Comparative evaluation of three calcium
phosphate synthetic block bone graft
materials for bone regeneration
in rabbit calvaria
Jae-SubPark
Department of Dental Science
The Graduate School, Yonsei University
i
Comparative evaluation of three calcium
phosphate synthetic block bone graft
materials for bone regeneration
in rabbit calvaria
Directed by Professor Seong-Ho Choi
The Master's Thesis
submitted to the Department of Dental Science
the Graduate School of Yonsei University
in partial fulfillment of the requirements for the degree of
Master of Dental Science
Jae-SubPark
October 2011
ii
This certifies that the Master’s thesis of
Jae-Sub Park is approved.
───────────────────────
Thesis Supervisor
:Seong-Ho Choi
───────────────────────
Chang-Sung Kim
───────────────────────
Ui-Won Jung
The Graduate School
Yonsei University
i
감사의 글
이 논문이 완성되기까지 부족한 저에게 지도와 격려를 아끼지
않으신 최성호 교수님께 깊은 감사를 드립니다. 그리고 언제나 따뜻한
관심과 진심 어린 조언을 아끼지 않으셨던 채중규 교수님, 조규성
교수님, 김창성 교수님, 정의원 교수님, 이용근 교수님께도 깊은 감사를
드립니다.
연구 내내 많은 도움을 주신 이중석, 박정철 교수님, 그리고 황지완
선생과 조아란 연구원에게도 특별한 감사를 드립니다. 저를 항상
지켜봐 주셨던 치주과 선후배님들 에게도 고마운 마음을 전합니다.
논문 영작에 많은 도움을 준 동서에게도 진심으로 고맙다는 말을
하고 싶습니다.
마지막으로 항상 제게 힘이 되어 주고 따뜻한 격려를 해주신 양가
부모님께 깊은 사랑과 감사를 드리며, 저를 항상 이해해주고 사랑해 준
아내와 딸 시현에게 이 논문을 바칩니다.
2011 년 12 월
저자 씀
i
Table of Contents
List of Figures ··· List of Tables··· ii iii Abstract (English) ··· iv I. Introduction ··· 1II. Materials and Methods ··· 4
1. Animals··· 4 2. Materials ··· 4 3. Study Design ··· 6 4. Surgical protocol ··· 6 5. Histologic processing ··· 7 6. Evaluating Methods ··· 8 7. Statistical analysis··· 9 III. Results ··· 10 1. Clinical findings ··· 10 2. Histologic findings··· 10 3. Histometric analysis··· 11 IV. Discussion ··· 13 V. Conclusion ··· 16 References ··· 17 Legends ··· 21 Tables ··· 22 Figures ··· 26 Abstract (Korean) ··· 30
ii
List of Figures
Figure 1. Clinical photograph of the experiment.
(a) Four 8-mm-diameter defects were made in rabbit calvaria. (b) HA, β-TCP, and BCP block bone graft materials in the
defects.··· Figure 2. Schematic drawing for the histometric analysis.···
26 27 Figure 3. Representative photomicrographs obtained at 4 weeks postsurgery.
(a, b) Control group; (c, d) HA group; (e, f) β-TCP group; (g, f) BCP group. Arrowheads = defect margin; NB = new bone; OB = original bone. (a, c, e, g) Goldner’s Masson trichrome stain, original magnification: ×40. (b, d, f, h) Hematoxylin and eosin stain, original magnification: ×100. ··· Figure 4. Representative photomicrographs obtained at 8 weeks postsurgery.
(a, b) Control group; (c, d), HA group; (e, f) β-TCP group; (g, h) BCP group. (a, c, e, g) Goldner’s Masson trichrome stain, original magnification: ×40, (b, d, f, h) Hematoxylin and eosin stain, original magnification: ×100.···
28
iii
List of Tables
Table 1. Area of augmented bone (mm
2) ···
Table 2. Area of new bone (mm
2) ···
Table 3. Bone density at each time interval(%) ···
22
23
24
iv
ABSTRACT
Comparative evaluation of three calcium phosphate synthetic
block bone graft materials for bone regeneration
in rabbit calvaria
Jae-Sub Park, D.D.S.
Department of Dental Science Graduate School, Yonsei University
(Directed by Professor Seong-Ho Choi, D.D.S., M.S.D., PhD.)
Various synthetic materials that maintain space and allow bone ingrowth have been developed for use in implant dentistry and periodontal treatments. Among various alloplastic bone substitutes, calcium phosphate ceramics have been investigated extensively because their mineral chemistry resembles that of human bone. In this work, we evaluated the bone formation of three calcium phosphate synthetic block-type bone grafts in rabbit calvarial defects. Four 8-mm-diameter defects were created in each of ten young adult New Zealand white rabbits. Each of three of the defects was randomly filled with one of three fabricated synthetic block-type bone graft materials: hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP), and biphasic calcium phosphate (BCP). BCP is a mixture of HA and β-TCP at a ratio of 7:3. The fourth defect, a sham-surgery control, was filled with blood clots. The specimens
v
were harvested at 4 and 8 weeks postsurgery for histologic and histomorphometric evaluation.
Our results indicate that the amount of newly formed bone and bone density were increased more for the BCP block bone substitute than for the other two types. Furthermore, the histologic and histometric findings revealed that the space-maintaining ability was significantly better for all three calcium phosphate block bone graft materials than for the control group at both 4 and 8 weeks. However, measurement of the absorption of each particle revealed a significant decrease in the residual particles for β-TCP, while there were only small decreases for HA and BCP.
1
Comparative evaluation of three calcium phosphate synthetic
block bone graft materials for bone regeneration
in rabbit calvaria
Jae-Sub Park, D.D.S.
Department of Dental Science Graduate School, Yonsei University
(Directed by Professor Seong-Ho Choi, D.D.S., M.S.D., PhD.)
I. Introduction
Clinicians often encounter an inadequate amount of bone during surgical procedures, which is due to several reasons, including injuries, periodontal diseases, and implant surgery. To overcome this difficulty, various bone substitutes have been used to reconstruct bony defects (Rojbani et al., 2011). Autogenous bone graft materials have been considered the gold standard in reconstructive surgery because of their osteogenic property (Bauer and Muschler, 2000). Despite this advantage, the autogenous bone graft has many disadvantages, including limited donor-site availability and associated morbidity. Conversely, allogenic bone graft and xenogenic bone graft materials can be obtained easily; however, they may provoke an immune response (Tudor et al., 2008). These limitations have led to extensive investigations
2
and the development of alloplastic materials. Alloplastic bone grafts are osteoconductive and do not induce immunogenicity (Daculsi et al., 2003).
Among various alloplastic bone substitutes, calcium phosphate ceramics have been investigated extensively because their mineral chemistry resembles that of human bone (Han et al., 1984; Trombelli et al., 2002). The first successful application of a calcium phosphate reagent [described as “triple calcium phosphate,” or tricalcium phosphate (TCP)] in humans was reported in 1920 (Albee, 1920).The clinical use of a TCP preparation in surgically created periodontal defects in animals was first reported 50 years later (Nery et al., 1975). Furthermore, the application of dense hydroxyapatite (HA) as an immediate tooth root replacement has been reported (Denissen and de Groot, 1979); synthetic HA and beta-TCP (β-TCP) have been used as commercially available bone substitute materials for dental and medical fields largely through efforts made in the early 1980s (LeGeros, 1988; Metsger et al., 1982).
In general, HA has low osteoconductive activity but has a good space-maintaining capacity, whereas β-TCP is more bioresorbable and is rapidly replaced by new bone material. Biphasic calcium phosphate (BCP) ceramics, which comprise mixtures of HA and β-TCP at various ratios, allow the resorption rate to be controlled without distorting the osteoconductive property of the bone (Nery et al., 1992; Yamada et al., 1997b).
There are two types of calcium phosphate bone substitute: particulated and block. The better space-providing abilities of block-type bone substitutes result in them showing better stability of augmented areas during the healing period(Kim et al.,
3
2011). Another advantage of the block-type bone substitute is its higher efficiency, with new bone apposition occurring in association with progressive material degradation (Daculsi and Passuti, 1990; Merkx et al., 1999).
Based on results obtained in recent studies, it is proposed that HA blocks should be used as a prefabricated scaffold in cell transplantation for periodontal regeneration. The block-type bone grafts may be a better solution than particulated bone graft materials in cases of vertical augmentation
(Chiapasco M et al., 1999; Cordaro L
et al., 2002).
However, few studies have assessed the performance of block-type bone grafts using different calcium phosphates. Hence, in the present study we evaluated the bone regeneration capabilities of three calcium phosphate synthetic block bone grafts in rabbit calvarial defects.4
II. Materials and methods
1. Animals
Ten New Zealand white rabbits weighing 3.0–3.5 kg were used in this study. The animals were housed in separate cages under standard laboratory conditions and fed a standard diet. Animal selection, management, surgical protocol, and preparation followed routines approved by the Institutional Animal Care and Use Committee, Yonsei Medical Center, Seoul, Korea.
2. Materials
Preparation of calcium phosphate glass
Calcium phosphate glass was prepared using the sol-gel process according to previous methods (Jang YJ et al,. 2011). In the present system CaO-CaF2-P2O5-MgO-ZnO was prepared using raw materials such as CaCO3, CaF2, H3PO4, MgO, and ZnO. The CaO: CaF2 molar ratio was fixed at 9. MgO and ZnO were both used at a concentration of 1%wt.
Mixed batches were dried for 12 h at 80ºC, calcined for 1 h at 450ºC, melted in a platinum crucible at 1250ºC in a Kanthal Super furnace, and then poured onto a
5
graphite plate at room temperature. As-quenched glass was crushed with an alumina pestle and attrition milled to an average size of less than 40 μm.
When preparing the glass the calcium: phosphorus ratio was 1.67 for HA and 1.5 for β-TCP. BCP was prepared with an HA: β-TCP glass ratio of 7:3.
Preparation of porous calcium phosphate blocks
The following procedure to prepare calcium phosphate blocks was applied to all HA, β-TCP, and BCP glasses. Porous calcium phosphate blocks were prepared using prefabricated calcium phosphate glass and a polymeric sponge according to previously described methods(Park YS et al., 2006). The reticulated polyurethane ester sponge used in this experiment (Regicell, Jehil Urethane Co., Korea), has 500 three-dimensionally interconnected open pores per linear millimeter. First, a calcium-phosphorus glass slurry was prepared by dispersing the prepared calcium phosphate glass powders into distilled water with organic additives such as binder, dispersant, and a drying chemical control additive (DCCA). The second procedure was infiltration. Prior to the infiltration process, the surface layer of the sponge was treated ultrasonically with a 2% solution of NaOH for 20 min to improve the surface layer’s hydrophilicity. After cleaning and drying, the porous sponge was immersed into the glass slurry and taken back several times, and then rolled through twin Teflon rollers, the spacing between which was controlled to reduce the thickness of the sponge by 75% to as to remove the excess residual slurry from the sponge.
6
Compressed air was blown into the pores of the sponge to perforate any clogged pores. After infiltration, the sponge was dried at room temperature and then heat-treated in a Kanthalfurnace, wherein the condition of the heat treatment was based upon a thermal analysis. The temperature was increased to 600ºC at 1ºC/min in order to burn out the sponge entirely, and then held constant for 2 h to remove the volatile organic additives such as the binder, dispersant, and DCCA. The remaining calcium phosphate glass was then sintered for 2 h at various temperatures from 650ºC to 850ºC. The full procedure described above was repeated twice to thicken the framework of the porous block. The finished block was trimmed into a cylindrical shape (8 mm diameter and 3 mm thick).
3. Study design
Four circular defects with a diameter of 8 mm were created in the calvaria of ten young adult New Zealand white male rabbits, and then three were filled with a fabricated HA, β-TCP, or BCP synthetic block-type bone graft (8 mm in diameter and 3 mm thick). The remaining (control) defect was filled with a blood clot (Fig.1a,b)
4. Surgical protocol
The animals were anesthetized with an intramuscular injection of a mixture of ketamine hydrochloride (Ketalar, Yuhan, Seoul, Korea) and xylazine (Rompun, Bayer
7
Korea Co., Seoul, Korea). The surgical site was shaved and then wiped with alcohol and povidone iodine, followed by local anesthesia with 2% lidocaine (LidocaineHCl, Huons, Seoul, Korea). An incision was made along the sagittal midline from the frontal bone to the occipital bone. The four circular defects were then created in each animal using 8-mm trephines under cool saline irrigation. Each of three of the defects was filled with one of the three synthetic block-type grafting materials, while the fourth control defect was filled with blood clots (see Study Design). The soft tissue was repositioned and then sutured layer by layer with a resorbable suture material (Monosyn®). The rabbits were sacrificed at either 4 weeks (n=5) or 8 weeks (n=5)
postsurgery.
5. Histologic processing
Block sections including the surgical sites were removed when the animals were killed. The sections were rinsed with sterile saline and fixed in 10% buffered formalin for 10 days. After being rinsed with water, the sections were decalcified in 5% formic acid for 14 days and then embedded in paraffin. Serial 5-μm-thick sections were cut through the center of the circular calvarial defects, as well as the subcutaneous sites. Two sections that contained the central portion were selected from each block, and stained with Goldner’s Masson trichrome andhematoxylin and eosin.
8
6. Evaluating methods.
Clinical and Histologic analysis
The animals were observed carefully for allergic reactions, inflammation, and other complications around the surgical site throughout the 8-week healing period. The specimens were examined under a binocular microscope (DM LB, Leica Microsystems, Wetzlar, Germany) equipped with a camera (DC300F, Leica Microsystems, Heerburgg, Switzerland). Images of the slides were acquired and saved as digital files.
Histometric analysis
After conventional microscopic examination, computer-assisted histometric measurements of the newly formed bone in the calvarial defect model were performed using an automated image analysis system (Image-Pro Plus; Media Cybernetics, Silver Spring, MD, USA). Four parameters were measured: the area of augmentation, the area of new bone, the new bone density, and the area of residual particles. Only the area with newly formed mineralized bone was measured as the new bone area (in mm2); marrow and fibrovascular tissues were excluded (Fig. 2).
9
7. Statistical analysis
The statistical analysis was performed using a commercially available software program (SPSS 15.0, SPSS, Chicago, IL, USA). Histomorphometric records from the calvarial defect samples were used to calculate the mean and standard deviation values of groups. The data were examined with the Kolmogorov-Smirnov test for conformance to a normal distribution. Analysis of variance was used to analyze the effects of time and experimental conditions. The Tukey test was used to analyze differences between the groups; these were considered significant when p<0.05 . A two-sample t-test was carried out to analyze the differences in parameters between the 4- and 8-week groups. The level of statistical significance was set at p<0.05.
10
III. Results
1. Clinical findings
Healing during the postoperative period was uneventful for all animals. There were no complications (i.e., inflammatory reactions, exposure of graft materials, or allergic reactions).
2. Histologic findings
In the control group, we observed that the defect areas had become filled with loose fibrous tissue. A thin layer of new bone had formed in the 4-weeks group, while a thicker layer of new bone was evident in the 8-weeks group. Bony islands that appeared in a few specimens at the center of the defects contained moderate amounts of bone marrow in the 8-weeks group (Figs. 3a and 4a).
One of the synthetic block bone grafts, HA, had induced limited formation of new bone at the boundary of the defect area after 4 weeks of postoperative healing. After 8 weeks, significantly more bone had been formed, although it was not thicker than the original bone (Figs. 3c and 4c).
New bone formation was also observed in the β-TCP group, but a notable distinguishing feature of this group was the presence of chronic inflammatory cells
11
and fibrovascular tissue filling the majority of the defects. One interesting difference between the 4- and the 8-weeks groups for this synthetic block bone graft material was new bone forming a bony island in the upper areas of the defects only in the latter group.
Finally, impressive bone ingrowth into the BCP particle was observed in all BCP-filled defects in the BCP group, with no visible inflammatory reaction. Similar to other groups, there was more new bone formation in the 8-weeks group than in the 4-weeks group (Figs. 3g and 4g). However, one difference was that the new bone was thicker than the original bone in the BCP group, while this was not the case in the HA group.
3. Histometric analysis
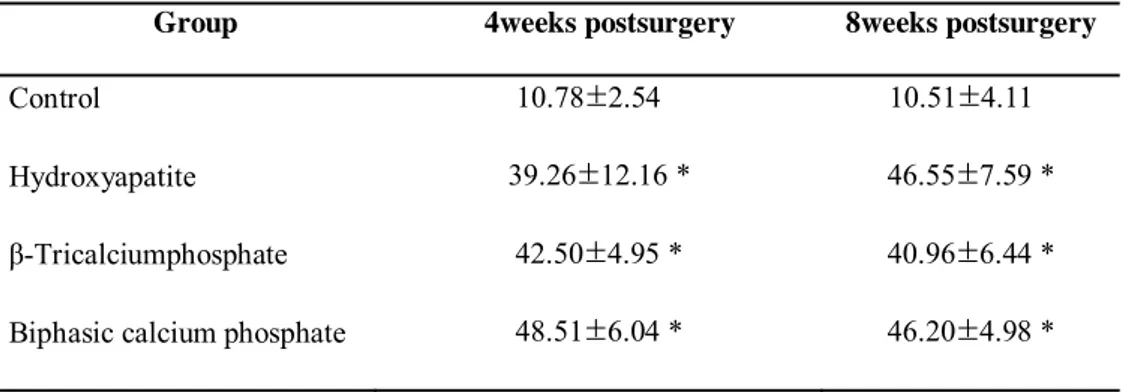
The histometric measurements are summarized in Tables 1–4 . Throughout our observations, the augmented areas (in mm2)—including bone areas, residual materials, and soft tissue area—were significantly larger in the three experimental groups than in the control group (Table 1). The augmented areas did not differ significantly among the three experimental groups at either 4 or 8 weeks of healing.
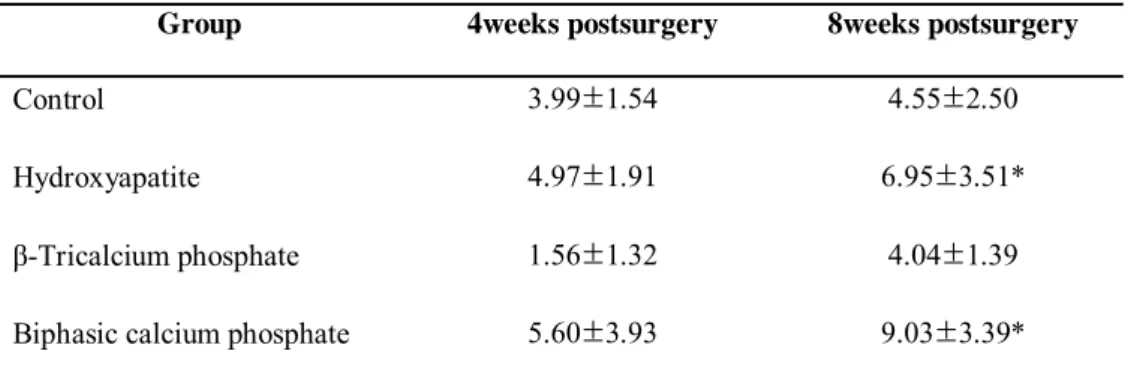
We observed that the new bone area (in mm2) was greater at 8 weeks than at 4 weeks in all groups. The β-TCP group exhibited the smallest area of new bone at both 4 and 8 weeks. There was a significant difference between the HA group at 8 weeks and the β-TCP group at 4 weeks. Furthermore, there was a significant difference
12
between the BCP group at 8 weeks and the β-TCP group at 4 weeks. However, there were no statistically significant differences among the other experimental groups and healing periods.
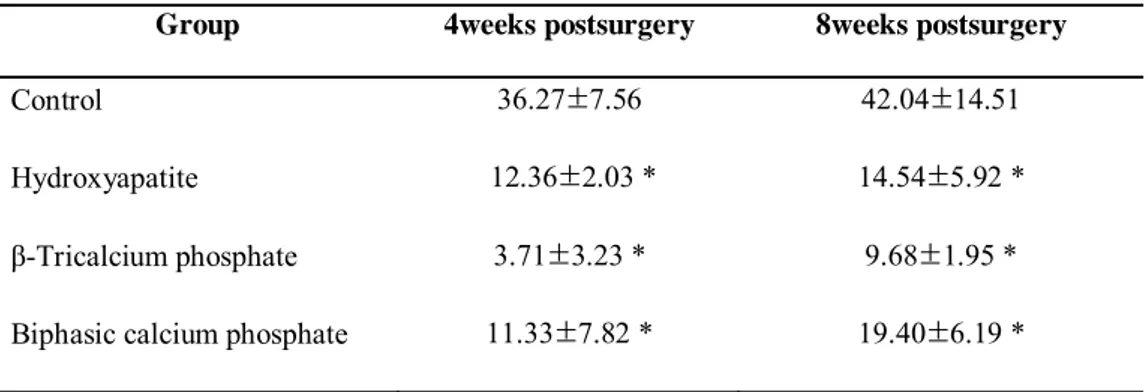
The findings regarding bone density (Table 3) show that the bone density was significantly higher in the control group than in the other groups. This may be because the control group had very small areas of new bone formation and because the defects were not filled with synthetic block-type bone graft material. The bone density in the three synthetic block bone grafts was lowest in the β-TCP group and highest in the BCP group at 8 weeks.
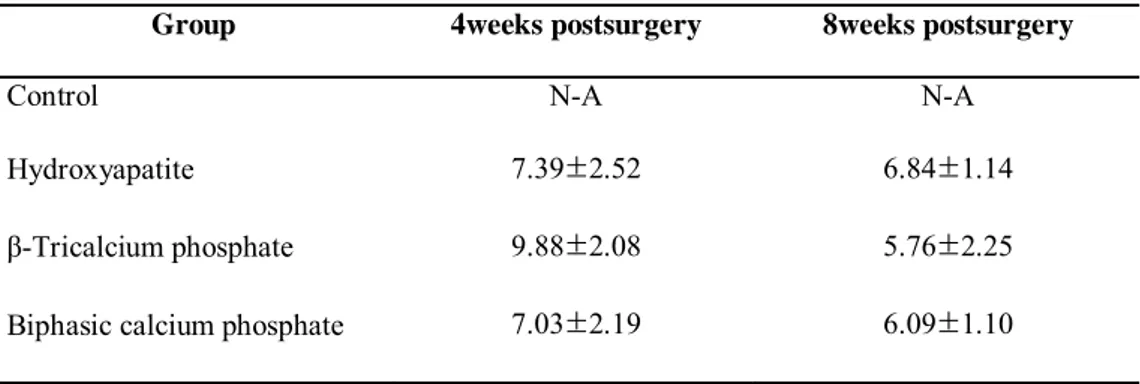
Table 4 lists the amount of residual particles of the synthetic block bone graft materials, demonstrating that the findings at 4 and 8 weeks did not differ significantly among the three types of graft materials. The HA and BCP groups exhibited a small decrease in residual particles between 4 and 8 weeks. The β-TCP group had a relatively large decrease in residual particles.
13
IV. Discussion
In the field of bone regeneration, autogenous bone for augmentation of bone defects is the gold standard. However, autogenous bone is not always available in sufficient volumes, and hence various methods have been proposed as alternatives, such as graft materials.Calcium phosphate ceramics have long been investigated as biologically compatible materials for use in the treatment of periodontal osseous defects and implant surgery. Calcium phosphate biomaterials allow the attachment, migration, proliferation, and phenotypic expression of bone cells, leading to bone apposition in direct contact with the implanted biomaterials.
In the present study we assessed the effectiveness of three types of calcium phosphate synthetic block bone graft material in bone regeneration using defects in the rabbit calvarium. Rabbit cranial defect models have been used in numerous studies for evaluating newly developed biomaterials due to the adequate amount of bone marrow. An 8-mm defect is known to be smaller than the critical defect size in rabbits for evaluating reossification, but has been suggested as a useful defect model for determining the effects of osteoinduction. The use of four 8-mm defects allowed comparison of an early-phase healing response for several materials, while avoiding individual variations. In the present study, the late and intermediate healing responses were analyzed at 8 and 4 weeks postsurgery, respectively.
The histologic and histometric results obtained in this study show that the three calcium phosphate block bone graft materials maintained space more effectively than
14
the control condition, which is consistent with the results of previous studies. For example, it has been demonstrated that the better space-providing abilities of block-type bone substitutes allows more stable augmented areas than particulated-block-type bone substitutes.
Previous studies found that HA did not support new bone formation, while the opposite result was found in the present study. This discrepancy is probably due to the use of a block-type bone substitute, which not only helps in the formation of new bone but is also more efficient than the particulated type of calcium phosphate bone substitute. In addition, block-type bone graft materials allow new bone apposition to occur in parallel with progressive material degradation.The histometric results of our study show that HA particles are not easily absorbed, since the amount of residual HA particles shows only small decreases between 4 and 8 weeks postsurgery.
We found that the amount of new bone formation was lowest in the β-TCP experimental group. β-TCP is known to be more readily bioresorbed than HA, and indeed our findings revealed the drastic absorption of β-TCP particles at 8 weeks postsurgery.
The amount of new bone formation at 8 weeks postsurgery was largest in the BCP group, which is consistent with the findings of previous studies. Moreover, the histometric analysis conducted in this study demonstrates that the BCP particles were absorbed more slowly than the β-TCP particles. This may be because the BCP group has the advantages of both the HA and β-TCP groups.
15
We tested three different block bone graft materials (HA, β-TCP, and BCP) in this study, and found that HA and BCP allowed considerably more new bone formation than either the β-TCP or control conditions. Evaluations of the performance of BCP should take into account that its bioreactivity and degradability can be controlled by altering the HA:β-TCP ratio. In previous studies, the HA:β-TCP ratio affected the reactivity of the material, such that a lower ratio resulted in higher reactivity. However, the optimal HA:β-TCP ratio for osteoconductivity has yet to be determined. Hence, additional studies assessing the efficacies of different HA:β-TCP ratios for block-type graft materials are necessary.
16
V. CONCLUSION
All three calcium phosphate synthetic block bone graft materials provided more space than the control natural-bone-healing group. Among the three synthetic graft materials, the BCP block bone graft was found to provide the most effective new bone formation at the 4- and 8-week postoperative periods.
17
References
Albee FH. Studies in Bone Growth: Triple Calcium Phosphate as a Stimulus to Osteogenesis. Ann Surg 71(1): 32-39, 1920.
Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science.
Clin Orthop Relat Res(371): 10-27, 2000.
Castaneda S, Largo R, Calvo E, Rodriguez-Salvanes F, Marcos ME, Diaz-Curiel M, Herrero-Beaumont G. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skeletal Radiol 35(1): 34-41, 2006.
Cavalcanti SC, Pereira CL, Mazzonetto R, de Moraes M, Moreira RW. Histological and histomorphometric analyses of calcium phosphate cement in rabbit calvaria. J Craniomaxillofac Surg 36(6): 354-359, 2008.
Chiapasco M, Abati S, Romeo E, Vogel G. Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res 10: 278-288, 1999
Cordaro L, Amade DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clini Oral Implants Res 13: 103-101, 2002
Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med 14(3): 195-200, 2003.
Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res 23(8): 883-894, 1989.
Daculsi G, Passuti N. Effect of the macroporosity for osseous substitution of calcium phosphate ceramics. Biomaterials 11: 86-87, 1990.
18
Denissen HW, de Groot K. Immediate dental root implants from synthetic dense calcium hydroxylapatite. J Prosthet Dent 42(5): 551-556, 1979.
Ellinger RF, Nery EB, Lynch KL. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: a case report. Int J Periodontics Restorative Dent 6(3): 22-33, 1986.
Han T, Carranza FA, Jr., Kenney EB. Calcium phosphate ceramics in dentistry: a review of the literature. J West Soc Periodontol Periodontal Abstr 32(3): 88-108, 1984.
Jang YJ, Jung IH, Park JC, Jung UW, Kim CS, Lee YK, et al. Effect of seeding using an avidin-biotin binding system on the attachment of periodontal ligament fibroblasts to nanohydroxyapatite scaffolds: three-dimensional culture. J
Periodontal Implan Sci 41: 73-78, 2011
Kalk WW, Raghoebar GM, Jansma J, Boering G. Morbidity from iliac crest bone harvesting. J Oral Maxillofac Surg 54(12): 1424-1429; discussion 1430, 1996. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, Cho KS. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112(3): 298-306, 2011.
Koerten HK, van der Meulen J. Degradation of calcium phosphate ceramics. J
Biomed Mater Res 44(1): 78-86, 1999.
LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent
Res 2(1): 164-180, 1988.
LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci
Mater Med 14(3): 201-209, 2003.
Lundgren D, Nyman S, Mathisen T, Isaksson S, Klinge B. Guided bone regeneration of cranial defects, using biodegradable barriers: an experimental pilot study in the rabbit. J Craniomaxillofac Surg 20(6): 257-260, 1992.
19
Merkx MA, Maltha JC, Freihofer HP, Kuijpers-Jagtman AM. Incorporation of three types of bone block implants in the facial skeleton. Biomaterials 20(7): 639-645, 1999.
Metsger DS, Driskell TD, Paulsrud JR. Tricalcium phosphate ceramic--a resorbable bone implant: review and current status. J Am Dent Assoc 105(6): 1035-1038, 1982.
Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants 12(6): 767-776, 1997.
Moskow BS, Lubarr A. Histological assessment of human periodontal defect after durapatite ceramic implant. Report of a case. J Periodontol 54(8): 455-462, 1983.
Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol 63(9): 729-735, 1992.
Nery EB, Lynch KL, Hirthe WM, Mueller KH. Bioceramic implants in surgically produced infrabony defects. J Periodontol 46(6): 328-347, 1975.
Nery EB, Pflughoeft FA, Lynch KL, Rooney GE. Functional loading of bioceramic augmented alveolar ridge--a pilot study. J Prosthet Dent 43(3): 338-343, 1980.
Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone 16(4 Suppl): 277S-284S, 1995.
Park Y-S, Kim K-N, Kim K-M, Choi S-h, Kim C-K, Legeros RZ, et al. Feasibility of three-dimensional macroporous scaffold using calcium phosphate glass and polyurethane sponge. Journal of Materials Science 41: 4357-4364, 2006 Rojbani H, Nyan M, Ohya K, Kasugai S. Evaluation of the osteoconductivity of
alpha-tricalcium phosphate, beta-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater
20
Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, Choi SH. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal
Implant Sci 40(4): 180-187, 2010.
Trombelli L, Heitz-Mayfield LJA, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. Journal of Clinical Periodontology 29: 117-135, 2002.
Tudor C, Srour S, Thorwarth M, Stockmann P, Neukam FW, Nkenke E, Schlegel KA, Felszeghy E. Bone regeneration in osseous defects - application of particulated human and bovine materials. Oral Surgery Oral Medicine Oral
Pathology Oral Radiology and Endodontology 105(4): 430-436, 2008.
Yamada S, Heymann D, Bouler JM, Daculsi G. Osteoclastic resorption of biphasic calcium phosphate ceramic in vitro. J Biomed Mater Res 37(3): 346-352, 1997a.
Yamada S, Heymann D, Bouler JM, Daculsi G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/beta-tricalcium phosphate ratios. Biomaterials 18(15): 1037-1041, 1997b.
21
Legends
Figure 1. Clinical photograph of the experiment.
(a) Four 8-mm-diameter defects were made in rabbit calvaria. (b) HA, β-TCP, and BCP block bone graft materials in the defects.
Figure 2. Schematic drawing for the histometric analysis.
Figure 3. Representative photomicrographs obtained at 4 weeks postsurgery.
(a, b) Control group; (c, d) HA group; (e, f) β-TCP group; (g, f) BCP group. Arrowheads = defect margin; NB = new bone; OB = original bone.
(a, c, e, g) Goldner’s Masson trichrome stain, original magnification: ×40. (b, d, f, h) Hematoxylin and eosin stain, original magnification: ×100.
Figure 4. Representative photomicrographs obtained at 8 weeks postsurgery.
(a, b) Control group; (c, d), HA group; (e, f) β-TCP group; (g, h) BCP group. (a, c, e, g) Goldner’s Masson trichrome stain, original magnification: ×40, (b, d, f, h) Hematoxylin and eosin stain, original magnification: ×100.
22
Tables
Table 1.Area of augmented bone- mm2
(group mean ±SD ; N=5)
Group 4weeks postsurgery 8weeks postsurgery
Control 10.78±2.54 10.51±4.11
Hydroxyapatite 39.26±12.16 * 46.55±7.59 *
β-Tricalciumphosphate 42.50±4.95 * 40.96±6.44 * Biphasic calcium phosphate 48.51±6.04 * 46.20±4.98 *
23
Table 2.Area of new bone - mm2
(group mean ±SD ; N=5)
Group 4weeks postsurgery 8weeks postsurgery
Control 3.99±1.54 4.55±2.50
Hydroxyapatite 4.97±1.91 6.95±3.51*
β-Tricalcium phosphate 1.56±1.32 4.04±1.39
Biphasic calcium phosphate 5.60±3.93 9.03±3.39*
24
Table 3.Bone density at each time interval - %
(group mean ±SD ; N=5)
Group 4weeks postsurgery 8weeks postsurgery
Control 36.27±7.56 42.04±14.51
Hydroxyapatite 12.36±2.03 * 14.54±5.92 *
β-Tricalcium phosphate 3.71±3.23 * 9.68±1.95 * Biphasic calcium phosphate 11.33±7.82 * 19.40±6.19 *
25
Table 4.Area of residual particle - mm2
(group mean ±SD ; N=5)
Group 4weeks postsurgery 8weeks postsurgery
Control N-A N-A
Hydroxyapatite 7.39±2.52 6.84±1.14
β-Tricalcium phosphate 9.88±2.08 5.76±2.25