저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Doctoral Thesis in Philosophy
Establishment of an acanthamoebic keratitis
mouse model
by
Hee Kyoung Kang
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School of Ajou University
Establishment of an acanthamoebic keratitis
mouse model
by
Hee Kyoung Kang
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements for the
Degree of Doctor of Biomedical Sciences
Supervised by
Ho-Joon Shin, Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School of Ajou University
i
-Abstract-Establishment of an acanthamoebic keratitis mouse model
Acanthamoeba castellanii invades the cornea and results in acanthamoebic keratitis (AK). It is mainly triggered by risk factors such as corneal injury, faulty eye surgery, and poor contact lens use. AK is typically chronic and progressive with inflammatory cells infiltrating the corneal stroma. Many in vitro studies of the pathogenesis of AK and development of therapeutic drugs need to be confirmed by the in vivo experiments. In this study, development of AK animal model was attempted. BALB/c mouse cornea was scratched using a syringe needle and ophthalmic surgical blade under anesthesia. A. castellanii (trophozoites equally mixed with cysts) cultures were serially diluted from 1 × 106 to 0.3 × 105 and placed on a 2-mm contact lens to be inserted into the mouse eye. To allow complete contact of A. castellanii with the damaged cornea, the eyelid was sutured. The keratitis symptoms in mouse eyes were grossly observed from day 1 to 7, post-inoculation. In addition, to confirm the AK development, the genomic DNA was extracted from the mouse ocular tissue with AK. For the amplification of acanthamoebic 18S-rRNA, PCR was performed with P-FLA primers. It was observed that the experimental mouse AK, characterized by typical hazy blurring and melting of the mouse cornea, developed on day 1 post-inoculation and was induced with at least 0.3 × 105 amoeba cells. With 0.5 × 105 amoeba inoculation, the experimental mouse AK was prolonged for 2 months (no more extended observation).
ii
On the contrary, the sham-operated mouse eye was clear during the observation periods. In addition, PCR products amplified from the extracted mouse eye DNA, using P-FLA primers confirmed the AK development during infection periods. Many commercial lens solutions are less effective against amoebicidal effects of Acanthamoeba. To confirm the amoebicidal effect of the commercial lens solutions, 0.5 × 105 amoebae were pre-treated with solutions A or B for 1, 6, 12 or 24 h, and inoculated into the eye of a previously established AK mouse model. AK occurrence by A. castellanii treated with lens solution A or B for 1 or 6 h was not grossly observed on day 1 and 2, but the infection progressed with a typical circulatory edema and keratitis, on days 3, 5, and 7. Upon pre-treatment for 12 or 24 h, AK development was not observed on days 1, 2, or 3 but progressed to corneal infection on days 5 and 7. Finally, the commercial contact lens solutions did not display a protective effect against AK development, although they affected the occurrence by delaying the AK. In conclusion, it is suggested that the present AK mouse model may serve as an important in vivo model for various future studies.
Keywords: Acanthamoeba castellanii, Acanthamoebic keratitis, Encystment, 18S-rRNA amplification, Commercial lens solution
iii
TABLE OF CONTENTS
ABSTRACT ……….. i
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· v
I. INTRODUCTION ··· 1
II. MATERIALS AND METHODS ··· 11
A. Cultivation of Acanthamoeba castellanii ··· 11
B. Encystation of A. castellanii trophozoites ··· 11
C. Experimental acanthamoebic keratitis development in mouse ··· 12
(1) Inoculation of A. castellanii into mouse eye ··· 12
(2) Observation of experimental mouse keratitis symptom ··· 14
D. Confirmation of experimental mouse keratitis ··· 15
(1) Genomic DNA extraction from keratitis-induced mouse eyeball ··· 15
(2) PCR with A. castellanii-specific primers ··· 15
(3) 18S-rDNA sequencing and homology analysis ··· 16
E. Optimal numbers of A. castellanii for the development of mouse keratitis ··· 16
iv
(1) Pre-treatment of commercial lens solutions on A. castellanii ··· 17
(2) Effect of commercial lens solutions on AK mouse model ··· 17
G. Ethics statement ··· 17
III. RESULTS ··· 18
A. Morphological observation of A. castellanii cyst formation ··· 18
B. The occurrence of acanthamoebic keratitis in mouse model ··· 20
C. A. castellanii DNA amplification from mouse eye tissue developed acanthamoebic keratitis ··· 22
D. Optimal numbers of A. castellanii for keratitis development in mouse ··· 26
E. Establishment of acanthamoebic keratitis mouse model ··· 31
F. Effect of commercial contact lens solution on the AK development in mice ··· 37
(1) Morphology of A. castellanii pre-treatment with solution A or B ··· 37
(2) Change of acanthamoebic keratitis development in mice ··· 40
IV. DISCUSSION ··· 46
V. CONCLUSION ··· 54
REFERENCES ··· 56
v
LIST OF FIGURES
Figure 1. Life of Acanthamoeba species. ··· 2
Figure 2. Acanthamoeba cysts morphological group II & III ··· 4
Figure 3. Acanthamoeba infection in human ··· 6
Figure 4. Procedure of the experimental acanthamoebic keratitis development ··· 13
Figure 5. Morphological changes of A. castellanii trophozoites into pre-cysts and cysts ··· 19
Figure 6.Induction and observation of acanthamoebic keratitis in experimental mouse models ··· 21
Figure 7. Amplification of A. castellanii 18S-rRNA from AK mouse eye tissue ··· 23
Figure 8. The nucleotide sequence and homology analysis of 18s-rRNA gene obtained from keratitis-induced mouse eye ··· 24
Figure 9. Observation of acanthamoebic keratitis development in mice inoculated with serially diluted A. castellanii (I) ··· 28
Figure 10. Observation of acanthamoebic keratitis development in mice inoculated with serially diluted A. castellanii (II) ··· 30
vi
Figure 11. Observation of Acanthamoebic keratitis infection with 0.5 × 105
A. castellanii, and 18S-rRNA amplification on mice eye ··· 32
Figure 12. The nucleotide sequence and homology analysis of 18S-rRNA gene
obtained from keratitis-induced mouse eye ··· 34
Figure 13. Morphological change of A. castellanii (trophozoites and cysts) treated
with commercial lens solution A or B ··· 38
Figure 14. Observation of acanthamoebic keratitis upon pre-treatment of solution A · 42
Figure 15. Observation of acanthamoebic keratitis upon pre-treatment of solution B · 44
1
I. INTRODUCTION
Acanthamoeba spp. are free-living amoebas, belonging to the genus Acanthamoeba. They are ubiquitously found in a variety of habitats, including soil, dust, fresh water, cooling tower of air conditioning, and domestic tap water. They also exist as a contaminant in tissue culture, contact lenses, and lens cases and containers
(YuGuang He, 1922; Auran et al., 1987; Visvesvara and Stehr-Green, 1990; De Jonckheere, 1991; Mergeryan, 1991; Szenasi et al., 1998; Marciano-Cabral et al., 2000; Schuster and Visvesvara, 2004a). Under environmental conditions, Acanthamoeba spp. feed on various Gram-negative bacteria, green algae, and yeast (Marciano-Cabral et al., 2000).
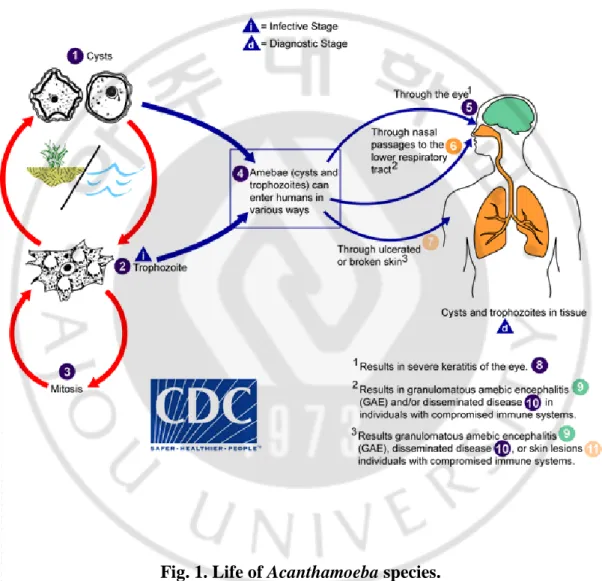
Acanthamoeba spp. exhibit a typical protozoan life cycle, consisting of an amoeboid trophozoite stage and a resting cyst stage. Adverse environmental and nutritional conditions, such as low temperatures, extreme dryness, and exhaustion of nutrition supply, induce the transformation of trophozoites into cysts. The cyst stage of Acanthamoeba spp. is resistant to physical, chemical, and radiological conditions (Aksozek et al., 2002; Moon et al., 2011). The trophozoite moves using pseudopodia of a size 25–50 μm. The resting cyst is 15–30 μm in size, and consists of thick double walls (Fig. 1) (Marciano-Cabral and Cabral, 2003).
2
3
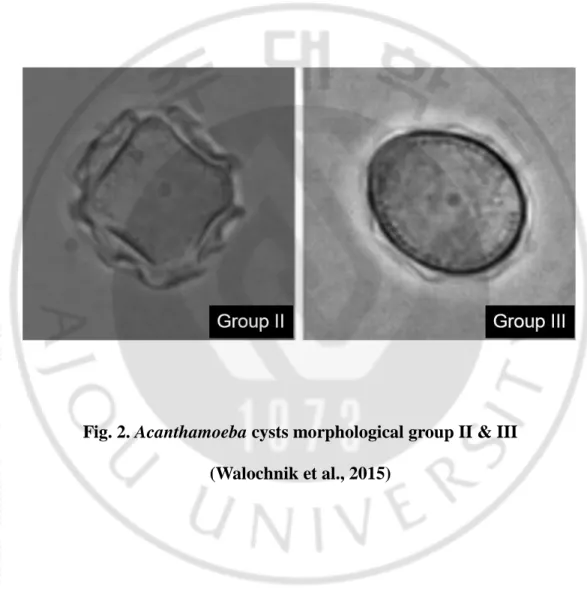
Acanthamoeba species are divided into three types of morphology groups, according to the size and shape of the cyst (Costas and Griffiths, 1985). Group I consist of species characterized by large amoebas, with cysts of 16–30 μm in size. The largest number of amoeba species belongs to Group II, with polygonal cysts of less than 18 μm in size. A. castellanii and A. polyphaga, causing acanthamoebic keratitis (AK) belong to Group II. Group III also has cysts less than 18 μm in size, but with subtle morphological differences (relatively round form of cyst) (Fig. 2).
4
Fig. 2. Acanthamoeba cysts morphological group II & III (Walochnik et al., 2015)
5
However, morphology-based species classification is unreliable due to changes in the cyst morphology. There have been efforts to use non-morphological criteria, such as protein and isoenzyme profiling, mitochondrial DNA polymorphism, and 18S-rDNA sequencing as adjuncts to species differentiation based on morphological characteristics (Jonckheere, 1983; Daggett et al., 1985; Johnson et al., 1990; Chung et al., 1998; Visvesvara et al., 2007).
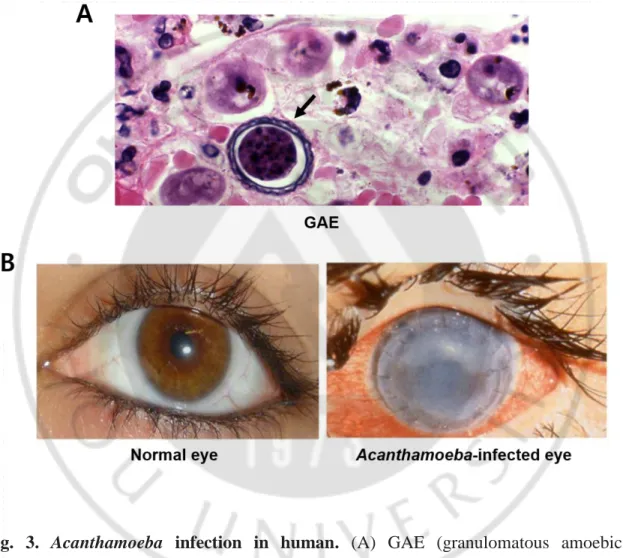
Currently, as a result of phylogenetic analysis based on the 18S-rRNA gene, Acanthamoeba exists in more than 24 species and 17 genotypes (T1–T17) (Stothard et al., 1998; Booton et al., 2005; Corsaro and Venditti, 2010; Nuprasert et al., 2010). Among them, T4 type is mainly associated with the human infections, and includes A. culbertsoni, which causes GAE (granulomatous amoebic encephalitis) (Fig. 3), A. castellanii, A. polyphaga which cause acanthamoebic keratitis (AK) (Fig. 3), and A. healyi and A. rhysodes(Byers et al., 1990; Visvesvara and Stehr-Green, 1990; van Klink et al., 1992; Im and Kim, 1998; Stothard et al., 1998; Corsaro and Venditti, 2010; Visvesvara, 2010; Alves et al., 2018).
6
Fig. 3. Acanthamoeba infection in human. (A) GAE (granulomatous amoebic encephalitis) due to Acanthamoeba sp. cyst in brain tissue (arrow). (B) Infection of acanthamoebic keratitis (Siddiqui and Khan, 2012).
7
AK can occur in non-contact lens users, but most commonly detected in people using contact lenses. The causes of amoebic keratitis include prolonged contact lens wear, lack of personal hygiene, contact lens contamination, and exposure to contaminated water (Khan, 2006; Siddiqui and Khan, 2012). Furthermore, the corneal stroma can be infected due to corneal injury and eye surgery. When a contact lens is worn for an extended time, oxygen is blocked, weakening the basal layer, and causing excessive irritation of the cornea, thereby creating a favorable environment for keratitis (Auran et al., 1987). Also, inadvertent care of contact lenses can cause amoeba contamination, wherein amoebas can infect eye through the contact lens or the lens solution in a contaminated storage case (Lindquist et al., 1988a; Kilvington and Larkin, 1990; Walochnik et al., 2000; Hiti et al., 2002; Hiti et al., 2005; Fears et al., 2018).
In AK, the amoeba infiltrates corneal stroma and epithelial cells, with progressive infiltration of the inflammatory cells. The symptoms of infection are unilateral red eye, severe unbearable pain, foreign body sensation, blurred vision, ring-shaped stromal infiltrates and photophobia, which are often misdiagnosed initially as herpes simplex virus infection, or bacterial or fungal keratitis (Fig. 3). However, once the disease develops, it is not accessible to treatment, and eventually progresses to chronic. AK is a painful infection that threatens the sight with excruciating pain. Early diagnosis and laboratory tests for AK are important for early treatment and prevention of the irreversible corneal damage. If the infection is not treated urgently, it can lead to corneal ulcers, eventual blindness and removal of the nucleus (Jones et al., 1975; Auran et al., 1987; Lindquist et al., 1988b; Bacon et al., 1993; Seal, 2003; Hiti et al., 2005; Khan,
8
2006; Behera and Satpathy, 2016).
Diagnosis of AK employs a variety of methods in the laboratory. The use of in vivo confocal microscopy has emerged as a valuable non-invasive tool for the clinical diagnosis of highly sensitive cases of severe infectious keratitis. Confirmatory evidence of infection is to detect amoebas using laboratory-based assays. The most common method is the cultivation of Acanthamoeba from corneal tissue biopsy or contact lenses and cases. Immunofluorescence assays and multiplex real-time PCR methods can also be used (Winchester et al., 1995; Qvarnstrom et al., 2006; Goldschmidt et al., 2009; Vaddavalli et al., 2011).
An effective therapeutic agent for the treatment of AK has not been developed yet. Therefore, it is often treated with antibiotics and antifungal agents. A variety of drugs have been used to treat AK, including propamidine isethionate, dibromopropamidine isethionate, polyhexamethylene biguanide, neomycin, chlorhexidine, polymyxin B, paromomycin, clotrimazole, miconazole, itraconazole and ketoconazole. Brolene, a commercial eye drop containing dibromopropamidine isethionate and propamidine isethionate, is effective in the treatment of Acanthamoeba infection, but may be accompanied by drug toxicity and resistance. A variety of diamidine compounds, synthetic maganins in combination with silver nitrate, triazole and imidazole compounds, phenothiazine, azithromycin and povidone-iodine have been screened in vitro for their efficacy against Acanthamoeba spp. Another drug, miltefosine (hexadecylphosphocholine), has been also shown to have potential activity against the amoeba. Clinically, the infection is treated with polyhexamethylene biguanide (PHMB)
9
or chlorhexidine gluconate, with or without brolene. In some cases, chlorhexidine and penetrating keratoplasty are done together when the treatment fails. Currently, the drugs selected for AK are chlorhexidine gluconate, PHMB, and brolene, which have improved the prognosis of AK patients. However, unwanted effects, such as corneal opacity or scarring may occur after treatment (Seal, 2003; Schuster and Visvesvara, 2004b; Schuster et al., 2006; Niyyati et al., 2009).
The incidence of AK is increasing worldwide, and since the first reported cases of AK in 1973, over 5,000 cases of amoebic keratitis have been reported in the United States in 2006 (Sotelo-Avila et al., 1974; Radford et al., 1995; Heffler et al., 1996; Visvesvara, 2010). In Korea, approximately 42 cases of amoebic keratitis were reported by 2008. In Austria, 89 % of AK patients were reported as contact lens users. Of these, 19 % were undergoing corneal transplantation (Walochnik et al., 2015).
The most common cause of AK infection in contact lens users is closely related to disinfection and storage of contact lenses. Most contact lens users use a convenient, commercial lens solution that primarily cleans, rinses, disinfects, and preserves the lens. However, commercial lens solutions are not completely insecticidal for Acanthamoeba. The Acanthamoeba cyst is known to be resistant to disinfection and washing solutions, as it consists of thick cell walls. Therefore, contact lens users should pay attention not only to tap water but also lens preservation containers and solutions. As prevention is most important, the contact lens should not be worn during swimming, underwater sports activities, or relaxing in the Jacuzzi or hot tub (Zanetti et al., 1995; Hiti et al., 2002; Visvesvara et al., 2007).
10
Contact lens use is steadily increasing AK incidence every year, raising the social health issues. Many patients also require corneal transplantation after treatment and may be re-infected with resting cysts (Hurt et al., 2003). Eighty percent of patients with keratitis are at constant risk as contact lens users, and the number of patients worldwide has increased over the past twenty years due to the increased contact lens use (Centers for Disease, 1986; Stopak et al., 1991; Srinivasan et al., 1993; Ren and Wu, 2010; Cope et al., 2016; Padzik et al., 2016). Number of patients with caustic amoebic keratitis is expected to increase in the future with the generalization and convenience of contact lenses, and amoebic keratitis has been reported worldwide, including in Korea.
Currently, many studies are under progress to treat AK. However, the results of pathologic mechanisms and lesion studies after inducing AK in in vivo animal models are insufficient. The development and establishment of in vivo animal models is essential to the development of therapeutic agents for the in vivo testing of AK's molecular biology, pathology and immunology. However, the research by foreign and domestic researchers is not adequate (Van Klink et al., 1997; Polat et al., 2007).
In this study, We established the mouse models of keratitis using trophozoites and cysts of A. castellanii to study the pathogenesis of AK in the future. We determined the minimum number of amoebas needed to induce keratitis in mice. and the efficacy of commercial lens solutions on removal of A. castellanii.
11
II. MATERIALS AND METHODS
A. Cultivation of Acanthamoeba castellanii
A. castellanii trophozoites were axenically cultured in 75-cm2 culture flask with PYG medium at 30°C (2% protease peptone, 0.2% yeast extract, 0.1 M glucose, 4 mM MgSO47H2O, 0.4 mM CaCl2, 3.4 mM sodium citrate 2H2O, 50 μM Fe(NH4)2(SO4)6H2O, 2.5 mM KH2PO4, 2.5 mM Na2HPO4, pH 6.5), in accordance with the previous reports (Visvesvara and Balamuth, 1975).
B. Encystation of A. castellanii trophozoites
The formation of A. castellanii cysts was induced by cultivation with encystment solution (95 mM NaCl, 5 mM KCl, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3, 20 mM Tris-HCl, pH 9.0) in accordance with the previous reports (Moon et al., 2008; Sohn et al., 2017). Briefly, A. castellanii trophozoites were harvested, washed twice with PBS (pH 7.4) and centrifugated at 1,500 rpm for 3 min. After the final step, the trophozoites were added into 75-cm2 culture flask (concentration adjusted as 1 × 106 cells/ml), and treated with 10 ml of encystment solution for 72 h. To confirm the formation of cysts, morphological changes were observed with an inverted microscope (Olympus, Shinjuku, Tokyo, Japan).
12
C. Experimental acanthamoebic keratitis development in mouse
(1) Inoculation of A. castellanii into mouse eye
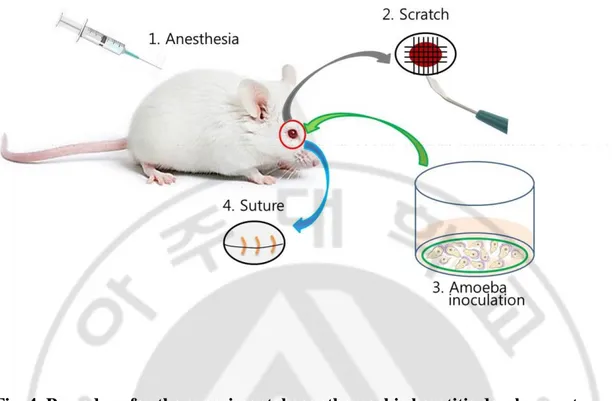
In order to establish AK mouse model, A. castellanii trophozoites and cysts were inoculated into mouse eyes. The commercial contact lenses (Proclear 1 day lens, Cooper Vision Inc, USA) were cut into 2 mm pieces using a punch, and placed in a 24-well plate with 10 μl PBS (pH 7.4). The harvested trophozoites and cysts of A. castellanii were mixed in equally at 5 × 105 cells/ml concentration. Total 1 × 106 cells/ml were placed in 1 ml tube, and centrifugated at 1,500 rpm for 3 min. The pellet was applied on a 2 mm contact lens, and incubated at 30°C for 1 h. 8 weeks old female BALB/c mice (Orient, Seongnam, Korea) were anesthetized with ketamine and rompun solution (Kang et al., 2015). The eyeballs of mouse were scratched with a syringe needle and ophthalmic blade. The A. castellanii-cultivated lens was put into the mouse eyelid and sutured with 6/0 nylon (Woorimedical, Namyangju, Korea) for the complete contact of A. castellanii (Fig. 4). The sham operation was carried out by inoculating only a clear lens into mouse eye without A. castellanii. And then, following procedures were same to above practices.
13
Fig. 4. Procedure for the experimental acanthamoebic keratitis development.
1. Mouse was anesthetized. 2. Eye balls of mouse were scratched. 3. A. castellanii trophozoites and cysts cultivated on the contact lens were inoculated. 4. Mouse eye-lid was sutured. Another eye was used for sham operation as control. (Figure was provided by Prof. Shin).
14
(2) Observation of experimental mouse keratitis symptoms
To observe the keratitis development, the sutures of the mouse eyelid were sequentially removed at intervals from day 1 to 7. The eyeballs were observed with the naked eye for symptoms, such as white ring corneal infection, circular edema with increased central corneal thickness, and possible keratitis circle.
15
D. Confirmation of experimental mouse keratitis
(1) Genomic DNA extraction from keratitis-induced mouse eyeball
To extract the ocular DNA of the keratitis-induced mouse, experiments were performed as follows. After the development of gross keratitis was observed, mouse eyeballs were removed under anesthesia for the periods of observation. Briefly, the eyeball tissue was chopped with a surgical scissor in 100 μl of PBS (pH 7.4) taken in 1 ml tube. Tissue lysis buffer and proteinase K were added in eyeball tissue-containing tubes, according to the instructions of DNA extraction kit (Qiagen, France). After lysis at 56°C, subsequent DNA extraction was performed.
(2) PCR with A. castellanii-specific primers
To observe whether acanthamoebic 18S-rDNA is amplified in the keratitis-induced mouse eyeball, PCR was performed with P-FLA primers, as described previously (Kang et al., 2015). The P-FLA primers (forward 5’-CGCGGTAATTCCAGCTCCAATAGC-3’ and reverse 5’-CAGGTTAAGGTCTCGTT CGTTAAC-3’) were prepared to amplify the 18S-rRNA gene specific for A. castellanii (Tsvetkova et al., 2004). Total 2 ng of genomic DNA was used as a template, and 10 μl of NoblezymeTM PCR Plus Premix 2x (Noble Bioscience Inc., Suwon, Korea) was
16
added to the PCR tube. The PCR conditions with P-FLA primers were as follows; 95°C
for 5 min, 40 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 3.5 min and a final
extension of 72°C for 10 min. After completion of the PCR reaction, the PCR products
were electrophoresed on 1% agarose gel containing ethidium bromide, 0.005% at 100 V for 20 min, and the image was analyzed by Gel Doc (Bio-Rad, USA).
(3) 18S-rDNA sequencing and homology analysis
DNA sequencing was performed to confirm whether the amplified PCR product matched with Acanthamoeba 18S-rDNA. The results were compared to Genebank database with Acanthamoeba 18-rDNA.
E. Optimal numbers of A. castellanii for the development of mouse keratitis
To determine the optimal numbers for the development of AK in mice, A. castellanii trophozoites and cysts (mixed in equal numbers) were serially diluted to 5 × 105, 2.5 × 105, 1.25 × 105, 0.625 × 105, 0.3125 × 105 or 0.1 × 105 cells/ml. The subsequent procedures were same as mentioned for the experimental mouse keratitis development (No. D).
17
F. Effect of commercial lens solutions on AK development in mouse
(1) Pre-treatment of commercial lens solutions on A. castellanii
To confirm the efficacy of the commercial lens solutions (called anonymously A and B), AK was induced with amoebas pre-treated with lens solution A or B. Briefly, total 0.5 × 105 cells of A. castellanii (equal numbers of trophozoites mixed with cysts) were placed in a 1 ml tube and centrifugated at 1,500 rpm for 3 min. The pellet was applied on a 2 mm contact lens in 24-well plate and incubated with commercial lens solutions A or B for 1, 6, 12 or 24 h.
(2) Effect of commercial lens solutions on AK mouse model
A. castellanii pre-treated with lens solution A or B were inoculated into mouse eye, as described previously (No. D). Also, the development of amoebic keratitis in mice was observed according to the previously described procedures (No. D).
G. Ethics statement
The present mouse experiments were reviewed and approved by the Animal Research Ethics committee of Ajou university school of medicine (AJ-IBC-17-09-10).
18
III. RESULTS
A. Morphological observation of A. castellanii cyst formation
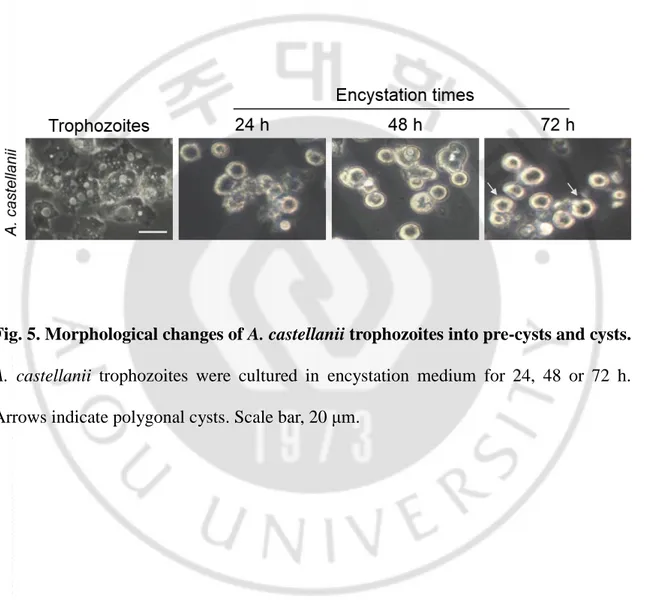
In order to induce the formation of cysts in A. castellanii, trophozoites were cultured for 24, 48, 72 h, and observed with an inverted microscope. It was observed that 24 h after the induction of cyst formation, half of the amoebas were transformed into a pre-cyst with single cell wall. Most of the amoebas were found in a round shaped cyst 48 h after induction. After 72 h induction of cyst formation, various forms of polygonal cysts and pre-cysts were observed as acanthamoeba cysts (Fig. 5).
19
Fig. 5. Morphological changes of A. castellanii trophozoites into pre-cysts and cysts.
A. castellanii trophozoites were cultured in encystation medium for 24, 48 or 72 h. Arrows indicate polygonal cysts. Scale bar, 20 μm.
20
B. The occurrence of acanthamoebic keratitis in mouse model
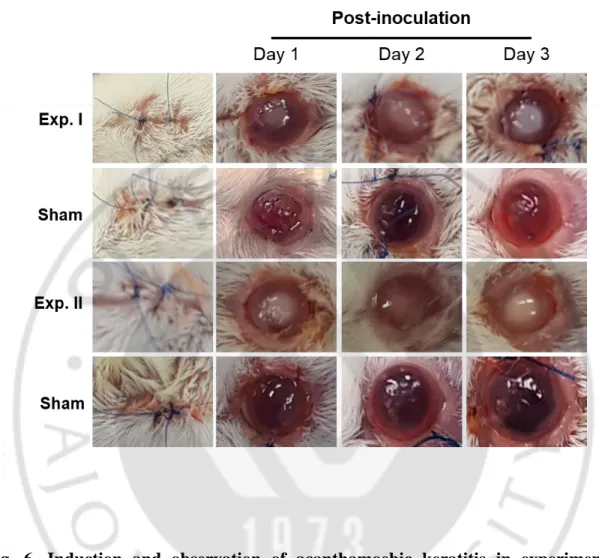
To induce acanthamoebic keratitis (AK), 2 mm lens cultivated with 1 × 106 A. castellanii (trophozoites and cysts mixed in equal numbers), was inserted into the mouse eye. The occurrence of keratitis symptoms was observed in mouse eyes between day 1 and 3. On the first day of AK induction, small, white, round edema was observed in the mouse eye, and typical round edema was diagnosed on the day 2 and 3. On the other hand, the sham operation did not show any symptoms of AK (Fig. 6).
21
Fig. 6. Induction and observation of acanthamoebic keratitis in experimental mouse models. To induce keratitis, 1 × 106 of A. castellanii (trophozoites and cysts mixed in equal numbers) were cultivated on the lens and inoculated into the mouse eye. AK symptoms were observed from day 1 to 3. All experiments were repeated (Exp. I and Exp. II). (Sham: sham operation, Exp: experiment).
22
C. A. castellanii DNA amplification from mouse eye tissue developed acanthamoebic keratitis
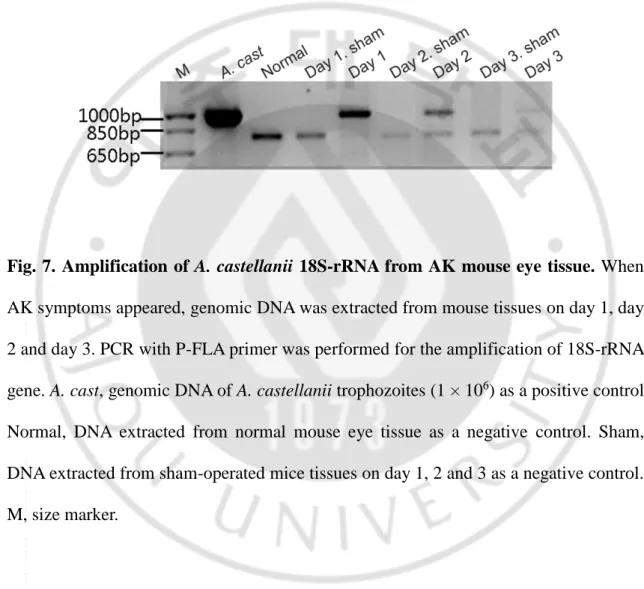
In order to identify the cause of AK development in mice, genomic DNA was extracted from eye tissues, and PCR with P-FLA primers was performed for the 18S-rRNA gene. DNA samples from the infected mouse eyeballs gave an amplicon of 1,080 bp size on day 1, 2 and 3, which was same as that of A. castellanii used as a positive control. On the other hand, the same amplicon was not obtained from the normal or sham-operated mouse DNA (Fig. 7).
PCR products amplified with P-FLA primers were subjected to DNA sequence analysis and gene homology search. On comparing the results with the 18S-rRNA of A. castellanii, the amplified products on day 1, 2 and 3 showed 97, 96 and 97 % homology, respectively (Fig. 8).
23
Fig. 7. Amplification of A. castellanii 18S-rRNA from AK mouse eye tissue. When
AK symptoms appeared, genomic DNA was extracted from mouse tissues on day 1, day 2 and day 3. PCR with P-FLA primer was performed for the amplification of 18S-rRNA gene. A. cast, genomic DNA of A. castellanii trophozoites (1 × 106) as a positive control. Normal, DNA extracted from normal mouse eye tissue as a negative control. Sham, DNA extracted from sham-operated mice tissues on day 1, 2 and 3 as a negative control. M, size marker.
25
Fig. 8. The nucleotide sequence and homology analysis of 18S-rRNA gene obtained from keratitis-induced mouse eye. PCR products (bands figures on left) on day 1 (A),
2 (B), and 3 (C) post-inoculation was sequenced and confirmed by NCBI BLAST search (right box), and the homology was analyzed with BLAST search (below box).
26
D. Optimal numbers of A. castellanii for keratitis development in mouse
To determine the optimal numbers for the development of AK in mice, serially diluted A. castellanii (trophozoites and cysts equally mixed) were inoculated into the mouse eye.
It was observed that with 5, 2.5, 1.25 and 0.625 × 105 number of A. castellanii, the keratitis symptoms were observed in all mice eyes between day 1 and 3. On the first day of AK induction, weak infection, characterized by cloudy eyeball, was observed in all mice eyes, along with a progressive corneal infection on day 2 and 3. On the other hand, the sham-operated mice did not show any apparent keratitis symptoms (Fig. 9).
In order to identify the cause of AK development in mice, genomic DNA extracted from mice eye tissues was amplified by PCR with P-FLA primers specific for 18S-rRNA gene. As a result, DNA samples of keratitis-induced mouse eyeballs gave 1,080 bp size of amplicon on day 1, 2 and 3, which was same as that of A. castellanii used as a positive control. On the other hand, same amplicon was not amplified from the normal or sham-operated mouse DNA (Fig. 9).
With 0.3125 or 0.1 × 105 of A. castellanii inoculation, no apparent keratitis induction in mice eyes was observed on day 1. Subsequently, white circular ulcer with increased central corneal infection of the mouse eye was observed on day 2 and 3. On the other hand, the sham-operated eye did not show any symptom (Fig. 10).
In the results of identification of 18s-rRNA gene for the cause of acanthamoebic keratitis development in mice, DNA samples of mouse eyeballs inoculated with 0.315
27
and 0.1 × 105 ofA. castellanii did not show an amplification of 1,080 bp corresponding to 18S-rRNA gene on day 1 and 3. On the other hand, same amplicon was not amplified from the normal or sham-operated mouse DNA (Fig. 10).
PCR products amplified with P-FLA primers were subjected to sequence analysis and gene homology search. On comparing with the 18S-rRNA of A. castellanii, the amplified bands in all samples showed the 87–99 % homology, respectively (Data not shown).
29
Fig. 9. Observation of Acanthamoebic keratitis development in mice inoculated with serially diluted A. castellanii (I). A. castellanii (equally mixed trophozoites and
cysts) were serially diluted to 5 × 105, 2.5 × 105, 1.25 × 105, and 0.625 × 105 and inoculated into mice eyes; and AK symptoms were observed from day 1 to 3 (Sham: sham operation). Regardless of the occurrence of keratitis symptoms, genomic DNA was extracted from all mice tissues on day 1, day 2, and day 3. Each band was PCR-amplified with P-FLA primers for the confirmation of AK development in mice. A. cast, genomic DNA of A. castellanii trophozoites (1 × 106) as a positive control; Normal, DNA extracted from normal mouse eye tissue as a negative control; sham, DNA extracted from sham-operated mouse tissues as a negative control. M, size marker.
30
Fig. 10. Observation of acanthamoebic keratitis development in mice inoculated with serially diluted A. castellanii (II). A. castellanii (equally mixed trophozoites and
cysts) diluted to 0.3125 × 105, and 0.1 × 105 were inoculated into mice eyes to induce keratitis; and AK symptoms were observed from day 1 to day 3 (S: sham operation). Regardless of the occurrence of keratitis symptoms, genomic DNA was extracted from all mice tissues on day 1, day 2, and day 3. The genomic DNA of mice eye was subjected to PCR with P-FLA primers for the amplification of 18S-rRNA gene (right for figures). A. cast, genomic DNA of A. castellanii trophozoites (1 × 106), as a positive control; Normal, DNA extracted from normal mouse eye tissue as a negative control; Sham, DNA extracted from sham-operated mouse tissues as a negative control. M, size marker.
31
E. Establishment of acanthamoebic keratitis mouse model
As a result of confirming the optimal concentration to cause acanthamoebic keratitis (AK) in mice, It was observed that the results of infection with 0.3125 × 105 and 0.1 × 105 of A. castellanii were not constant in repeated experiments. The sufficient number of amoebas that can cause mouse eye keratitis was 0.5 × 105 amoebas. Finally, 0.5 × 105 amoebas on 2 mm lens were inoculated into mice eyes, and the keratitis symptoms were observed from day 1 to 7 post-inoculation. white ring corneal infection, circular edema the mouse eye was observed on day 1, which progressed to corneal infection on day 2 to 7. On the other hand, the sham-operated mice did not show the keratitis symptoms (Fig. 11A).
To confirm the cause of AK development in mice, PCR products from DNA samples of mouse eyeballs on day 1 to 7 post-inoculation were obtained, giving an amplicon of 1,080 bp size, corresponding to 18S-rRNA gene, which was same as that of A. castellanii used as a positive control. On the other hand, the same PCR product from normal and sham-operated mouse DNA was not amplified (Fig. 11B).
PCR products amplified with P-FLA primers were subjected to sequence analysis and gene homology search. On comparing with the 18S-rRNA of A. castellanii, the amplified bands on day 1, 2, 3, 5 and 7 showed 96, 97, 97, 91, 94 and 93 % homology, respectively (Fig. 12).
33
Fig. 11. Observation of acanthamoebic keratitis infection with 0.5 × 105 A.
castellanii, and 18S-rRNA amplification on mice eye. (A) Total 0.5 × 105 A. castellanii (trophozoites with cysts mixed equally) applied on the contact lens were inoculated into mice eye; AK symptoms were observed from day 1 to 7 (S: sham operation, E: experiment). (B) To confirm the causative agent of AK, genomic DNA extracted from the eye tissues on day 1, 2, 3, 4, 5, 6, and 7 was subjected to PCR with P-FLA primers for the amplification of 18S-rRNA gene. A. cast, genomic DNA of A. castellanii trophozoites (1 × 106) used as a positive control; Normal, DNA extracted from normal mouse eye tissue as a negative control; Sham, DNA extracted from the sham-operated eye tissues as a negative control. Exp I, II, III, experiments were practice three times. M, size marker (below bands figure).
36
Fig. 12. The nucleotide sequence and homology analysis of 18S-rRNA gene obtained from keratitis-induced mouse eye. PCR products (bands figures on left) on
day 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), 6 (F), and 7 (G) post-inoculation were sequenced and confirmed by NCBI BLAST search (right box), and the homology was analyzed with BLAST search (below box).
37
F. Effect of commercial contact lens solutions on the AK development in mice
(1) Morphology of A. castellanii pre-treatment with solution A or B
To investigate the effect of the commercial lens solutions, 0.5 × 105 A. castellanii were treated with either solution A or solution B, and cultured for 1, 6, 12, or 24 h. On the observation with an inverted microscope, round morphological shapes were observed from 1 to 6 h, some pre-cystic form appeared at 12 h, and cysts were observed at 24 h (Fig. 13A, B).
39
Fig. 13. Morphological change of A. castellanii (trophozoites and cysts) treated with commercial lens solution A or B. A. castellanii (trophozoite and cyst, mixed equally)
were cultured in commercial lens solution A (A), or B (B) for 1, 6, 12 and 24 h. Arrows indicate pre-cyst and cyst. Scale bar, 20 μm.
40
(2) Change of acanthamoebic keratitis development in mice
To investigate the effect of the commercial contact lens solution, 0.5 × 105 of A. castellanii (trophozoites mixed equally with cysts) pre-treated with solution A or B were inoculated into mouse eye, and keratitis symptoms were observed for 1, 2, 3, 5, or 7 days (Fig. 14).
As a result of the pre-treatment for 1 or 6 h, solution A and B did not show any change in mice eyeball, in comparison with the established AK mouse model (Result E in this paper). Very weak keratitis symptoms were observed on day 1, and a progressive corneal infection on day 2 to 5. On the 7th day, it was confirmed that the typical ulcer circle had worsened (Fig. 14, 15). Subsequently, it was confirmed by PCR with P-FLA primers with an amplicon of the same size (1080 bp) as that of A. castellanii. In addition, all PCR products were sequenced and identified as A. castellanii 18S-rDNA gene (data not shown). On the other hand, control mice (0.5 × 105 amoeba infected) showed the keratitis symptoms.
On pre-treatment of lens with solution A or B for 12 h, the mice did not show the keratitis symptoms on day 1, even though a weak corneal infection was present (Fig. 14, 15). A mild infection was observed on day 2 and 3, and the typical ulcer circle developed on day 5 and 7 (Fig. 14, 15). The results were also confirmed by PCR with P-FLA primers (Fig. 14, 15) and DNA sequencing (data not shown). On the other hand, control mice (0.5 × 105 amoeba infected) showed the keratitis symptoms (Fig. 14, 12h).
41
symptoms were not observed on day 1 and 2. A mild infection was observed on day 3 and the circular edema with increased central corneal involvement developed on the day 5 and 7 (Fig. 14, 15), which was same as the previous established AK mouse model (Result E) (Fig. 14, 15). Subsequently, it was also confirmed by PCR with P-FLA primers (Fig. 14, 15) and DNA sequencing (data not shown). Otherwise, keratitis symptoms observed in control mice (0.5 × 105 amoeba infected) (Fig. 14, 15).
43
Fig. 14. Observation of acanthamoebic keratitis upon pre-treatment with solution A. Solution A was used to treat 0.5 × 105 A. castellanii (trophozoite and cyst equally mixed) for 1, 6, 12 and 24 h. AK was induced in mouse eyes and observed for 1, 2, 3, 5 and 7 days (Sham: sham operation). Genomic DNA of mouse eye tissues on day 1, 2, 3, 4, 5 and 7 was subjected to PCR with P-FLA primers for the amplification of 18S-rRNA gene. A. cast, genomic DNA of A. castellanii trophozoites (1 × 106) as a positive control; Normal, DNA extracted from normal mouse eye tissue as a negative control; (+) control, DNA extracted from AK-mouse eye tissue (inoculated 0.5 × 105 amoeba) as a positive control. M, size marker (below band figures).
45
Fig. 15. Observation of acanthamoebic keratitis upon pre-treatment with solution B. Solution B was used to treat 0.5 × 105 A. castellanii (trophozoite and cyst equally mixed) for 1, 6, 12 and 24 h. AK was induced in mouse eyes and observed for 1, 2, 3, 5 and 7 days (Sham: sham operation). Genomic DNA of mouse eye tissues on day 1, 2, 3, 5 and 7 was subjected to PCR with P-FLA primers for the amplification of 18S-rRNA gene. A. cast, genomic DNA of A. castellanii trophozoites (1 × 106) as a positive control; Normal, DNA extracted from normal mouse eye tissue as a negative control; (+) control, DNA extracted from AK-mouse eye tissue (inoculated 0.5 × 105 amoeba) as a positive control. M, size marker (below band figures).
46
IV. DISCUSSION
Ubiquitous free-living A. castellanii and A. polyphaga cause acanthamoebic keratitis (AK) in animals and in humans, and 85–88% of patients with AK use contact lenses. Furthermore, the number of AK patients is increasing because of the increasing use of contact lenses worldwide (Cerva et al., 1973; Lyons and Kapur, 1977; Brown et al., 1982; Auran et al., 1987; Visvesvara and Stehr-Green, 1990; De Jonckheere, 1991; Paszko-Kolva et al., 1991; Abjani et al., 2016).
Contact lens users are mainly exposed to AK because Acanthamoeba species can attach themselves to the soft surface of contact lenses and the cornea, resulting in progression of infection. The amoebae attached to the surface of the cornea destroy epithelial cells, penetrate the corneal stroma, and cause infection deep inside the stroma (Hadas and Mazur, 1993; Illingworth et al., 1995; Cao et al., 1998; Cho et al., 2000; Marciano-Cabral and Cabral, 2003; Por et al., 2009). The symptoms of AK include photophobia, ring-like stromal infiltrates, ulceration, development of retinal lesions, epithelial defects, lid edema, and severe pain (Kilvington and Larkin, 1990; Lorenzo-Morales et al., 2015).
Since AK is mostly chronic, early diagnosis and treatment are important. Sometimes, AK is misdiagnosed as a fungal or herpes simplex infection, which only involves 10–23% of coinfection by Acanthamoeba (Bacon et al., 1993; Tu et al., 2008b; Szentmary et al., 2012; Bouheraoua et al., 2013). Currently, there is no effective
47
treatment for AK; imidazole, voriconazole, polymyxin B, and polyhexamethylene biguanide as well as mixed drugs that contain antibiotics, antifungals, antiprotozoals, and antivirals are mainly prescribed (Lim et al., 2008; Dart et al., 2009; Lorenzo-Morales et al., 2013).
Furthermore, preventing AK infection is important, which can be done by keeping the contact lens case clean and not wearing lenses while swimming or showering. In addition, exposure to contaminated water and injury to the cornea should be avoided (Maycock and Jayaswal, 2016).
Although there has been some progress in research regarding the infection mechanism of AK in vitro, in vivo studies using animal models have rarely been conducted. In addition, in these previous studies, AK was induced by direct intrastromal injection of the amoebae into mouse cornea using a microneedle (Matthaei et al., 2012). However, this procedure is complicated because the intrastromal cornea needs to be injected precisely, for which ophthalmologists are required. Moreover, this method of inducing keratitis is artificial. Therefore, in this study, I attempted to construct an animal model of AK which can be used easily and quickly. In this model, AK can be induced in mice using contact lenses in a manner similar to that of natural infection.
Other previous studies used pigs, rabbits, and rats as animal models for AK (Alizadeh et al., 1995; Said et al., 2004; Awwad et al., 2007; Vural et al., 2007). In this study too, experiments were first performed using rats. However, these animals were difficult to handle, only a limited number of the animals were available, and the cornea
48
was too thick for AK induction (data not shown). Therefore, the experiment was conducted with mice, which are widely used, cheap, and easy to handle and can be used in large numbers in experiments (Ren and Wu, 2010).
In this study, I constructed an AK mouse model by mixing trophozoite and cyst forms of A. castellanii because in natural AK infections, both trophozoite and cyst forms of A. castellanii are detected in contact lenses. The eyes of the mice were scratched with a syringe needle and ophthalmic blade during AK induction because it is difficult to infect the cornea without any damage. Previous studies have shown that AK incidence is affected by corneal damage before infection (van Klink et al., 1993).
After scratching the mouse eyeball, contact lenses with A. castellanii were placed on the eyes. Next, mouse eyelids were sutured. This is because it is difficult to naturally attach contact lenses to mouse eyeballs as they are rounder than human eyeballs, and it was necessary to ensure complete attachment of the lenses to the cornea. To detect the incidence of AK symptoms, the eyelid sutures were sequentially removed on the days of observation.
To determine the minimum number of Acanthamoeba necessary to induce AK in mice, equal numbers of trophozoites and cysts of A. castellanii were mixed and serially diluted from 1 × 106 to 0.1 × 105 to induce AK. Infection was induced with a minimum of 0.3 × 105 and 0.1 × 105 cells. However, it was not the same as the result of grossly observation and PCR results using the ocular DNAs that caused keratitis in repeated experiments. Therefore, 0.5 × 105 was determined to be the most stable number
49
of A. castellanii for AK induction. After AK induction, symptoms were observed for up to two months and were confirmed to persist and worsen (data not shown). AK induction experiments were at least practiced over three times. Therefore, it was determined that 0.5 × 105 A. castellanii can be used to establish an AK mouse model.
The diagnosis of AK is mainly based on in vitro experiments, in which lenses and lens solution in lens case of patients with AK are cultured or corneal biopsies are performed (Stothard et al., 1998; Stothard et al., 1999; Khan, 2001; Schuster and Visvesvara, 2004a; Qvarnstrom et al., 2006). For accurate diagnosis, PCR can also be used to confirm the amplification of Acanthamoeba DNA (Stothard et al., 1998; Stothard et al., 1999; Khan, 2001; Schuster and Visvesvara, 2004a; Qvarnstrom et al., 2006; Tu et al., 2008a; Tu et al., 2008b). In this study, eye tissues and contact lenses of the mice induced with AK were cultured on PYG medium and non-nutrient (NN) plates. However, the presence of A. castellanii was not confirmed because of rapid fungal growth, even after treatment with antifungal reagents. In addition, the number of amoebae remaining in the tissues and contact lenses was too small to culture. Next, eyes of mice that developed keratitis were stained with hematoxylin-eosin (HE) to confirm the presence of amoebae between the cornea and underneath the cornea. However, A. castellanii was not observed on the mouse eye balls (Fig. 17). It is impossible to observe amoebae under these conditions because the cornea of AK mice is too thin, and it would be difficult to obtain a tissue specimen.
51
Fig. 16. Histology of AK-induced mouse eye.
Corneal stroma and polymorphic inflammatory infiltrates consisting of many lymphocytes were observed on tissue slides with Hematoxylin-eosin staining (100 µm and 200 µm)
52
To address these problems, DNA was extracted from the eye tissue of the AK mice, and PCR was performed with P-FLA primers that amplify the 18S rRNA of Acanthamoeba (Tsvetkova et al., 2004). PCR of DNA from the AK mouse tissue produced an amplicon of the same size (1080 bp) as that of Acanthamoeba 18S-rRNA, and sequence analysis of the obtained PCR products revealed that AK was indeed induced by A. castellanii. The bands below 750 bp were identified as the 18S rRNA of Mus musculus.
Contact lens users mostly use commercial multiple lens solutions. However, commercially available solutions are ineffective in preventing infections because of their poor amoebicidal effects (Radford et al., 2002; Kilvington et al., 2004; Joslin et al., 2006; Lorenzo-Morales et al., 2013). It is known that the effects of antibiotics, antifungal agents, and antiviral agents in commercial lens solutions may affect the trophozoites of the Acanthamoeba but have little effect on the cyst form (Zanetti et al., 1995; Hurt et al., 2001; Hiti et al., 2002). Therefore, to confirm the effectiveness of the AK mouse model established in the present study, the effects of commercial lens solutions A and B, which are widely used worldwide, on AK incidence were tested. After pretreatment with 0.5 × 105 A. castellanii (equal number of trophozoites and cysts) with commercial lens solutions A or B for 1, 6, 12, and 24 h, the shape of all pretreated amoebae changed to that of the pre-cyst and cyst types. The number of amoebae was counted over time to determine if the commercial lens solutions affected amoeba population, but no significant differences were observed between 1 to 24 h (data not
53
shown). Then, the AK mouse model was used to test whether commercial lens solutions A or B could inhibit AK over time. AK mice treated with solutions A or B showed delayed development of AK between day 1 and day 3, but AK was induced in mice treated with solutions A or B by day 5 and day 7. These results indicated that the use of commercial lens solutions may be effective in the short term, but they cannot inhibit AK induction over a long period of use.
In this study, an AK mouse model was established. This mouse model will be an important platform for in vivo testing of AK, for in vitro molecular biology, pathology, and immunology studies, and for the development of new pharmacological drug agents for the treatment of AK.
54
V. CONCLUSION
Acanthamoeba castellanii causes acanthamoebic keratitis (AK). To decipher the exact pathogenesis of AK and development of therapeutic drugs, in vivo model needs to be established. In this study, BALB/c mouse cornea was scratched using a syringe needle and ophthalmic surgical blade under anesthesia. A. castellanii (trophozoites mixed equally with cysts) were serially diluted from 1 × 106 to 0.3 × 105, placed on a 2-mm contact lens, and put into the mouse eye. After the eyelid was sutured, the occurrence of keratitis symptoms was observed from day 1 to 7 post-inoculation. In addition, to confirm the AK development, the genomic DNA was extracted from the infected mouse ocular tissue. Amplification of acanthamoebic 18S-rRNA gene by PCR using P-FLA primers was performed. In conclusion, the experimental mouse AK, characterized by typical hazy blurring and melting of mouse cornea, was developed from day 1 to 7 post-inoculation with 0.5 × 105 amoeba. PCR products from DNA samples of mouse eyeballs on day 1 to 7 were amplified to be same as 18S-rRNA gene of A. castellanii used a positive control. Subsequently, the amplified bands were identified as 18S-rDNA of A. castellanii, which showed 91–97 % homology, by BLAST search. To confirm the effect of the commercial lens solution, 0.5 × 105 amoebas (trophozoites mixed equally with cysts) were treated with solutions A or B for 1, 6, 12 or 24 h, and inoculated into mouse eye (previously established AK mouse model). Thereafter, AK development was observed for 7 days post-inoculation. AK
55
occurrence by A. castellanii treated with lens solution A or B for 1 or 6 h was not grossly observed on day 1 and 2, but the infection progressed gradually with a typical circulatory edema and keratitis on day 3, 5 and 7. Upon pre-treatments for 12 or 24 h, the development of AK was not observed for 1, 2 or 3 days, but progressed to corneal infection at 5 and 7 days. Finally, commercial contact lens solutions did not show the protective effect against AK pathogenesis, although they delayed the occurrence of AK. In conclusion, it is suggested that the present AK mouse model is an important in vivo model for future studies.
56
REFERENCES
1. Abjani F, Khan NA, Yousuf FA, Siddiqui R: Targeting cyst wall is an effective strategy in improving the efficacy of marketed contact lens disinfecting solutions against Acanthamoeba castellanii cysts. Cont Lens Anterior Eye 39: 239-243, 2016
2. Aksozek A, McClellan K, Howard K, Niederkorn JY, Alizadeh H: Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J Parasitol 88: 621-623, 2002
3. Alizadeh H, He Y, McCulley JP, Ma D, Stewart GL, Via M, Haehling E, Niederkorn JY: Successful immunization against Acanthamoeba keratitis in a pig model. Cornea 14: 180-186, 1995
4. Alves D, Goncalves GS, Moraes AS, Alves LM, Carmo Neto JRD, Hecht MM, Nitz N, Gurgel-Goncalves R, Bernardes G, Castro AM, Chalita MR, Vinaud MC: The first Acanthamoeba keratitis case in the Midwest region of Brazil: diagnosis, genotyping of the parasite and disease outcome. Rev Soc Bras Med Trop 51: 716-719, 2018
5. Auran JD, Starr MB, Jakobiec FA: Acanthamoeba keratitis. A review of the literature. Cornea 6: 2-26, 1987
6. Awwad ST, Petroll WM, McCulley JP, Cavanagh HD: Updates in Acanthamoeba keratitis. Eye Contact Lens 33: 1-8, 2007
57
7. Bacon AS, Frazer DG, Dart JK, Matheson M, Ficker LA, Wright P: A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye (Lond) 7 ( Pt 6): 719-725, 1993
8. Behera HS, Satpathy G: Characterisation and expression analysis of trophozoite and cyst proteins of Acanthamoeba spp. isolated from Acanthamoeba keratitis (AK) patient. Mol Biochem Parasitol 205: 29-34, 2016
9. Booton GC, Visvesvara GS, Byers TJ, Kelly DJ, Fuerst PA: Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J Clin Microbiol 43: 1689-1693, 2005
10. Bouheraoua N, Gaujoux T, Goldschmidt P, Chaumeil C, Laroche L, Borderie VM: Prognostic factors associated with the need for surgical treatments in acanthamoeba keratitis. Cornea 32: 130-136, 2013
11. Brown TJ, Cursons RT, Keys EA: Amoebae from antarctic soil and water. Appl Environ Microbiol 44: 491-493, 1982
12. Byers TJ, Hugo ER, Stewart VJ: Genes of Acanthamoeba: DNA, RNA and protein sequences (a review). J Protozool 37: 17S-25S, 1990
13. Cao Z, Jefferson DM, Panjwani N: Role of carbohydrate-mediated adherence in cytopathogenic mechanisms of Acanthamoeba. J Biol Chem 273: 15838-15845, 1998
14. Centers for Disease C: Acanthamoeba keratitis associated with contact lenses--United States. MMWR Morb Mortal Wkly Rep 35: 405-408, 1986
58
15. Cerva L, Serbus C, Skocil V: Isolation of limax amoebae from the nasal mucosa of man. Folia Parasitol (Praha) 20: 97-103, 1973
16. Cho JH, Na BK, Kim TS, Song CY: Purification and characterization of an extracellular serine proteinase from Acanthamoeba castellanii. IUBMB Life 50: 209-214, 2000
17. Chung DI, Yu HS, Hwang MY, Kim TH, Kim TO, Yun HC, Kong HH: Subgenus classification of Acanthamoeba by riboprinting. Korean J Parasitol 36: 69-80, 1998
18. Cope JR, Collier SA, Schein OD, Brown AC, Verani JR, Gallen R, Beach MJ, Yoder JS: Acanthamoeba Keratitis among Rigid Gas Permeable Contact Lens Wearers in the United States, 2005 through 2011. Ophthalmology 123: 1435-1441, 2016
19. Corsaro D, Venditti D: Phylogenetic evidence for a new genotype of Acanthamoeba (Amoebozoa, Acanthamoebida). Parasitol Res 107: 233-238, 2010
20. Costas M, Griffiths AJ: Enzyme composition and the taxonomy of Acanthamoeba. J Protozool 32: 604-607, 1985
21. Daggett PM, Lipscomb D, Sawyer TK, Nerad TA: A molecular approach to the phylogeny of Acanthamoeba. Biosystems 18: 399-405, 1985
22. Dart JK, Saw VP, Kilvington S: Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol 148: 487-499 e482, 2009
59
23. De Jonckheere JF: Ecology of Acanthamoeba. Rev Infect Dis 13 Suppl 5: S385-387, 1991
24. Fears AC, Metzinger RC, Killeen SZ, Reimers RS, Roy CJ: Comparative in vitro effectiveness of a novel contact lens multipurpose solution on Acanthamoeba castellanii. J Ophthalmic Inflamm Infect 8: 19, 2018
25. Goldschmidt P, Degorge S, Benallaoua D, Saint-Jean C, Batellier L, Alouch C, Laroche L, Chaumeil C: New tool for the simultaneous detection of 10 different genotypes of Acanthamoeba available from the American Type Culture Collection. Br J Ophthalmol 93: 1096-1100, 2009
26. Hadas E, Mazur T: Proteolytic enzymes of pathogenic and non-pathogenic strains of Acanthamoeba spp. Trop Med Parasitol 44: 197-200, 1993
27. Heffler KF, Eckhardt TJ, Reboli AC, Stieritz D: Acanthamoeba endophthalmitis in acquired immunodeficiency syndrome. Am J Ophthalmol 122: 584-586, 1996 28. Hiti K, Walochnik J, Faschinger C, Haller-Schober EM, Aspock H: One- and
two-step hydrogen peroxide contact lens disinfection solutions against Acanthamoeba: how effective are they? Eye (Lond) 19: 1301-1305, 2005
29. Hiti K, Walochnik J, Haller-Schober EM, Faschinger C, Aspock H: Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br J Ophthalmol 86: 144-146, 2002
60
30. Hurt M, Apte S, Leher H, Howard K, Niederkorn J, Alizadeh H: Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect Immun 69: 2988-2995, 2001
31. Hurt M, Niederkorn J, Alizadeh H: Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Invest Ophthalmol Vis Sci 44: 3424-3431, 2003
32. Illingworth CD, Cook SD, Karabatsas CH, Easty DL: Acanthamoeba keratitis: risk factors and outcome. Br J Ophthalmol 79: 1078-1082, 1995
33. Im K, Kim DS: Acanthamoebiasis in Korea: two new cases with clinical cases review. Yonsei Med J 39: 478-484, 1998
34. Johnson AM, Fielke R, Christy PE, Robinson B, Baverstock PR: Small subunit ribosomal RNA evolution in the genus Acanthamoeba. J Gen Microbiol 136: 1689-1698, 1990
35. Jonckheere JF: Isoenzyme and Total Protein Analysis by Agarose Isoelectric Focusing, and Taxonomy of the Genus Acanthamoeba. The Journal of Protozoology 30: 701-706, 1983
36. Jones DB, Visvesvara GS, Robinson NM: Acanthamoeba polyphaga keratitis and Acenthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc U K 95: 221-232, 1975
37. Joslin CE, Tu EY, McMahon TT, Passaro DJ, Stayner LT, Sugar J: Epidemiological characteristics of a Chicago-area Acanthamoeba keratitis outbreak. Am J Ophthalmol 142: 212-217, 2006
61
38. Kang H, Seong GS, Sohn HJ, Kim JH, Lee SE, Park MY, Lee WJ, Shin HJ: Effective PCR-based detection of Naegleria fowleri from cultured sample and PAM-developed mouse. Eur J Protistol 51: 401-408, 2015
39. Khan NA: Pathogenicity, morphology, and differentiation of Acanthamoeba. Curr Microbiol 43: 391-395, 2001
40. Khan NA: Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev 30: 564-595, 2006
41. Kilvington S, Gray T, Dart J, Morlet N, Beeching JR, Frazer DG, Matheson M: Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci 45: 165-169, 2004
42. Kilvington S, Larkin DF: Acanthamoeba adherence to contact lenses and removal by cleaning agents. Eye (Lond) 4 ( Pt 4): 589-593, 1990
43. Lim N, Goh D, Bunce C, Xing W, Fraenkel G, Poole TR, Ficker L: Comparison of polyhexamethylene biguanide and chlorhexidine as monotherapy agents in the treatment of Acanthamoeba keratitis. Am J Ophthalmol 145: 130-135, 2008 44. Lindquist TD, Doughman DJ, Rubenstein JB, Moore JW, Campbell RC:
Acanthamoeba-contaminated hydrogel contact lenses. Susceptibility to disinfection. Cornea 7: 300-303, 1988a
45. Lindquist TD, Sher NA, Doughman DJ: Clinical signs and medical therapy of early Acanthamoeba keratitis. Arch Ophthalmol 106: 73-77, 1988b
46. Lorenzo-Morales J, Khan NA, Walochnik J: An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 22: 10, 2015
62
47. Lorenzo-Morales J, Martin-Navarro CM, Lopez-Arencibia A, Arnalich-Montiel F, Pinero JE, Valladares B: Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol 29: 181-187, 2013
48. Lyons TB, Kapur R: Limax amoebae in public swimming pools of albany, schenectady, and rensselaer counties, new york: their concentration, correlations, and significance. Appl Environ Microbiol 33: 551-555, 1977
49. Marciano-Cabral F, Cabral G: Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16: 273-307, 2003
50. Marciano-Cabral F, Puffenbarger R, Cabral GA: The increasing importance of Acanthamoeba infections. J Eukaryot Microbiol 47: 29-36, 2000
51. Matthaei M, Meng H, Bhutto I, Xu Q, Boelke E, Hanes J, Jun AS: Systematic assessment of microneedle injection into the mouse cornea. Eur J Med Res 17: 19, 2012
52. Maycock NJ, Jayaswal R: Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 35: 713-720, 2016
53. Mergeryan H: The prevalence of Acanthamoeba in the human environment. Rev Infect Dis 13 Suppl 5: S390-391, 1991
54. Moon EK, Chung DI, Hong Y, Kong HH: Expression levels of encystation mediating factors in fresh strain of Acanthamoeba castellanii cyst ESTs. Exp Parasitol 127: 811-816, 2011
63
55. Moon EK, Chung DI, Hong YC, Ahn TI, Kong HH: Acanthamoeba castellanii: gene profile of encystation by ESTs analysis and KOG assignment. Exp Parasitol 119: 111-116, 2008
56. Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, Valladares B: Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res 106: 279-281, 2009
57. Nuprasert W, Putaporntip C, Pariyakanok L, Jongwutiwes S: Identification of a novel t17 genotype of acanthamoeba from environmental isolates and t10 genotype causing keratitis in Thailand. J Clin Microbiol 48: 4636-4640, 2010 58. Padzik M, Starosciak B, Szaflik JP, Pietruczuk-Padzik A, Siczek P, Chomicz L:
Assessment of in vitro dynamics of pathogenic Acanthamoeba strains originating from contact lens wearers with infectious keratitis. Ann Parasitol 62: 331-336, 2016
59. Paszko-Kolva C, Yamamoto H, Shahamat M, Sawyer TK, Morris G, Colwell RR: Isolation of amoebae and Pseudomonas and Legionella spp. from eyewash stations. Appl Environ Microbiol 57: 163-167, 1991
60. Polat ZA, Ozcelik S, Vural A, Yildiz E, Cetin A: Clinical and histologic evaluations of experimental Acanthamoeba keratitis. Parasitol Res 101: 1621-1625, 2007
61. Por YM, Mehta JS, Chua JL, Koh TH, Khor WB, Fong AC, Lim JW, Heng WJ, Loh RS, Lim L, Tan DT: Acanthamoeba keratitis associated with contact lens wear in Singapore. Am J Ophthalmol 148: 7-12 e12, 2009
64
62. Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ: Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol 44: 3589-3595, 2006 63. Radford CF, Bacon AS, Dart JK, Minassian DC: Risk factors for acanthamoeba
keratitis in contact lens users: a case-control study. BMJ 310: 1567-1570, 1995 64. Radford CF, Minassian DC, Dart JK: Acanthamoeba keratitis in England and
Wales: incidence, outcome, and risk factors. Br J Ophthalmol 86: 536-542, 2002 65. Ren M, Wu X: Evaluation of three different methods to establish animal models
of Acanthamoeba keratitis. Yonsei Med J 51: 121-127, 2010
66. Said NA, Shoeir AT, Panjwani N, Garate M, Cao Z: Local and systemic humoral immune response during acute and chronic Acanthamoeba keratitis in rabbits. Curr Eye Res 29: 429-439, 2004
67. Schuster FL, Guglielmo BJ, Visvesvara GS: In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 53: 121-126, 2006
68. Schuster FL, Visvesvara GS: Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34: 1001-1027, 2004a
69. Schuster FL, Visvesvara GS: Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist Updat 7: 41-51, 2004b