKs (P38, JNK and ERK) in BV2 cells treated with Nf-ESPs. Mouse BV2 cells were treated with Nf-ESPs (100 μg/ml) for varying time periods (0-9 h). The phosphorylation levels of MAPKs were analyzed by western-blot (a). The densitometric analysis graph (b; P38, c; JNK, d; ERK). The graph values were shown phosphorylation /total rate.
28
IV. Discussion
Naegleria fowleri is the causal agent of PAM in experimental animals and humans. Naegleria spp. are widely found in soil and water (Schuster and Visvesvara, 2004). N. fowleri usually propagates in warm, stagnant bodies of freshwater (typically during the summer season), and opportunistically infects the central nervous system via insufflation of water conjugated with the amoeba (CDC, 2008). The amoeba attaches to the olfactory nerve, migrates through the cribriform plate and then reaches to the olfactory bulbs of the forebrain (Cervantes-Sandoval et al., 2008), where it actively multiplies by feeding the nerve tissue. During this stage, usually occurring approximately 3–7 days post-infection, the infected person showed typical symptoms such as parosmia, rapidly progressing anosmia (with resultant ageusia) as the nerve cells of the olfactory bulbs are disrupted and replaced with necrotic lesions.
Microglial cells are the resident macrophages of the brain and spinal cord, and play as first-like immune defense cells in the CNS. The primary role of microglia, involves the engulfing of various materials including cellular debris, lipid apoptotic cell death in the non-inflamed state, and invading virus, bacteria, or other foreign materials in the inflamed state. In addition, microglia also play various roles in the CNS including cytotoxicity for infectious organisms, antigen presenting, synaptic stripping, promotion of repair and extracellular signaling and homeostasis in
non-29
infected region and promoting inflammation infected or damaged tissue (Gehrmann et al., 1995). Extracellular signaling is complicated connected with other microglia, astrocytes, nerves, T-cells and progenitor cells. Microglia activation is induced by IFN-γ, and this activation increases IFN-γ release into the extracellular environment. The activated microglia induces expressions of several cytokines, and they rapidly activate nearby microglia. Microglia-induced TNF-α causes neural tissue to undergo apoptosis and induces inflammation. Another cytokine IL-1 inhibits IL-10 and TGF-β, which down-regulate antigen presentation and pro-inflammatory signaling. The pro-inflammatory cytokines IL-1α, IL-1β and TNF-α induced by microglia stimulation of CNS, which play a potential role in neurodegeneration when microglia remain in a sustained activated state (Wood, 1998; Aloisi, 2001).
Elucidation of pathogenicity-related factors in PAM is important for understanding the mechanism of N. fowleri-host interactions. Amoebic pathogenicity may consist of complex processes that include both contact-dependent and contact-incontact-dependent mechanisms that lead to host cell death. In contact-dependent mechanism, Nfa1 protein, HSP 70s and Nf-actin, which are closely related with phagocytic food-cup formation, seem to play critical roles (Shin et al., 2001; Song et al., 2006; Sohn et al., 2010). Meanwhile, in a contact-independent mechanism, Nf-ESPs, which consisted of peroxiredoxins, proteases and thrombin receptor, may play a role in host cell invasion and lytic activity for host immunoglobulin and immune evasion (Kim et al., 2009).
30
IL1-β and IL-6 are produced in primary astrocytes stimulated with N. fowleri lysate (Kim et al., 2013). It is also reported that IL1-β, IL-6 and TNF-α were produced in primary microglia co-cultured with N. fowleri trophozoite. IL-18 and IFN-γ were also induced in microglial cell line by N. fowleri lysate (Oh et al., 2005). However, no studies have been on inflammatory responses against Nf-ESPs., In this study, the changes and immune responses in microglia cell line, BV2 cells, treated with Nf-ESPs were analyzed. BV2 cells treatment with Nf-ESPs markedly reduced cell viability in dose dependent manners, which supporting that Nf-ESPs is a significance effector factor for cell damages observed in PAM. The BV2 cells secreted various cytokines and chemokines in response to Nf-ESPs. Especially, expressions of pro-inflammatory cytokines IL-1α and TNF-α were highly increased. To confirm mRNA expressions of IL-1α and TNF-α, RT-PCR was performed. Both IL-1α and TNF-α expressions was increased in BV2 cells in a time dependent manner by treatment of Nf-ESPs.
IL-1α is the prototypic pro-inflammatory cytokine and effective nearly every cell type, often in concert with another pro-inflammatory cytokine, TNF-α (Dinarello, 1997). Previous study of Leshmaniasis, IL-1 production was induced in monocytes (Crawford et al., 1985). Leshmania mexicana lipophosphoglican (LPG) induced the production of TNF-α, IL-1β, IL-12P70 and IL-10 when human macrophage was stimulated with L. mexicana LPG (Rojas-Bernabe et al., 2014). Another study showed that IL-1α and TNF-α production increased in human oral
31
and vaginal epithelial cells during Candida albicans infection (Steele and Fidel, 2002). Pro-inflammatory cytokine such as IL-1α, IL-1β and TNF-α mRNA expression increased in primary rat-microglia co-cultured with Acanthamoeba castellanii (Marciano-carbral et al., 2000). In experiment of chlamydia trachomatis, IL-1α was increased in both apical and basolaterial of C. trachomatis infected polarized, immotalized, endocervical epithelial cell model (polA2EN) (Buckner et al., 2013). Some case report of carmeroonians infected by Onchocerca volvulus, observed that IL-1α, IL-6, IL-10 and IL-13 were detected from blood sample by Enzyme-linked immunoabsorbant assay (ELISA) (Nmorsi et al., 2012). In addition, some reports have described that proinflammatory cytokines IL-1, IL-6 and TNF-α level were increased mainly in the aged hippocampus but also in cortical regions (Murray et al., 1997; Katafuchi et al., 2003; Maher et al., 2004; Sierra et al., 2007). Moreover, the peripheral blood mononuclear cells (PBMC) from parturient and non-pregnant woman was exposed with live tarchyzoites of Toxoplasmma gondii, pro-inflammatory cytokines such as TNF-α and IL-2 was produced significantly higher level (Rezende-Oliveira et al., 2012). Moreover, release of IL-1α, IL-6 and GM-CSF activity were increased in the astroglial cells infected by T. gondii (Fischer et al., 1997). So I suggest that pro-inflammatory cytokine IL-1α and TNF-α may play as an inducer of inflammatory response during the N. fowleri infection. MAPK are serine/threonine/tyrosine-specific protein kinase belonging to the CDK, MAPK, GSK and CLK kinase group (Manning et al., 2002). MAPKs
32
signaling pathway is essential in regulating diverse cellular processes including inflammatory response, cell differentiation, proliferation and death. MAPKs signaling modulated which mediate extracellular signals into the nucleus to turn on the responsive genes in mammalians cells, including P38, JNK and ERK kinase. MAPKs phosphorylate a variety of intracellular targets including transcription factors, nuclear pore proteins, membrane transporters, cytoskeletal elements, and other protein kinases. Especially, MAPKs activation was related with pro-inflammatory response in astrocyte (Kim et al., 2013). Activation of MAPKs is also crucial for regulating cytokine expressions. Treatment of Nf-ESPs induced high level expressions of several cytokines, especially IL-1α and TNF-α.
IL-1 is responsible for inflammation as well as the promotion of fever and sepsis. Especially, IL-1α is mainly produce by activated macrophages, neutrophils, epithelial cells and endothelial cells. It plays one of the central roles in the regulation of immune response and possesses metabolic, physiological, haematopoietic activities (Bankers-Fulbright et al., 1996; Dinarello, 1997) and it is on the pathway that activates TNF-α. The role of TNF-α is regulation of immune cells, being an endogenous pyrogen, can induce fever, apoptotic cell death, inflammation and inhibit tumorgenesis. In addition, TNF-α activation closely relate with various human disease including Alzheimer’s disease (Locksley et al., 2001), cancer (Dowlati et al., 2010), major depression (Bryuskov et al., 2002) and inflammatory bowel disease (IBD) (Mikocka-walus et al., 2007). In conclusion,
33
Nf-ESPs induced inflammatory responses in BV2 microglial cells. Increased levels of several cytokines and chemokines were identified and IL-1α and TNF-α were the most predominant cytokines effected by Nf-ESPs. The expression of these cytokines is likely to be regulated by MAPKs signal pathway, but more detailed study on interaction of transcription factors such as AP-1 and NF-κB would be necessary to fully understand the precise mechanism for up-regulation of cytokine production. The result obtained, in this study suggest that Nf-ESPs may play an important role in contact-independent pathogenicity of N. fowleri in PAM.
34
V. Conclusion
To understand contact-independent pathogenesis of N. fowleri in PAM, the change and immune responses in BV2 cells induced by Nf-ESPs were analyzed. The treatment of Nf-ESPs to BV2 cells markedly reduced cell viability of the cells, which suggesting possible roles of Nf-ESPs in cell damages observed in PAM. Treatment of Nf-ESPs induced increased expressions of, especially IL-1α and TNF- α, various cytokine and chemokines in BV2 cells. To further understand the mechanism of expression of pro-inflammatory cytokines, MAPK such as P38, JNK and ERK activation were slightly increased by treatment of Nf-ESPs. These results suggested that Nf-ESPs induced productions of pro-inflammatory cytokines, which will further activate inflammation responses in the microglia during the PAM. Therefore, Nf-EPSs can be an important factor in contact-independent pathogenic mechanism of N. fowleri. Considering to Nf-ESPs is a mixture of various proteins secreted by the amoeba, further detailed studies to determine which protein is closely related to the pathogenic mechanism of Nf-ESPs should be needed.
35
References
1. Aichelburg AC, Walochnik J, Assadian O, Prosch H, Steuer A, Perneczky G, Visvesvara GS, Aspock H, Vetter N: Successful treatment of disseminated Acanthamoeba sp. infection with miltefosine. Emerg Infect Dis 14(11): 1743-1746, 2008
2. Aloisi F: Immune function of microglia. Glia 36(2): 165-179, 2001.
3. Bankers-Fulbright JL, Kalli KR, McKean DJ: Interleukin-1 signal transduction. Life Sci 59(2): 61-83, 1996
4. Bauman RW; "Microbial Diseases of the Nervous System and Eyes". Microbiology, With Diseases by Body System (2nd ed.). San Francisco: Pearson Education. p. 617, 2009
5. Brown T: Observations by immunofluorscence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo culutres. J Med Microbiol 12:355-362, 1979
6. Brynskov J, Foegh P, Pedersen G, Ellervik C. Kirkegaard T, Bingham A, Saemark T: Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 51(1): 37-43, 2002
7. Buckner LR, Lewis ME, Greene SJ, Foster TP, Quayle AJ: Chlamydia trachomatis infection results in a modest pro-inflammatory cytokine response
36
and a decrease in T cell chemokine secretion in human polarized endocervical epithelial cells. Cytokine 63(2): 151-165, 2013
8. Carter RF: Description of a Naegleria species isolated from two cases of primary amoebic meningoencephalitis and of the experimental pathological changes induced by it. J Pathol 100: 217-244, 1970
9. Carter RF: Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg 66: 193-213, 1972
10. Carter RF: Primary amoebic meningoencephalitis: clinical, pathological and epidemiological features of six fatal cases. J Pathol Bacteriol 96: 1-25, 1968 11. Centers for Disease Control Prevention (CDC): Primary amebic
meningoencephalitis—Arizona, Florida, and Texas, 2008. MMWR Morb Mortal Wkly Rep 57: 573-577, 2007
12. Cervantes-Sandoval L, Serrano-Luna Jde J, Garcia-Latorre E, Tsutsumi V, Shibayama M: Mucins in the host defence against Naegleria fowleri and mucinolytic activity as a possible means of evasion. Microbiology 154(Pt 12): 3895-3904, 2008
13. Chao CC, Anderson WR, Hu S, Gekker G, Martella A, Peterson PK: Acvated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin Immunol Immunopathol 67: 178-183, 1993
14. Chao CC, Gerkker G, Hu S, Peterson PK: Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol 152: 1246-1252,
37 1994
15. Chao CC, Hu S, Close K, Choi CS, Molter TW, Novick WJ, Peterson PK: Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis 166: 847-853, 1992
16. Chao CC, Hu S, Peterson PK: Glias: the not so innocent bystanders. J Neurovirol 2: 234-239, 1996
17. Crawford GD,Whler DJ, Dinarello CA: Parasite-monocyte interactions in human leishmaniasis: production of interleukin-1 in vitro. J Infect Dis 152(2): 315-322, 1985
18. Culberson GC: The pathogenicity of soil amoebas. Annu Rev Microbiol 25: 231-254, 1971
19. da Rocha-Azevedo B, Tanowitz HB, Marciano-Cabral F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip Perspect Infect Dis 251406, 2009
20. De Jonckheere JF: Characterization of Naegleria species by restriction endonuclease digestion fo whole-cell DNA. Mol Biochem Parasitol 24: 55-66, 1987
21. De Jonckheere JF: Isoenzyme patterns of pathogenic and non-pathogenic Naegleria spp. Using agarose isoelectirc focusing. Ann Microbiol 133(2): 3193-3142, 1982
38
treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clinical Infect Dis 37 (10): 1304-1312, 2003
23. Derr-Harf and De Jonckheere JF: Isolation of pathogenic Naegleria australiensis (Amoebida, Vahlkampfidae) from the Rhine. Protistologica 302: 489-494, 1984
24. Dinarello CA: Interleukin-1 and its biologically related cytokines. Adv Immunol 44: 153-205, 1989
25. Dinarello CA: Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117(14): 3720-3732, 2011
26. Dinarello CA: Interneukin-1. Cytokine Growth Facor Rev 8(4): 253-265, 1997 27. Dinarello CA: Role of pro- and anti-inflammatory cytokines during
inflammation: experimental and clinical findings. J Biol Regul Homeost Agents 11(3): 91-103, 1997
28. Dingle AD and Fulton C: Development of the flagellar apparatus of Naegleria. J Cell Biol 31: 43-54, 1966
29. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL: A meta-analysis of cytokines in major depression. Biol Psychiatry 67(5): 446-457, 2010
30. Ferrant A and Bates EJ: Elastase in the pathogenic free-living amebae Naegleria and Acanthamoeba spp. Infec Immun 56: 3320-3321, 1988
39
induced by Toxoplsma gondii in astrocytes and microglial cells. Eur J Immunol 27(6): 1539-1548, 1997
32. Fulton C: Cell differentiation in Naegleria gruberi. Annu Rev Microbiol 31: 597-629, 1977
33. Gehrmann J, Matsumoto Y, Kreutzberg GW: Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 20(3): 269-287, 1995 34. Hadas E and Mazur T: Characterization of ameboid microglia isolated from
developing mammalian brain. Trop Med Parasitol 44: 197-200, 1993
35. Hu S, Sheng WS, Peterson PK, Chao CC: Cytokine modulation of rat microglial cell superoxide production. Glia 13: 45-50, 1995
36. John DT, Cole TB Jr, Bruner RA: Amebostomes of Naegleria fowleri. J protozool 32: 12-19, 1985
37. John DT, Cole TB Jr, Marciano-Cabral FM: Sucker-like structures on the pathogenic amoeba Naegleria fowleri. Appl Environ Microbiol 47: 12-14, 1984 38. John DT: Primary amebic meningoencephalitis and the biology of Naegleria
fowleri. Annu Rev Microbiol 36: 101-123, 1982
39. Jung SY, Kim JH, Lee YJ, Song KJ, Kim K, Park S, Im KI, Shin HJ: Naegleria fowleri: nfa1 gene knock-down by double-stranded RNAs. Exp Parasitol 118: 208-213, 2008
40. Kaminsky R: Miltefosine Zentaris. Curr Opin Investig Drug 3: 550-554, 2002 41. Katafuchi T, Takaki A, Take S, Kondo T, Yhshimura M: Endotoxin inhibitor
40
blocks heat exposure-induced expression of brain cytokine mRNA in aged rat. Brain Res Mol Brain Res 118(1-2): 24-32, 2003
42. Khan NA, Jarrol EL, Panjwani N, Cao Z, Paget TA: Protease as markers for differentiation of pathogenic and non-pathogenic species of Acanthamoeba. J Clin Microbiol 43: 391-318, 2000
43. Kim JH, Jung SY, Lee YJ, Song KJ, Kwon D, Kim K, Park S, Im KI, Shin HJ: Effect of therapeutic chemical agents in vitro and on experimental meningoencephalitis due to Naegleria fowleri. Antimicrob Agents Chemother 52: 4010-4016, 2008
44. Kim JH, Song AR, Sohn HJ, Lee J, Yoo JK, Kwon D, Shin HJ: IL-1beta and IL-6 activate inflammatory response of astrocytes against Naegleria fowleri infection via the modulation of MAPKs and AP-1. Parasite Immunol 35(3-4): 120-128, 2013
45. Kim JH, Yang AH, Sohn HJ, Kim D, Song KJ, Shin HJ: Immunodominant antigens in Naegleria fowleri excretory--secretory proteins were potential pathogenic factors. Parasitol Res 105(6):1675-1681, 2009
46. Lattyak M, Cabral G, Marciano-Cabral F: Scanning electron microscopy of trophozoites of Naegleria species. Proc Elect Microsc Soc Am 43: 642-643, 1985
47. Lether H, Silvany R, Alizadeh H, Huang J, Niederkorn JY: Mannose induce the release of cytophathic factors from Acanthamoeba Keratitis. Infect Immun 66:
41 5-10, 1998
48. Locksley RM, Killeen N, Leonardo MJ: The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104(4): 487-501, 2001 49. Ma P, Visvesvara GS, Martinez AJ, Theodore FH, Daggett PM, Sawyer TK:
Naegleria and Acanthamoeba infections: review. Reviews infect dis 12(3): 490-513, 1990
50. Maher FO, Martin DS, Lynch MA: Increased IL-1beta in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiol Aging 25(6): 795-806, 2004
51. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S: The portein kinase complement of the human genome. Science 298(5600): 1912-1934, 2002
52. Marciano-Cabral F and Fulford DE: Cytopathology of pathogenic and nonpathogenic Naegleria species for cultured rat neuroblastoma cells. Appl Eviron Microbiol 51: 1133-1137, 1986
53. Marciano-Cabral F and John DT: Cytophathologenicity of Naegleria fowleri for rat neuroblastoma cell cultures: scanning electron microscopy study. Infect Immun 40: 1214-1217, 1983
54. Marciano-Cabral F, MacLean R, Mensah A, LaPat-Polasko L: Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl Envion Microbiol 69: 5864-5869, 2003
42
55. Marciano-Cabral F, Patterson M, John DT, Bradley SG: Cytopathogenicity of Naegleria fowleri and Naegleria gruberi for established mammalian cell cultures. J Parasitol 68: 1110-1116, 1982
56. Marciano-Cabral F, Puffenbarger R, Cabral GA: The increasing importance of Acanthamoeba infections. J Eukaryot Microbiol 47(1): 29-36, 2000
57. Marciano-Cabral F: Biology of Naegleria spp. Microbiol Rev 52: 114-133, 1988
58. Martinez AJ: Free living amebas: natural history, prevention, diagnosis, pathology and treatment of the disease. Boca Raton: CRC Press Inc 156, 1985 59. Martinez DY, Seas C, Bravo F, Legua P, Ramos C. Cabello AM, Gotuzzo E:
Successful treatment of Balamuthia mandrillaria amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis 51(2): e7-e11, 2010
60. Mikocka-Walus A, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ: Controversies surrounding the comorbidity of depression and anxiety in inflammatory bowel disease patients: a literature review. Inflamm Bowel Dis 13(2): 225-234, 2007
61. Mitra MM, Alzadeh H, Gerard HR, Niederkorn JY: Characterization of plasminogen activator produced by Acanthamoeba castellanii. Mol Biochem Parasitol 73:157-164, 1995
43
Chokshi R, Chokshi B, James ER: Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol 78: 377-385
63. Murray-Calderon P and Connolly MA: Interleukin-1 beta inhibits glutamate release in hippocamous of young, but not aged, rats. Neurobiol Aging 18(3): 343-348, 1997
64. Nakajuma K and Kohsaka S: Function roles of microglia in the brain: (Review). Neurosci Res 17: 187, 1993
65. Nmorsi OP, Nkot BP, Che J: Relationship between pro-and anti-inflammatory cytokines profiles and some haematological parameters in some Cameroonians infected with Onchocerca volvulus. Asian Pac J Trop Med 5(9): 713-717, 2012 66. Oh YH, Jeong SR, Kim JH, Song KJ, Kim K, Park S, Sohn S, Shin HJ:
Cytopathic changes and pro-inflammatory cytokines induced by Naegleria fowleri trophozoites in rat microglial cells and protective effects of an anti-Nfa1 antibody. Parasite Immunol 27(12): 453-459, 2005
67. Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ: Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp. Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol 44(10): 3589-3595, 2006 68. Rezende-Oliveria K, Silva NM, Mineo JR, Rodrigues Junior V: Cytokine and
chemokines production by mononuclear cells from paturient woman after stimulation with live Toxoplasma gondii. Placenta 33(9): 682-687, 2012
44
identification of Naegleria spp. by real-time PCR and melting-curve analysis. Appl Environ Microbiol 72(9): 5857-5863, 2006
70. Rojas-Bernabe A, Garcia-Hernamdez O, Maldonado-Bernal C, Delegado-Dominguez J, Ortega E, Gutierrez-Kobeh L, Beker I, Aguirre-Garcia M: Leishmania mexicana lipophosphoglycan activate ERK and p38 MAP kinase and induces production of proinflammatory cytokines in human macrophages through TLR2 and TLR4. Parasitology 141(6): 788-800, 2014
71. Scheld WM, Whitley RJ, Durack DT: Infections of the central nervous system. Raven Press, 43: 403-447, 1991
72. Schuster FL and Rechthand E: In vitro effects of amphotericin B on growth and ultrastructure of the amoeboflagellates Nagleria gruberi and Naegleria fowleri. Antimicrob Agents Chemother 8: 591-605, 1975
73. Schuster FL and Visvesvara GS: Amebae and ciliated protozoa as causal agents of waterborne zoonotic disease. Veter Parasitol 126(1-2): 91-120, 2004
74. Schuster FL and Visvesvara GS: Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34(9): 1001-1027, 2004
75. Schuster FL, Guglielmo BJ, Visvesvara GS: In-vitro activity of miltefosine and voriconazole in clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 53: 121-126, 2006
45
76. Sedgwick JD, Schwender S, Gregersen R, Dorries R: Resident macrophages (ramified microglia) of the adult brown norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med 177: 1145, 1993
77. Shakoor S, Beg MA, Mahmood SF, Bandea R, Sriram R, Noman F, Visvesvara GS, Zafar A: Primary amoebic meningoencephalitis caused by Naegleria fowleri, Karachi, Pakistan. Emerg Infect Dis 17(2): 258-61, 2011
78. Shin HJ, Cho MS, Jung SU, Kim HI, Park S, Kim HJ, Im KI: Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of Naegleria fowleri. J Eukaryot Microbiol 48(6): 713-717, 2001
79. Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K: Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55(4): 412-424, 2007
80. Sleigh MA: Protozoa and other protists. Edward Arnold, London, UK 3429, 1989
81. Sohn HJ, Kim JH, Shin MH, Song KJ, Shin HJ: The Nf-actin gene is an important factor for food-cup formation and cytotoxicity of pathogenic Naegleria fowleri. Parasitol Res 106(4): 917-924, 2010
82. Song KJ, Song KH, Kim JH, Sohn HJ, Lee YJ, Park JE, Shin HJ: Heat shock protein 70 of Naegleria fowleri is important factor for proliferation and in vitro cytotoxicity. Parasitol Res 103(2): 313-317, 2008
46
83. Song KJ, Jeong SR, Park S, Kwon MH, Im KI, Pak JH, Shin HJ: Naegleria fowleri: functional expression of the Nfa1 protein in transfected Naegleria gruberi by promoter modification. Exp Parasitol 112(2):115-120, 2006
84. Steele C, Fidel PL Jr: Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun 70(2): 577-583, 2002
85. Suzumura A, Sawada M, Yamamoto H, Marrunouchi T: Transforming growth factor-β suppresses activation and proliferation of microglia in vitro. J Immunol 151: 2150-2158, 1993
86. Townsend GC and Scheld WM: Infections of the celtral nervous system. Adv Intern Med 43: 403-447, 1998
87. Visvesvara GS: Amebic meningoencephalitides and keratitis: challenges in dignosis and treatment. Curr Opin Infect Dis 23: 590-594, 2010
88. Wood P: Neuroinflammation: Mechanisms and Management. Humana Press, 375, 2998
89. Yang Z and Panjwani N: Pathogenesis of Acanthamoeba keratitis: carbohydrate mediated host-parasite interactions. Infect Immun 65: 439-445, 1997
90. Yoder JS, Eddy BA, Visvesvara GS, Capewell L, Beach MJ: The epidemiology of primary amoebic memingoencephalitis in the USA, 1962-2008. Epidemiol Infect 138(7): 968-975
47
91. Zielasek J, Archelos JJ, Toyka KV, Hartung HP: Expression of intercellular adhesion molecule-1 on rat microglial cells. Neurosci Lett 153: 136, 1993
48 -국문요약-
BV2 마이크로글리아 세포에 대한 파울러자유아메바 배설-분
비 단백질의 효과에 관한 연구
아주대학교 대학원 의생명과학과 이 진 영 (지도교수: 신 호 준) 자유생활을 하는 파울러자유아메바 (Naegleria fowleri)는 전 세계에 걸치는 다양한 서식 환경에서 발견되고, 사람과 실험 동물에서 급성 중 증의 출혈성 원발성 아메바성 수막뇌염의 원인이 되며, 접촉성 또는 비 접촉성 병원성 기작에 의해 숙주 세포를 죽음에 이르게 하는 것으로 알 려져 있다.파울러자유아메바 감염의 접촉성 기작에는 Nfa1, Nf-actin 및 heat shock protein 70s 같은 다양한 유전자(단백질)들이 병원성과 연관되어 있고, 파울러자유아메바로 부터 분비되는 몇몇 단백질들은 비접촉성 병 인 기작에 관여하는 것으로 알려져 있는데, 파울러자유메바의 배설-분 비 단백질에는 포스포리파아제 (phospholipase), 단백질 분해효소 (protease), peroxiredoxin 및 thrombin receptor 같은 다양한 다양한 단백질들이 포함되어 있다. 한편, 원발성 아메바성 수막뇌염 발생시에
49 파울러자유아메바 배설-분비 단백질의 작용 기작은 정확히 밝혀지지 않 은 상태이다. 이 연구에서는 파울러자유아메바 배설-분비 단백질에 의 해 표적세포에서 유도되는 염증 반응의 변화를 관찰하여, 원발성 아메바 성 수막뇌염을 야기하는 파울러자유아메바의 비접촉성 병인기전을 이해 하고자 하였다. 파울러자유아메바의 배설-분비 단백질에 의해 표적세포 로 사용된 BV2 마이크로글리아 세포에서 부분적으로 IL-1α 과 TNF-α 과 같은 몇몇 사이토카인의 발현이 증가하였고, 이 사이토카 인들의 발현은 MAPKs (Mitogen activated protein kinase) 경로에 의 해 매개되었다. 이런 연구 결과에서 파울러자유아메바의 배설-분비 단 백질은 BV2 마이크로글리아 세포에서 MAPKs 신호 전달 경로를 경유 하여 염증 반응을 유도하고 파울러자유아메바에 의한 원발성 아메바성 수막뇌염 유도시 비접촉성 병인 기전에 중요한 역할을 할 것으로 생각 된다. Keywords: 파울러자유아메바, 단백분해효소, 배설-분비 단백질, 원발성 아메바성 수막뇌염, 사이토카인, 신경소교세포, MAPK 신호전달
50
PART II
A novel cathepsin B and cathepsin B-like cysteine protease
from Naegleria fowleri excretory secretory proteins
51
I. Introduction
A. ESP in Naegleria fowleri
Naegleria fowleri is a free-living amoeba, which causes acute, fulminating, hemorrhagic primary amoebic meningoencephalitis (PAM) in healthy children and young adults. N. fowleri has been isolated from freshwater lakes, hot springs, chlorinated swimming pools and soil (Cerva, 1971; Brown et al., 1983; Kyle and Noblet, 1985; Marciano-cabral and Cabral, 2007). A characteristic of PAM is the rapid onset of symptoms following exposure. N. fowleri trophozoites migrate to the brain tissue, meninges, and CSF through the olfactory bulb by penetrating the nasal epithelium (Ma et al., 1990). The disease progresses rapidly, and without prompt diagnosis and intervention, death usually occurs within one week or earlier (Visvesvara et al., 2007).
Elucidation of pathogenicity-related factors in N. fowleri is important for understanding the mechanism of parasite-host interactions in PAM. Amoebic pathogenicity may be resulted by complex processes including contact-dependent and contact-independent mechanisms, both which lead to host cell death. In previous studies, several agents have been reported that they are involved in the contact-dependent pathogenic mechanism of N. fowleri pathogenic related factors such as the Nfa1 protein, Heat shock protein 70, and Nf-actin, by participating in
52
the formation of phagocytic food-cup (Song et al., 2006; Song et al., 2008; Sohn et al., 2010).
In the contact-independent mechanism, the phospholipase A and B activity or a cytolytic factor causing destruction of cell membranes, the neuraminidase or elastase activity facilitating destruction of tissue culture cells, a perforin-like, pore-forming protein that lyses target cells, and the presence of a cytopathic protein within Naegleria amoebae that triggers the apoptosis pathway in susceptible tissue culture cells, were induced in amoebic pathogenesis (Schuster and Visvesvara, 2004; Marciano-Cabral and Cabral, 2007).
The excretory/secretory proteins of parasite are related with pathogenicity in contact-independent mechanism. The important roles for parasites ESP in pathobiological events of parasitic organisms have been largely addressed. Echinococcus granulosus secretes a protein, which inhibits neutrophil chemotaxis and thereby affects the function of host cells (Shepherd et al., 1991). The excreted/secreted antigens of Toxoplasma gondii play critical roles in the regulation of host’s immune responses and induce protective immune responses in the hosts (Darcy et al., 1988), which collectively suggest that these antigens are attractive candidates for the development of vaccine against toxoplasmosis. N. fowleri also secrets large number of proteins into outside of the parasite and these proteins may play various pathobiological functions required for survival of the parasite and host-parasite interactions. Treatment of Nf-EPSs to Chinese hamster ovary (CHO)
53
cells resulted in cytotoxic effect (Lee et al., 2012). It was also reported that Nf-ESPs may play a critical role for host cell invasion by the parasite. Nf-Nf-ESPs also degraded host immunoglobulins, which suggesting its possible role in host immune evasion for parasite survival in the host (Kim et al., 2008; Kim et al., 2009). However, only limited information on the nature of Nf-ESPs and biological roles of each component in the ESP is currently available. And therefore, characterization of biological and functional properties of the Nf-EPSs would be important to understand the pathobiogical events occurred by the parasite infection.
B. Cysteine proteases
Cysteine proteases, also known as thiol proteases, are ubiquitous enzymes that found in all living organisms from viruses to mammals. They are involved in diverse biological events in the organisms and play pivotal roles for the maintenance of homeostasis and survival of the organisms. They are classified into 14 superfamilies and several currently unassigned families based on MEROPS classification criteria, but they share a common catalytic mechanism, in which the sulphur atom of a cysteine residue in a catalytic triad or dyad acts as an attacking nucleophile (Smooker et al., 2010). Also, a histidine side chain is involved in a hydrogen acceptor/shuttle role. They are usually synthesized as preproenzymes, which are comprised with an N-terminal signal peptide, a prodomain, and a mature