저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Regimen for Target-controlled Infusion
Decreasing Remifentanil-induced Cough
by
In Kyong Yi
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
Regimen for Target-controlled Infusion
Decreasing Remifentanil-induced Cough
by
In Kyong Yi
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of Master of Medicine
Supervised by
Young Joo Lee, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of In Kyong Yi is approved.
SUPERVISORY COMMITTEE
Young Joo Lee
Bong Ki Moon
Jong Yeop Kim
The Graduate School, Ajou University
June 23rd, 2011
- ABSTRACT -
Regimen for Target-controlled Infusion Decreasing
Remifentanil-induced Cough
Background: This study evaluated the effectiveness of target-controlled infusion (TCI) of
remifentanil using different stepwise increases of central effect-site concentration (Ceff) on
cough prevention.
Methods: As a preliminary study, 140 patients were randomly assigned to receive
remifentanil targeting 2-stepwise increase of Ceff (1.0 to 4.0, or 2.0 to 4.0 ng/ml). From the
result of the preliminary study, 3-stepwise increase TCI (1.0 to 2.0, then to 4 ng/ml) was postulated and compared to direct targeting with 4.0 ng/ml TCI, randomly applied to another 140 patients. Episodes of cough were recorded and graded as mild (1–2), moderate (3–4), or severe (5 or more).
Results: In Group R1-4, one patient (1.5%) coughed at the first step, and the other 5
patients (7.3%) coughed during the second step. In Group R2-4, 9 patients (13.2%) coughed
during the first step; but none coughed during the next step. Only 1 patient showed a mild cough during the 3-stepwise increase TCI in Group R1-2-4, which significantly reduced the
Conclusions: Stepwise increase of TCI of remifentanil could reduce the incidence of
remifentanil-induced coughing, and 3-stepwise increase of TCI could nearly abolish remifentanil-induced coughing.
Key words : Remifentanil, Cough, Target-controlled infusion, Opioid
TABLE OF CONTENTS
ABSTRACT ··· ⅰ
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· iv
LIST OF TABLES ··· v
Ⅰ. INTRODUCTION ··· 1
Ⅱ. MATERIALS AND METHODS ··· 2
A. MATERIALS ··· 2 1. SUBJECTS ··· 2 2. PREPARATION ··· 2 B. METHODS ··· 3 1. SETTING ··· 3 2. STATISTICS ··· 4 III. RESULTS ··· 6 IV. DISCUSSION ··· 14 Ⅴ. CONCLUSION ··· 18 REFERENCES ··· 19 국문요약 ··· 22
LIST OF FIGURES
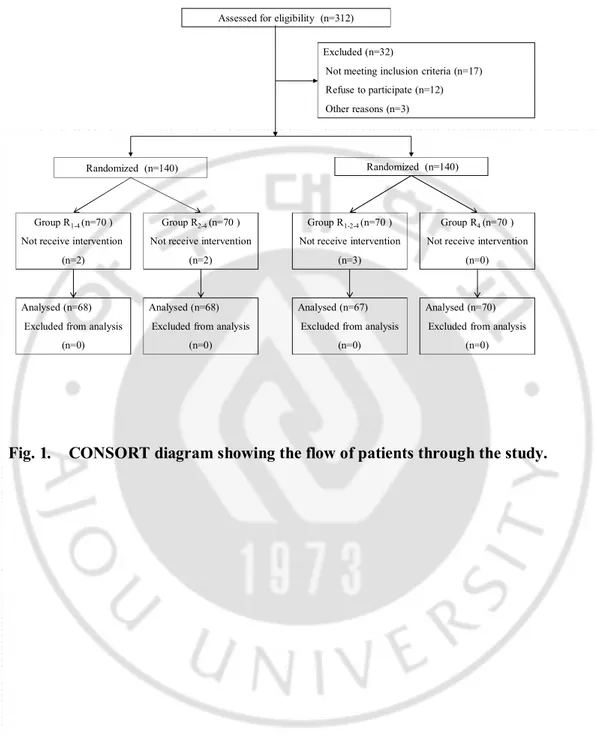
Fig. 1.
CONSORT diagram showing the flow of patients through the study.
··· 7
Fig. 2.
Time course of the predicted plasma (black dotted curve) and central
effect-site (gray curve) concentration of remifentanil during target
controlled-infusion.
··· 12Fig. 3.
Time course of prediced plasma (black dotted curve) and central effect-site
(gray curve) concentration of remifentanil during target
controlled-infusion.
··· 13Table 1.
Characteristics of patients receiving different remifentanil infusions
to target effect-site concentrations.
··· 8Table 2.
Bolus dose for target-controlled infusion of remifentanil.
··· 9Table 3.
Incidence, severity, and characteristics of remifentanil-induced
I. INTRODUCTION
Remifentanil, one of the narcotic opioids traditionally known as an analgesic and antitussive agent, sometimes causes cough during induction of anaesthesia, as with other opioids of the fentanyl series.( Agarwal, 2007; Kim, 2010; Lim, 2010; Cho, 2010) The incidence of cough after intravenous administration of remifentanil varies between 25 and 34%.( Kim, 2010; Lim, 2010; Cho, 2010) Although remifentanil-induced cough is usually benign and self-limiting, it may disrupt the smooth induction of anaesthesia and might cause harm to predisposed patients with increased intracranial, intra-ocular, and intra-abdominal pressures, and unstable haemodynamics.
Pretreatment of drugs known to reduce airway reactivity, such as lidocaine or propofol, could reduce incidence of remifentanil-induced cough, as in fentanyl-induced cough.( Pandey, 2004; Kim, 2008; Kim, 2010) However, these pharmacological preventive interventions do not appear to have achieved the maximal avoidance of cough. Our previous study demonstrated the relationship between the occurrence of cough and the time course of the plasma (Cp) and central effect-site (Ceff) concentration of remifentanil during
target-controlled infusion (TCI) of remifentanil.(Kim, 2010) Also, pharmacokinetic (PK) and pharmacodynamic (PD) approaches, maintaining the balance between the tussive and antitussive arm of remifentanil, were thought to be essential for prevention of cough. Therefore, this study was designed to build a remifentanil TCI regimen for maximal avoidance of remifentanil-induced cough, without any requirement of additional pharmacologic preventive measures.
II.
MATERIALS AND METHODS
A. Material
1. Subjects
This study was approved by the institutional review board, and written informed consents were obtained from all patients. A total of 280 patients, ASA physical status I or II, ages 18-70 years, undergoing general anaesthesia for gynaecologic surgery, were enrolled in the study. Exclusion criteria included a body weight exceeding 20% of ideal, a history of bronchial asthma or chronic obstructive pulmonary disease, respiratory tract infection, or hypertension treated with angiotensin converting enzyme inhibitors. Patients who smoked were included in the study.
2. Preparation
No premedication was administered prior to surgery. A 20-gauge cannula was inserted into the forearm or dorsum of the hand, and connected to a three-way stopcock prior to arrival in the operating theatre. Upon arrival in the operating theatre, all patients were monitored with electrocardiogram, pulse oximeter, noninvasive blood pressure, and capnography.
Infusion of remifentanil was prepared in a 60 ml syringe (BD 60ml Syringe, Luer-LokTM Tip, BD, USA) using 2.0 mg of remifentanil (UltivaTM, GlaxoSmithKline, Parma,
Italy) diluted with 50ml normal saline to make a 40 μg/ml solution. A TCI pump (Orchestra®, Fresenius Vial, Brezins, France) with the pharmacokinetic model of Minto and
syringe pump was set to 1,200 ml/hr and maximal permissible plasma concentration of remifentanil to 50.0 ng/ml, thus, an overshoot of plasma concentration was permitted, avoiding interference in maximal delivery by the TCI pump. Following insertion of the syringe into the TCI pump, we performed ‘purge the syringe and infusion line’, and connected the syringe extension line to the three-way stopcock of the patient just before initiation of infusion.
B. Methods
1. Setting
As a preliminary study, 140 patients were randomly assigned to one of the two groups using computer-generated random numbers. Group R1-4 received remifentanil TCI, initially
targeting 1.0 ng/ml of Ceff, and Group R2-4 targeting 2.0 ng/ml of Ceff. When Ceff reached
each target effect-site concentration (Ct-eff), Ct-eff was increased to 4.0 ng/ml. Immediately
after infusion of remifentanil, an observer, blinded to the regimens of remifentanil infusion, recorded the occurrence of cough as 'yes' or 'no', and the onset time of cough (from the start of infusion to the first cough). And the duration (from the start of cough to cessation of cough) was also recorded. Cough was assessed until 1 min after Ceff reached the final Ct-eff,
while maintaining pseudo steady-state concentration. Depending upon the number of coughs observed, cough severity was graded as no (0), mild (1–2), moderate (3–5), or severe (> 5). The amount of remifentanil infused at each step, and total duration to final target were recorded during the study. Assisted mask ventilation with oxygen was applied if desaturation was observed (SpO2 < 95%).
Using PHARMACOKINETIC (PK) and PHARMACODYNAMIC (PD) software (Asanpump, ver 1.5, Ulsan University, Seoul, Korea), we performed TCI simulations for patients. Individual covariates were entered and the Minto PK/PD model of remifentanil was also used, and data refresh interval was set to 2 s. Relationships between the occurrence of coughing and the time course of Cp and Ceff-CNS of remifentanil were evaluated.
Based on the results of this preliminary study, we have built a regimen in anticipation of maximal reduction of the incidence of coughing (less than 5% of incidence), which was a 3-stepwise increase of Ct-eff, starting at 1.0 ng/ml of Ct-eff, increased to 2.0 ng/ml after 30 s,
when Ceff reached the target, and was finally increased to 4.0 ng/ml. Another 140 patients
were randomly assigned to one of the two groups. Group R1-2-4 received remifentanil using
this 3-stepwise increase TCI, and Group R4 received a one-step increase TCI with direct
targeting of 4.0 ng/ml of Ceff from the start of infusion. Evaluation of cough and TCI
simulations were performed in the same manner described above.
Loss of consciousness (LOC), apnoea, and chest wall rigidity after remifentanil injection were observed. Mean arterial pressure (MAP), heart rate (HR), and SpO2 were compared
upon arrival in the operating theatre (baseline) with those at 5 min after remifentanil infusion. Following assessment of cough, propofol TCI was titrated to induce loss of consciousness and rocuronium was administered for intubation.
2. Statistics
Considering the incidence of remifentanil-induced cough to be 27.6%, (Kim, 2008) and assuming the incidence of cough to be less than 5% after application of a regimen, this study required at least 63subjectsper group at the 5% level of significance and 90% power of test. The sample size was increased to 70 patients per group in order to account for up to 10% losses from the study. Statisticalanalyses were performed using SPSS 13.0 for Windows (SPSS Inc, Chicago, IL, USA). Data are reported as the mean ± SD or number of patients. Continuous data were compared using a two tailed Student’s t-test or by repeated measures ANOVA with Bonferroni correction. Categorical data were analysed using a Fisher’s exact test or chi-square test where appropriate. A P value < 0.05 was considered significant.
III. RESULTS
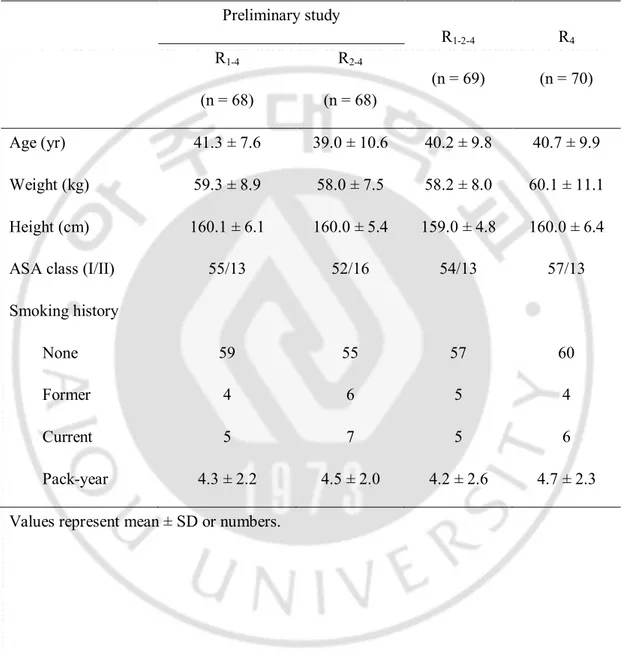
Among 280 enrolled patients, 7 patients did not complete study due to technical problems, such as IV line obstruction, disconnection, and TCI device alarm (Fig. 1). There were no differences between groups in terms of patient characteristics and smoking status (Table 1). Infused amounts of remifentanil and total duration to 4.0 ng/ml of Ceff during
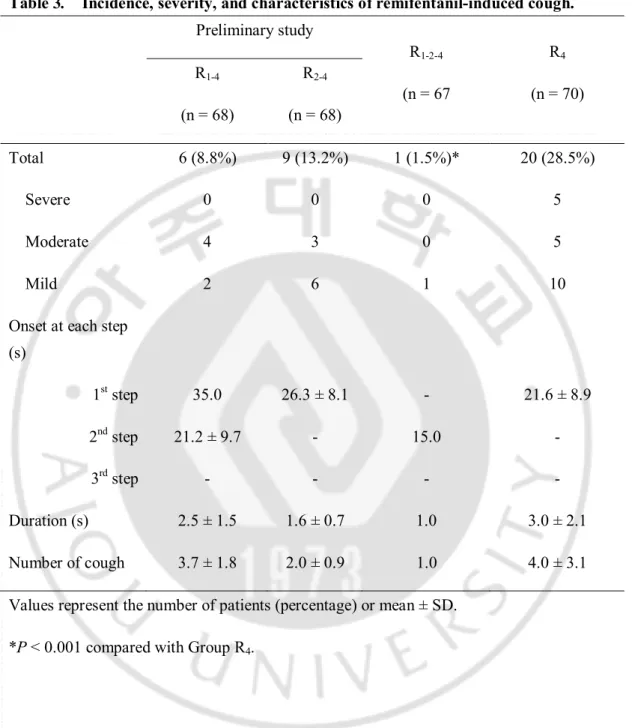
remifentanil TCI of the preliminary studies are shown in (Table 2). Incidence, severity, and characteristics of the cough are shown in (Table 3). In Group R1-4, one patient (1.5%)
coughed at the first step, and the other 5 patients (7.3%) coughed during the second step, increasing to 4.0 ng/ml of Ct-eff. Mean onset time of cough of these patients was 111.2 s
after the start of infusion; however, this was also 21.2 s after the start of the second step. In Group R2-4, 9 patients (13.2%) coughed during the first step; but none coughed during the
next step. Neither group showed severe cough. Time courses of the Cp and Ceff, and the
occurrence of cough in Group R1-4 and R2-4 are illustrated in (Fig. 2). Cp and Ceff of
Assessed for eligibility (n=312)
Randomized (n=140)
Excluded (n=32)
Not meeting inclusion criteria (n=17) Refuse to participate (n=12) Other reasons (n=3)
Group R1-4 (n=70 )
Not receive intervention (n=2)
Randomized (n=140)
Analysed (n=68) Excluded from analysis
(n=0)
Group R2-4 (n=70 )
Not receive intervention (n=2)
Group R1-2-4 (n=70 )
Not receive intervention (n=3)
Group R4 (n=70 )
Not receive intervention (n=0) Analysed (n=68)
Excluded from analysis (n=0)
Analysed (n=67) Excluded from analysis
(n=0)
Analysed (n=70) Excluded from analysis
(n=0)
Table 1. Characteristics of patients receiving different remifentanil infusions to target effect-site concentrations. Preliminary study R1-2-4 (n = 69) R4 (n = 70) R1-4 (n = 68) R2-4 (n = 68) Age (yr) 41.3 ± 7.6 39.0 ± 10.6 40.2 ± 9.8 40.7 ± 9.9 Weight (kg) 59.3 ± 8.9 58.0 ± 7.5 58.2 ± 8.0 60.1 ± 11.1 Height (cm) 160.1 ± 6.1 160.0 ± 5.4 159.0 ± 4.8 160.0 ± 6.4
ASA class (I/II) 55/13 52/16 54/13 57/13
Smoking history
None 59 55 57 60
Former 4 6 5 4
Current 5 7 5 6
Pack-year 4.3 ± 2.2 4.5 ± 2.0 4.2 ± 2.6 4.7 ± 2.3
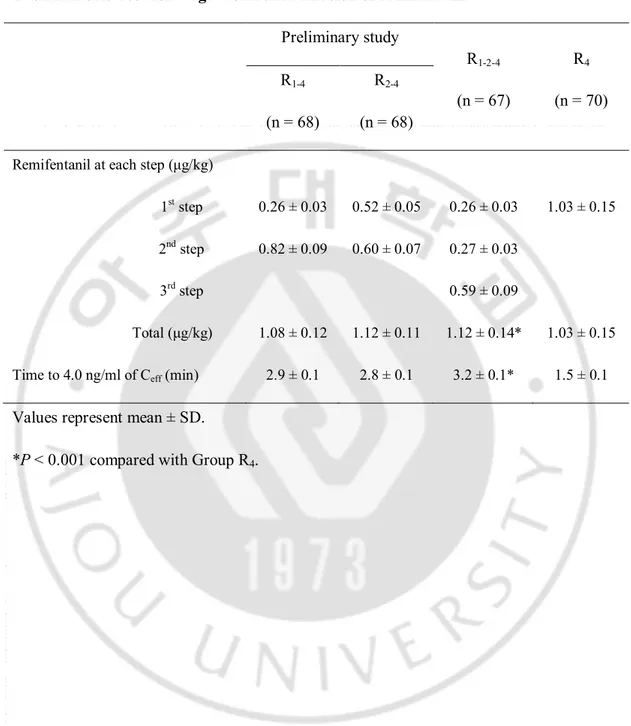
Table 2. Bolus dose for target-controlled infusion of remifentanil. Preliminary study R1-2-4 (n = 67) R4 (n = 70) R1-4 (n = 68) R2-4 (n = 68)
Remifentanil at each step (μg/kg)
1st step 0.26 ± 0.03 0.52 ± 0.05 0.26 ± 0.03 1.03 ± 0.15
2nd step 0.82 ± 0.09 0.60 ± 0.07 0.27 ± 0.03
3rd step 0.59 ± 0.09
Total (μg/kg) 1.08 ± 0.12 1.12 ± 0.11 1.12 ± 0.14* 1.03 ± 0.15 Time to 4.0 ng/ml of Ceff (min) 2.9 ± 0.1 2.8 ± 0.1 3.2 ± 0.1* 1.5 ± 0.1
Values represent mean ± SD.
Table 3. Incidence, severity, and characteristics of remifentanil-induced cough. Preliminary study R1-2-4 (n = 67 R4 (n = 70) R1-4 (n = 68) R2-4 (n = 68) Total 6 (8.8%) 9 (13.2%) 1 (1.5%)* 20 (28.5%) Severe 0 0 0 5 Moderate 4 3 0 5 Mild 2 6 1 10
Onset at each step (s) 1st step 35.0 26.3 ± 8.1 - 21.6 ± 8.9 2nd step 21.2 ± 9.7 - 15.0 - 3rd step - - - - Duration (s) 2.5 ± 1.5 1.6 ± 0.7 1.0 3.0 ± 2.1 Number of cough 3.7 ± 1.8 2.0 ± 0.9 1.0 4.0 ± 3.1
Values represent the number of patients (percentage) or mean ± SD. *P < 0.001 compared with Group R4.
Infused amounts of remifentanil and total duration to the final targets in Group R1-2-4
and R4 are also shown in (Table 2). The total amount of infused remifentanil and time to
4.0 mg/ml of Ceff were significantly greater in Group R1-2-4 compared to Group R4 (P <
patient showed a mild cough during the 3-stepwise increase TCI in Group R1-2-4, which
significantly reduced the incidence of cough, compared with Group R4 (P < 0.001). Time
courses of the Cp and Ceff, and the occurrence of cough of Group R1-2-4 and R4 are illustrated
in (Fig. 2). Cp and Ceff of patients who coughed in Group R4 were 9.9 ± 1.72 ng/ml and 2.3
± 0.73 ng/ml, respectively.
In all groups, coughing started between 5 and 40 s after the start of each step. The maximum number of coughs was 12, and one patient of Group R4 showed symptoms
suggesting opioid-induced muscle rigidity, which was relieved after administration of propofol and rocuronium. None of the patients showed LOC or apnoea during the study. MAP, HR, and SpO2 did not differ significantly between before and after remifentanil TCI,
Fig.2. Time course of the predicted plasma (black dotted curve) and central effect-site (gray curve) concentration of remifentanil during target controlled-infusion. Target
effect-site concentration (gray dotted line) was increased in a step-wise manner from 1.0 to 4.0 ng/ml (left graph), and 2.0 to 4.0 ng/ml (right graph). The scattered plot shows the predicted plasma (○) and central effect-site (●) concentration of each individual at the onset of coughing.
Fig.3. Time course of the predicted plasma (black dotted curve) and central effect-site (gray curve) concentration of remifentanil during target controlled-infusion. Target
central effect-site concentration (gray dotted line) was increased in a step-wise manner from 1.0 to 2.0 and to 4.0 ng/ml (left graph), and was directly increased to 4.0 ng/ml (right graph). The scattered plot shows the predicted plasma (○) and central effect-site (●) concentration of each individual at the onset of coughing.
IV. DISCUSSION
When initiating intravenous infusion of remifentanil targeting Ceff during induction of
anaesthesia, the regimen using multiple stepwise increase of Ceff could prevent severe
remifentanil-induced coughing and significantly reduce the incidence of coughing, compared to the method of direct increase to a final target of 4.0 ng/ml of Ceff.
Among previous studies of opiod-induced cough, several are based on different methods of opioid injection. Schapermeier et al. reported that intravenous injection speeds of 1.5 μg/kg fentanyl did not influence the incidence of cough.(Schapermeier, 2008) However, Lin et al. reported that prolonged injection time of fentanyl decreased the incidence of cough.(Lin, 2005) Chen et al. demonstrated a decreasing incidence of cough for the intravenous injection rate of fentanyl and the influence of injection site on the cough.(Chen, 2009) A pre-emptive small dose of 25 μg/kg of fentanyl was reported to significantly reduce cough, followed by 125 μg/kgof fentanyl.(Hung, 2010) Lim et al. reported that administration of graded escalation of Ceff of remifentanil effectively suppressed the cough,
and their method and result were quite similar to those of our study.(Lim, 2010) However, most of these studies are focused on simply decreasing the incidence. Therefore, we attempted to investigate the relationship between provocation and suppression of cough in the context of PK/PD.
In putting the results of our preliminary study together with the previous simulation work,1 we were able to make tentative conclusions as follows: (1) Remifentanil-induced
ng/ml (bolus of 0.82 μg/kg) while Ceff was maintained at 1.0 ng/ml, 7.3% (5/68) of patients
coughed. That is, 1.0 ng/ml of Ceff was not considered sufficient for prevention of coughing.
(3) When there was no remifentanil in the body, 0.52 μg/kg of remifentanil, in order to increase to 2.0 ng/ml of Ct-eff, made 13.2% of patients cough. However, if only Ceff was
maintained at 2.0 ng/ml, none of the patients coughed, even though a similar amount was administered (0.60 μg/kg) in order to increase to 4.0 ng/ml of Ceff. As a result, we were
able to anticipate that with addition of one more step, infusion of 1/3 increment Ceff in the
second step of R1-4, incidence at the second step might decrease by less than 2%, and overall
incidence might decrease by less than 5%, which was proposed as a final endpoint of cough incidence in this study. Using this 3 stepwise increase of TCI, one patient showed just one cough, and we were able to significantly decrease the incidence of cough. This regimen, of course, would be somewhat cumbersome in a routine clinical setting, because it requires that the targets be properly increased following the time elapsed. However, all TCI pumps display Cp, Ceff, and Ct-eff in real time, and some delay of Ct-eff increase might be beneficial
because it offers enough time for reaching the pseudo-steady state of remifentanil.
Simulations of the infusion regimens used during the study have shown the time courses of Cp and Ceff. Coughing also showed a tendency to develop while Cp was maintained
higher than Ceff, and as discrepancies between Cp and Ceff became smaller, or during the
steady-steady equilibrium state, coughing never occurred (Fig. 2, 3). Meanwhile, we found that the concentration-response relationship was difficult to account for, because the scattered plots of Cp of patients who coughed were not distributed around the peaks on visual
of Cp was also widely distributed. Intravascular space might not be the action site of
coughing, and that in order to trigger cough, remifentanil should diffuse from the intravascular space into the cough receptor sites, somewhere in the extra-vascular pulmonary structures. Therefore, in order to describe the concentration at the cough-triggering site, additional tussive effect-site compartment would be helpful to describe the PK/PD relationship of cough.
One of the findings associated with remifentanil-induced coughing that attracted our attention is its very rapid onset. Occurrence of coughing was as fast as 5-6 s in 3 patients of Group R4. Coughing occurred prior to completion of the injection of a whole bolus amount
of remifentanil. During this short interval, it could not be believed that remifentanil, injected into the vein of the dorsum of the hand or forearm, reached the central nervous system (CNS). Hoffmann et al. reported that ‘arm-to-head time’, the interval between the start of infusion of the echo-contrast agent injected into the antecubital vein and the beginning of signal amplification in the carotid artery, was 14.3 ± 3.0 s (min-max 9.0-22.0 s).(Hoffmann, 2000) Blumgart et al. reported that an active deposit of radium injected into the antecubital vein had been detected in the right chamber of the heart at 2.5- 14.0 s (arm-to-heart time), and pulmonary circulation time was estimated as 5.5 - 17.5 s.(Blumgart, 1927) Therefore, the possibility that remifentanil could have entered the CNS within 5 s is considered to be very low. Rather, it is supposed that remifentanil triggered coughing during pulmonary circulation. These cough-onset data might hold some important clues to a tussive effect via a peripheral acting mechanism. This peripheral tussive mechanism offers certain logic to our multi-stepwise increase regimen. Priming a small bolus amount
of remifentanil that is not sufficient to trigger cough, while passing the pulmonary circulation, eventually enters the systemic circulation and the drug will reach the CNS. Thereafter, if only the Ceff of the CNS increases up to a level sufficient for inhibition of
cough, coughing will never occur, even though the infusion of a large amount of remifentanil follows.
Several caveats require discussion here. First, we must consider the large variation of remifentanil concentration in the body during the initial rapidly mixing period immediately after injection into the vein.(Egan, 1996) In particular, considering its onset time, the predicted concentrations in the plasma and lung could be much different from the measured concentrations. Prior to entry into the central circulation, remifentanil might be diluted into the volume, which is different from that of the central compartment. Second, the target concentrations of remifentanil of this study were limited only to 1.0, 2.0, and 4.0 ng/ml. And we considered 2.0 ng/ml of Ceff as an effective antitussive concentration. However, it
is not guaranteed for higher targets. Therefore, more attention should be paid when high concentrations of remifentanil are targeted in clinical settings.
V. CONCLUSION
In conclusion, in this study, we wanted to build a regimen for remifentanil in anticipation of reducing the incidence of remifentanil-induced cough, based on analysis using PK/PD approaches, and, as a result, a multiple-stepwise increased TCI of remifentanil could effectively prevent coughing without other pharmacologic interventions.
REFERENCE
1. Agarwal A, Gautam S, Nath SS, Gupta D, Singh U: Comparison of the incidence and severity of cough induced by sufentanil and fentanyl: a prospective, randomised, double-blind study. Anaesthesia 62: 1230-2, 2007
2. Blumgart HL, Weiss S: STUDIES ON THE VELOCITY OF BLOOD FLOW: VII. The Pulmonary Circulation Time in Normal Resting Individuals. J Clin Invest 4: 399-425, 1927
3. Chen YM, Chen WT, Liang SW, Gu MN: Intravenous injection rate and site of fentanyl affect the incidence and onset time of fentanyl-induced cough. Nan Fang Yi
Ke Da Xue Xue Bao 29: 339-40, 2009
4. Cho HB, Kwak HJ, Park SY, Kim JY: Comparison of the incidence and severity of cough after alfentanil and remifentanil injection. Acta Anaesthesiol Scand 54: 717-20, 2010
5. Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL: Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology 84: 821-33, 1996
6. Hoffmann O, Weih M, Schreiber S, Einhaupl KM, Valdueza JM: Measurement of cerebral circulation time by contrast-enhanced Doppler sonography. Cerebrovasc Dis 10: 142-6, 2000
7. Hung KC, Chen CW, Lin VC, Weng HC, Hsieh SW: The effect of pre-emptive use of minimal dose fentanyl on fentanyl-induced coughing. Anaesthesia 65: 4-7, 2010 8. Kim JY, Lee SY, Kim DH, Park SK, Min SK: Effect-site concentration of propofol for
reduction of remifentanil-induced cough. Anaesthesia 65: 697-703, 2010
9. Kim JY, Park KS, Kim JS, Park SY, Kim JW: The effect of lidocaine on remifentanil-induced cough. Anaesthesia 63: 495-8, 2008
10. Lin JA, Yeh CC, Lee MS, Wu CT, Lin SL, Wong CS: Prolonged injection time and light smoking decrease the incidence of fentanyl-induced cough. Anesth Analg 101: 670-4, 2005
11. Lim JH, Ryu SJ, Lim YS: The incidence of cough induced by remifentanil during anesthetic induction was decreased by graded escalation of the remifentanil concentration. Korean J Anesthesiol 58: 117-21, 2010
12. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, Billard V, Hoke JF, Moore KH, Hermann DJ, Muir KT, Mandema JW, Shafer SL: Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology 86: 10-23, 1997
13. Minto CF, Schnider TW, Gregg KM, Henthorn TK, Shafer SL: Using the time of maximum effect site concentration to combine pharmacokinetics and pharmacodynamics. Anesthesiology 99: 324-33, 2003
14. Pandey CK, Raza M, Ranjan R, Lakra A, Agarwal A, Singh U, Singh RB, Singh PK: Intravenous lidocaine suppresses fentanyl-induced coughing: a double-blind, prospective, randomized placebo-controlled study. Anesth Analg 99: 1696-8, 2004 15. Schapermeier U, Hopf HB: Fentanyl-induced cough does not depend on injection
speed: a randomized study. Acta Anaesthesiol Scand 52: 1071-5, 2008
Remifentanil 유발 기침을 줄이기 위한 목표농도주입의 Regimen 아주대하교 대학원 의학과 이 인 경 (지도교수 : 이영주) 연구 목적 : 본 연구는 remifentanil 의 목표농도주입시 중심효과처 농도를 여러 가지 regimen 으로 단계적으로 증가시켰을 때 remifentanil 유발 기침을 예방하는데 효과적인지 알아보고자 하였다. 연구 방법 : 이전 연구에서 140 명의 환자들을 무작위로 2 군으로 나누었다. 두 군 모두 2 단계의 중심 효과처 목표 농도 증가를 이용하여 remifentanil 을 투여받았는데, 한 군은 1.0 에서 4.0ng/ml 로, 한 군은 2.0 에서 4.0ng/ml 로 증가시켰다. 이 연구 결과를 바탕으로 본 연구에서는 3 단계의 목표농도주입을 시행하였고(1.0 에서 2.0, 그리고 4.0ng/ml), 처음부터 4.0ng/ml 로 즉시 목표농도주입을 시작한 군과 비교하기 위해 새로운 140 명의 환자를 무작위로 두 군으로 나누어 연구를 진행하였다. 기침이 발생했을 경우를 기록하였고, 그 정도를 약함(1-2), 중간(3-4), 심함(5 회 이상)으로 분류하여 기록하였다. 결과 : R1-4 군의 1 명(1.5%)의 환자가 1 단계에서 기침을 하였으며, 5 명(7.3%)의 환자가 2 단계에서 기침을 하였다. R2-4 군에서는 9 명(13.2%)의 환자가 1 단계에서 기침을 하였고, 2 단계에서는 기침을 하는 환자가 없었다. 1 명의 환자만이 R1-2-4 군의 3 단계 목표농도주입시에 기침을 하였고, 이는
R4 군에 비교하였을 때 기침의 발생율과 정도에서 통계적으로 유의하게 감소된
결과를 보였다. (P < 0.001)
결론 : Remifentanil 의 목표농도주입시 단계적 증량은 remifentanil 유발 기침의 발생율을 줄여주고, 특히 3 단계 증량 시에는 거의 발생하지 않았다.
Key words: Remifentanil, Cough, Target-controlled infusion, Opioid.