Photorejuvenation Induced by
5-Aminolevulinic Acid Photodynamic
Therapy in the Patients with Actinic
Keratosis: A Histological Analysis
by
Min Young Park
Major in Medicine
Department of Medical Sciences
Photorejuvenation Induced by
5-Aminolevulinic Acid Photodynamic
Therapy in the Patients with Actinic
Keratosis: A Histological Analysis
by
Min Young Park
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Master of Medicine
Supervised by
You Chan Kim, M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Min Young Park is approved.
SUPREVISORY COMMITTEE
You Chan Kim
Eun-So Lee
Seonghyang Sohn
The Graduate School, Ajou University
December 22nd, 2008
감사의
감사의
감사의
감사의
글
글
글
글
본 논문을 완성할 때까지 지도와 격려를 베풀어 주셨던 지도 교수이신 김
유찬 교수님께 먼저 진심으로 감사 드립니다. 또한 논문 지도를 위하여 많은
조언를 아끼지 않으신 이은소 교수님, 손성향 교수님께 감사의 마음을 전합
니다.
연구가 진행되는 동안 많은 시간과 노력으로 도와주신 피부과학 교실원 여
러분과, 실험을 위하여 필요한 조직 염색과 분석을 도와주신 김영배 선생님
께 감사의 마음을 전합니다. 그리고 언제나 저에게 아낌없는 사랑으로 힘이
되어주신 부모님, 가족들에게 깊은 감사의 마음을 전합니다.
2009 년 1 월
저자씀
- ABSTRACT-
Photorejuvenation Induced by 5-Aminolevulinic Acid
Photodynamic Therapy in the Patients with Actinic Keratosis
: A Histological Analysis
Background: Repeated exposure to ultraviolet radiation from the sun results in premature
photoaging. Photodamaged skin is histologically characterized by reduced collagen synthesis,
increased collagen destruction, and accumulation of abnormal elastotic material in the
dermis. Photodynamic therapy (PDT) is one of many treatment options available for
photoaging; it has been shown to be effective, whereas the data on most other studies is
based on clinical observation.
Objectives: I investigated whether 5-aminolevulinic acid (ALA)-PDT induced histological
changes suggesting photorejuvenation.
Materials and Methods: Fourteen patients with one to three actinic keratosis on the face
were treated twice with ALA-PDT using a 1200W metal halogen lamp at one month
intervals. Skin biopsy before and one month after the PDT was performed. Twenty five pairs
of specimens were obtained. I examined the specimens with routine and
immunohistochemical staining and evaluated the parameters associated with photoaging
Results: After ALA-PDT, the mean epidermis thickness and inflammatory infiltrate in the
dermis were reduced. The total collagen volume in the dermis was significantly increased
with expression of type I and III procollagen. The level of transforming growth factor β and transforming growth factor β type II receptors were also increased. The elastotic material with co-localizing fibrilline-1 and tropoelastin expression in the dermis tended to decrease after
treatment. The expression of matrix metalloproteinases -1, -3, and -12 was also decreased.
Conclusion: The histological evidence showed beneficial effects of ALA-PDT for
photodamaged skin. Further controlled split-face studies are required to determine the
precise mechanism underlying the effects of ALA-PDT on photoaging.
Key words: Photodynamic therapy, Photorejuvenation, Histological changes
TABLE OF CONTENTS
ABSTRACT ··· ⅰ
TABLE OF CONTENTS ··· iii
LIST OF FIGURES ··· iv
LIST OF TABLES··· v
. Ⅰ INTRODUCTION ··· 1
. Ⅱ MATERIALS AND METHODS··· 3
A. SUBJECTS ··· 3
B. MATERIALS AND METHODS··· 3
1. Photodynamic therapy ··· 3
2. Hematoxylin and eosin (H&E) staining··· 4
3. Masson-trichrome staining and Verhoeff’s elastic staining··· 5
4. Immunohistochemical staining··· 5 5. Statistical analysis··· 6 . Ⅲ RESULTS ··· 7 . Ⅳ DISCUSSION ··· 19 . Ⅴ CONCLUSION ··· 24 REFERENCES ··· 25 국문요약 ··· 29
LIST OF FIGURES
Fig. 1. Clearance of AK after ALA-PDT··· 9
Fig. 2. Decreased epidermal thickness and dermal inflammatory infiltrate after ALA-PDT ··· 10
Fig. 3. Marked increased collagen deposition in the dermis after ALA-PDT ··· 11
Fig. 4. Increased expression of procollagen type I and III after ALA-PDT ··· 12
Fig. 5. Increased expression of TGF-β and TβR II in the epidermis after ALA-PDT ·· 13
Fig. 6. Accumulative effect of ALA-PDT on the expression of TGF-β, TβR II, procollagen - I, procollagen III, and total collagen volume ··· 14
Fig. 7. Improvement of solar elastosis by ALA-PDT··· 15
Fig. 8. Decreased fibrillin-1 and tropoelastin after ALA-PDT ··· 16
LIST OF TABLES
Table 1. Baseline characteristics of patients and course of treatment of AKs ··· 8
I. INTRODUCTION
Ultraviolet (UV) irradiation from the sun damages human skin and causes premature skin aging
(photoaging) (Gilchrest, 1995). Clinically, photoaged skin differs from sun-protected, naturally
aged skin; it is thickened and rough, with course wrinkles, mottled pigmentation, and
precancerous lesions including actinic keratosis (AK) (Gilchrest, 1989).
There is growing interests in reversing the signs of photoaging. Photodynamic therapy (PDT),
with a 5-aminolevulinic acid (ALA) a photosensitizing agent, using a variety of lasers and light
sources, enhances the treatment of photodamaged skin and associated AK (Alster et al., 2005;
Gold et al., 2006; Touma et al., 2004). However, the histological changes that occur after ALA-PDT, have not been fully assessed.
The main constituent of dermal extracellular matrix (ECM) is collagen, particularly collagens I and III, which provide skin with its strength and resilience. However, in photoaged skin, the production of procollagen, the precursors of collagen is reduced; an impaired transforming growth factor -β (TGF-β)/Smad pathway, caused by UV irradiation, might play a role in the pathology (Fisher et al., 2000; Quan et al., 2004). TGF-β is the major regulator of ECM synthesis in human skin; it stimulates fibroblast proliferation in the dermis to enhance collagen
synthesis (Inagaki et al., 1994). Down-regulation of the TGF-β type II receptor (TβRII) and ensuing lower response of TGF-β, by UV radiation result in reduced of collagen synthesis (Quan et al., 2004). Moreover, dermal collagen is destroyed by the UV-induced matrix
metalloproteinases (MMPs) in human skin (Fisher et al., 1996). MMP-1(fibroblast collagenase)
Once MMP-1 breaks down collagen, further degradation is followed by MMP-3 (stromelysin 1),
and other MMPs (Sternlicht and Werb, 2001).
Solar elastosis, the deposition of dystrophic elastotic material within the reticular dermis is also a characteristic of photoaged skin (Montagna et al., 1989). The mechanism causing this damage
remains unclear. However, it may be related to the increased production of elastic fibers which
consist of elastin and fibrillin-rich microfibrils (Bernstein et al., 1994; Seo et al., 2001). The
degradation of elastin by MMP-12 (human macrophage metalloelastase) may contribute to the
development of solar elastosis in photoaged skin (Chung et al., 2002).
In the present study, I examined pre- and post-ALA-PDT biopsy specimens for AK, and determined whether ALA-PDT induced histological changes reversing the destructive
II. MATERIALS AND METHODS
A. SUBJECTS
From December 2006 to February 2008, fourteen patients with one to three AKs on the face presenting to the outpatient clinic at the Department of Dermatology, Ajou University Hospital, Korea were treated with ALA-PDT. All patients diagnosed with AK by skin biopsy. The study was approved by the institutional review board (IRB number: AJIRB-CRO-08-021) and all subjects provided written informed consent. All patients were Korean with skin types III to V.
Among these subjects were five (35.8%) males and nine (64.2%) females. The age ranged from
52 to 85, with a mean age of 62.07. There was no history of previous treatment for AKs.
B. MATERIALS AND METHODS 1. Photodynamic therapy
All patients received the same therapeutic procedure. Twenty percent ALA (MEDAC GmbH, Hamburg, Germany) was applied to the lesions under an occlusive and light-shielding dressing.
Scales and crusts were gently removed before application of the cream. Four hours later, the dressings were removed, and the ALA was washed off with 0.9% saline solution. The lesions were illuminated with red light from a noncoherent light source (Waldmann PDT 1200; Waldmann-Medizin-Technik, Villingen-Schwenningen, Germany, emission wavelength
580~740m) at a light dose of 100 J/cm2 and a fluence rate of 100 mW/cm2.
The treatments were performed twice, at one month intervals. One month after the last
performed for histological examination to assess the response. Patients with residual lesions
received an additional two cycles of PDT or cryotherapy. The post-cryotherapy specimens were
excluded from the analysis. Finally, 25 pairs of skin specimens were obtained.
2. Hematoxylin and eosin (H&E) staining
Skin biopsies were fixed in 10% formalin, embedded in paraffin, and sectioned into 5-µm sections for routine H&E staining. The epidermal thickness, degree of solar elastosis, and
inflammatory infiltrate were measured. The epidermal thickness was the mean length between
the outer sides of the epidermis, excluding the stratum corneum, and the dermo-epidermal
junction through the entire section; computer-based software (Image-Pro Plus, MEDIA
CYBERNETICSA, Silver Spring, MD, USA) was used for the measurements. For analysis of
the solar elastosis and the inflammatory infiltrate, the sections were randomized, and examined
under an Olympus microscope (Tokyo, Japan) by blinded investigators. Two individual
dermatologists scored the samples using a five-point semi-quantitative scale (0 = no staining and
4 = maximal expression within the view) of three high power fields per section; the average
score was calculated for each section.
3. Masson-trichrome staining and Verhoeff’s elastic staining
Masson-trichrome stained collagen fibers and Verhoeff’s stained elastic fibers were quantified using computer software as mentioned above. For the scanning view of the papillary and upper reticular dermis, low magnification (x100) was used. The epidermis and epidermal appendages
stained target versus the unstained background, presented as the percent expression.
4. Immunohistochemical staining
Using the immunoperoxidase technique, 5 µm sections from formalin-fixed, paraffin embedded skin samples were stained with antibodies as follows: Rabbit monoclonal anti-procollagen type I
antibody (diluted 1:1000, Chemicon, Temecula, CA, USA), rabbit polyclonal anti-procollagen
type III antibody (diluted 1: 500, CEDARLANE, Ontario, Canada), mouse monoclonal
anti-TGF-β antibody (diluted 1: 500, Gene Tex, San Antonio, TX, USA), rabbit polyclonal anti-TβRII antibody (diluted 1: 100, Spring Bioscience, Fremont, CA, USA), rabbit polyclonal anti-fibrillin-1 antibody (diluted 1: 50, Abcam, Cambridge, UK), rabbit polyclonal anti-tropoelastin
antibody (diluted 1: 50, Abcam, Cambridge, UK), rabbit polyclonal anti-MMP-1 antibody
(diluted 1: 50, NeoMarker, Fremont, CA, USA), rabbit monoclonal anti- MMP-3 antibody
(diluted 1: 100, Epitomics, Burlingame, CA, USA), rabbit monoclonal anti- MMP-12 antibody
(diluted 1: 100, Epitomics, Burlingame,CA, USA), and mouse monoclonal anti-TIMP-1
antibody (diluted 1: 50, Santa Cruz, CA, USA).
For quantitative evaluation of the immunoreactivity of procollagen I, III, TGF-β, and TβRII, the previously mentioned software was used for the immunohistochemically stained sections in
the same way it was used for collagen and elastin fiber measurements. The TGF-β and TβRII, was evaluated in the epidermis where it is the predominant expressed. The expressing pattern of
fibrillin-1, tropoelastin, MMP-1, 3, 12, and TIMP-1 were discontinuous, not appropriate to apply
5. Statistical analysis
A paired Student’s t-test was used to determine statistical significance of the differences
between the staining patterns using the SPSS 11.0 statistics program (SPSS, Inc., Chicago, IL,
USA). All P values were two-tailed, and differences were considered significant when the
III. RESULTS
1. Clearance of AKs
The ALA-PDT was tolerated in all patients. During the treatment, variable degrees of erythema and edema occurred, but resolved within a few days. Some patients reported mild to moderate pain, but interruption of procedure or topical anesthesia were not required.
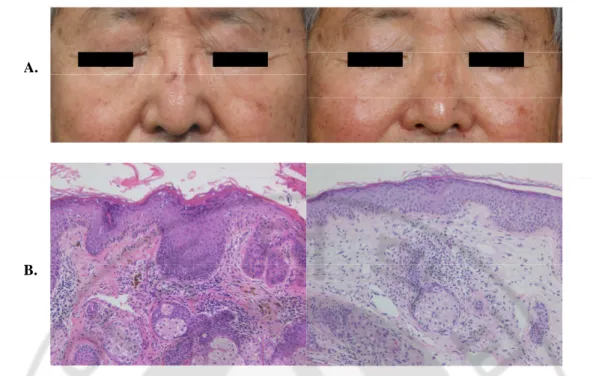
Initially, 23AKs were detected among the patients. One month after 2 sessions of PDT, 19 lesions from 11 patients showed a complete clinical and histological response (remission rate: 82.6%) (Fig. 1A, B). Clinically, there was no significant scarring or pigmentary changes after treatment (Fig. 1A). Among four patients with persistent lesions, one patient with two lesions received two additional PDT treatments, the AKs in the follow up biopsy resolved. The other two patients underwent cryotherapy twice with liquid nitrogen, and the lesions cleared (Table 1).
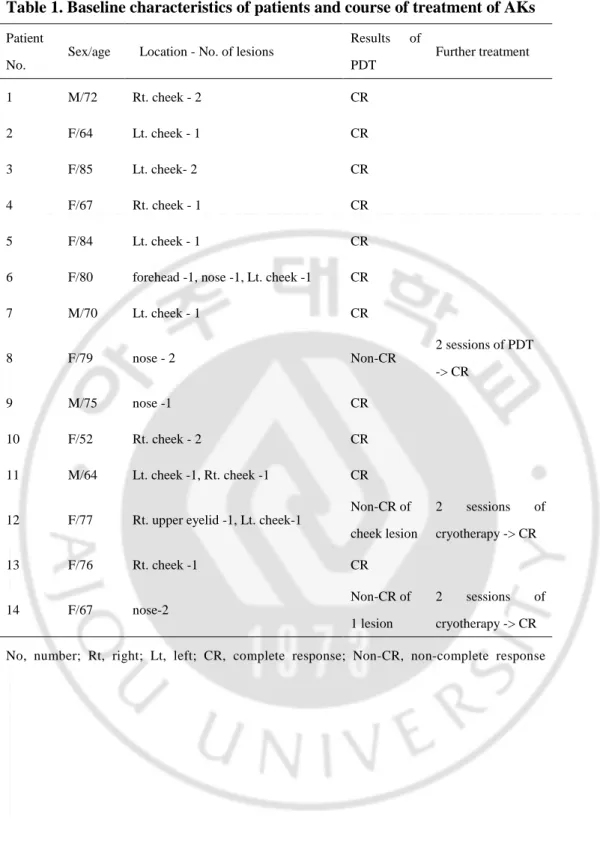
Table 1. Baseline characteristics of patients and course of treatment of AKs
Patient No.
Sex/age Location - No. of lesions
Results of PDT Further treatment 1 M/72 Rt. cheek - 2 CR 2 F/64 Lt. cheek - 1 CR 3 F/85 Lt. cheek- 2 CR 4 F/67 Rt. cheek - 1 CR 5 F/84 Lt. cheek - 1 CR
6 F/80 forehead -1, nose -1, Lt. cheek -1 CR
7 M/70 Lt. cheek - 1 CR 8 F/79 nose - 2 Non-CR 2 sessions of PDT -> CR 9 M/75 nose -1 CR 10 F/52 Rt. cheek - 2 CR 11 M/64 Lt. cheek -1, Rt. cheek -1 CR
12 F/77 Rt. upper eyelid -1, Lt. cheek-1
Non-CR of cheek lesion 2 sessions of cryotherapy -> CR 13 F/76 Rt. cheek -1 CR 14 F/67 nose-2 Non-CR of 1 lesion 2 sessions of cryotherapy -> CR
pre- PDT post- PDT
Fig. 1. Clearance of AK after ALA-PDT. Patient 9 with actinic keratosis on the nose showed complete clinical clearance of lesion (A). The atypical keratinocytes in the epidermis were all disappeared after treatment in the same patient (H&E, x 200) (B).
A.
2. Mean epidermal thickness and inflammatory infiltrates
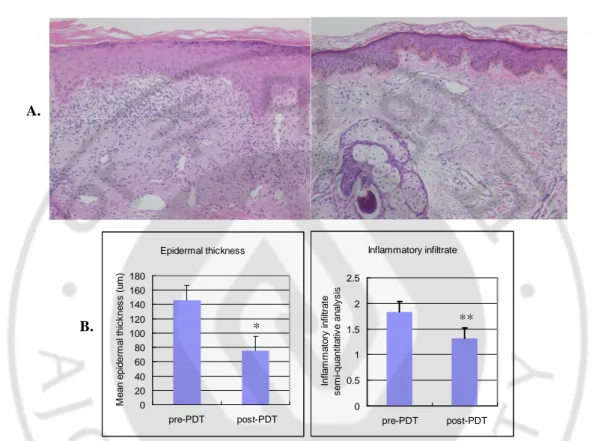
The pre-treatment specimens showed thick epidermis and a large number of infiltrating cells in the dermis. However, after treatment, the mean epidermal thickness and the inflammatory infiltrate were significantly reduced (Fig. 2 A, B).
pre- PDT post- PDT Epidermal thickness 0 20 40 60 80 100 120 140 160 180 pre-PDT post-PDT M e a n e p id e rm a l th ic k n e s s ( u m ) Inflammatory infiltrate 0 0.5 1 1.5 2 2.5 pre-PDT post-PDT In fl a m m a to ry i n fi lt ra te s e m i-q u a n ti ta ti v e a n a ly s is
Fig. 2. Decreased epidermal thickness and dermal inflammatory infiltrate after ALA-PDT. The epidermal thickness and numerous dermal inflammatory infiltrate decreased after PDT (patient 2, H&E, x 200) (A). The mean epidermal thickness in photodamaged facial skin was significantly reduced from 146.10 ±173.20 um to 75.26±29.70 um after treatment. And in the semi-quantitative analysis, the dermal inflammatory infiltrate also significantly decreased (B). (*p<0.00, **p<0.02)
A.
3. Total collagen volume
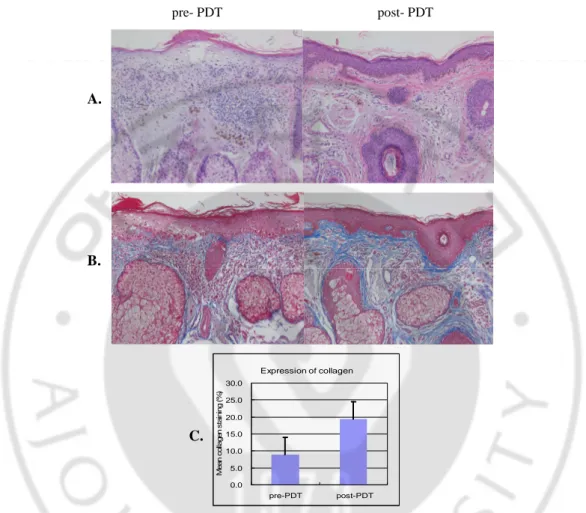
After PDT, almost all biopsy specimens showed impressive collagen deposition in the upper dermis by H&E staining that was more clearly demonstrated by Masson-trichrome staining (Fig. 3 A, B). The differences in staining density were significant (Fig. 3 C).
pre- PDT post- PDT Expression of collagen 0.0 5.0 10.0 15.0 20.0 25.0 30.0 pre-PDT post-PDT M e a n c o lla g e n s ta in in g ( % )
Fig. 3. Marked increased collagen deposition in the dermis after ALA-PDT. H&E (A) and
Masson-trichrome (B) stained specimens demonstrated up-regulated collagen fibers in the upper dermis after PDT (patient 7, x 200). Staining with Masson-trichrome demonstrated a 10.49
±9.93 % average increase of collagen in staining density in the post-treatment than in the pre-treatment specimens (p<0.00) (C).
A.
B.
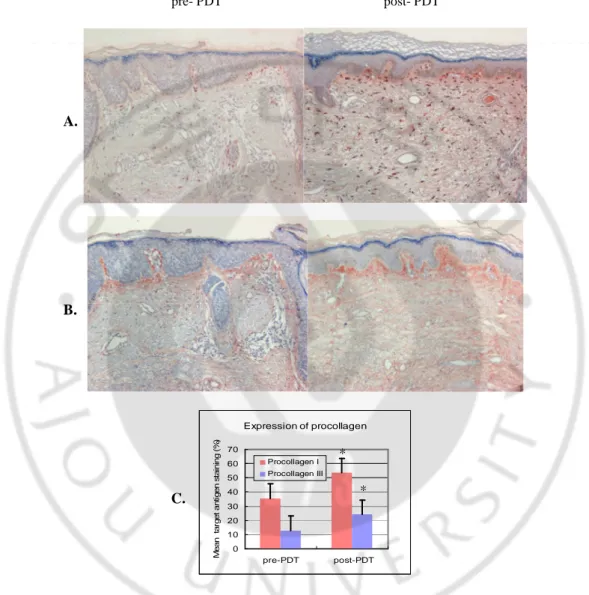
4. Procollagen type I and type III
The expression of collagen precursors was also increased. Staining with procollagen type I and type III antibodies demonstrated an 18.03±16.41% and 11.50±9.97% average increase in their staining density, respectively (Fig. 4 A-C).
pre- PDT post- PDT Expression of procollagen 0 10 20 30 40 50 60 70 pre-PDT post-PDT M e a n ta rg e t a n ti g e n s ta in in g ( % ) Procollagen I Procollagen III
Fig. 4. Increased expression of type I and III procollagen after ALA-PDT. Type I (A) and
type III (B) procollagen expression was increased in skin sections after ALA-PDT (patient 10, lesion 1, x 200). The average increase was significantly different (C). (*p<0.05)
A.
B.
C.
* *
5. TGF-ββββ and TββββR II
In the pre-treated skin sections, the expression of TGF-β and TβR II was insignificant. However, after treatment, the mean staining density with TGF-β and TβR II antibodies showed a significant increase mainly in the epidermis.
pre- PDT post- PDT TGF-b 0 10 20 30 40 50 60 70 80 pre-PDT post-PDT M e a n t a rg e t a n ti g e n s ta in in g ( % ) TGF-b TbRII C. TβR II TGF-β Expression of TGF-β and TβR II * * B) A)
Fig. 5. Increased expression of TGF-ββββ and TββββR II in the epidermis after treatment. TGF-β
(patient 3, x 200) (A) and TβR II (patient 14, x 200) (B) was more expressed in the epidermis after ALA-PDT. The mean staining with TGF-β and TβR II antibodies demonstrated a 23.57±15.16% and 6.61±9.56% average increase in staining density, respectively (C). (*p<0.05)
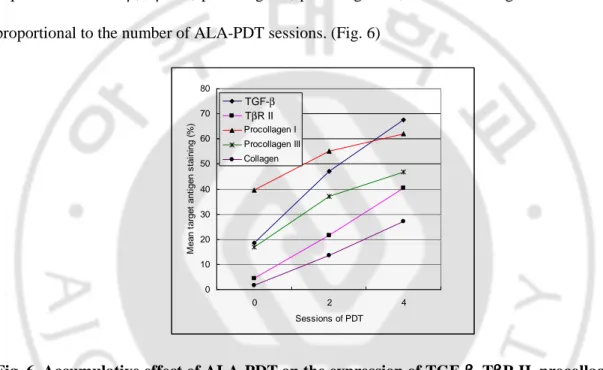
In one patient with two AK lesions that underwent two additional PDT sessions, the mean
expression of TGF-β, TβR II, procollagen I, procollagen III, and total collagen increased more proportional to the number of ALA-PDT sessions. (Fig. 6)
0 10 20 30 40 50 60 70 80 0 2 4 Sessions of PDT M e a n t a rg e t a n ti g e n s ta in in g ( % ) TGF-b TbRII Procollagen I Procollagen III Collagen
Fig. 6. Accumulative effect of ALA-PDT on the expression of TGF-ββββ, TββββR II, procollagen I,
procollagen III, and total collagen volume. The mean expression of TGF-β, TβR II,
procollagen I, procollagen III, and total collagen volume was more and more increased with
sessions of ALA-PDT (patient 8).
TGF-β
6. Elastotic material
In the H&E stained sections, a marked elastotic mass was visible in the papillary and reticular
dermis of the pre-PDT specimens that were pathognomonic for photoaging. After ALA-PDT,
there was a tendency for decrease of these elastotic masses compared to the pre-treatment
specimens. For patient 8 who received four treatment sessions, the change was more pronounced
(Fig. 7A). Verhoeff’s elastic stain demonstrated that after the PDT, the thickened and amorphous elastotic materials disappeared and were restored to more normal horizontally arranged fibers in
the dermis (Fig. 7B).
pre- PDT post- PDT
Fig. 7. Improvement of solar elastosis by ALA-PDT. After ALA-PDT, solar elastosis was
improved (patient 8, lesion 1, H&E, x 200) (A), and elastin fiber reorganization was more clearly
presented with Verhoeff’s stain (B) (same patient, x 400).
A.
7. Fibrillin-1 and tropoelastin
In the dermis, expression of fibrillin-1 and tropoelastin were mainly co-localized with elastotic
material, found in the epidermis to a lesser extent. After treatment, the immunoreactivity of
fibrillin-1 and tropoelastin was reduced along with resolution of the solar elastosis; the epidermal
expression also decreased (Fig. 8A-C).
pre- PDT post- PDT
Expression of fibrillin-1 and tropoelastin
0 0.5 1 1.5 2 2.5 3 3.5 pre-PDT post-PDT M e a n t a rg e t a n ti g e n s ta in in g s e m i-q u a n ti ta ti v e a n a ly s is Fibrillin-1 Tropoelastin A. B. C * *
Fig. 8. Decreased fibrillin-1 and tropoelastin after ALA-PDT. The fibrillin-1 (A) and
tropoelastin (B) expression decreased in the dermis after treatment (patient 8, lesion 2, x 200).
The mean difference was 1.32±0.90 and 0.56±0.58 in the semi-quantitative analysis after
ALA-PDT (C). (*p<0.05)
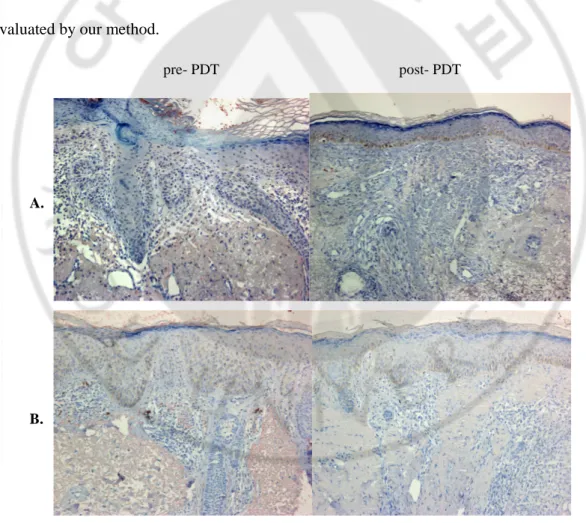
8. MMPs and TIMP
I also investigated changes in the MMPs, degradating enzymes of ECM, and TIMP-1, an
inhibitor of MMPs. The expression of MMP-1(Fig. 9A), MMP-3(Fig. 9B), and MMP-12 (Fig.
9C) tended to decrease after treatment. The immunoreactivity of TIMP-I was minimal when
evaluated by our method.
pre- PDT post- PDT
A.
Expression of MMP-1,-3,-12 0 0.5 1 1.5 2 2.5 pre-PDT post-PDT M e a n t a rg e t a n ti g e n s ta in in g s e m i-q u a n ti ta ti v e a n a ly s is MMP-1 MMP-3 MMP-12
Fig. 9. Decreased expression of MMP1, 3, and 12 after ALAPDT. The degree of MMP1,
-3, and -12 expression reduced after ALA-PDT (A-C). The differences were 0.60±0.91,
0.64±0.91, and 0.68±1.14 in the semi-quantitative analysis, respectively (D). (*p<0.05)
C.
D.
*
IV. DISCUSSION
Topical ALA-PDT was originally used for superficial non-melanoma skin cancers and their
precursors (Fritsch et al., 1998). However other benign diseases, such as acne vulgaris,
sebaceous gland hyperplasia, and hidradenitis suppurativa have been shown to improve with this
treatment (Szeimies et al., 2002). Moreover, previous studies have demonstrated the
effectiveness of ALA-based PDT treatments using a variety of lasers and light sources for
photorejuvenation (Table 2). However, most prior data has been based on clinical observation,
without histopathological confirmation. The results of the present study provide histopathological evidence for photorejuvenation with ALA-PDT in patients with AKs.
Table. 2 Summary of recent studies of ALA-PDT in photorejuvenation
Author year
Light source
Methods Results of ALA-PDT
Touma et al. 2004 (Touma et al., 2004) Blue light (417±5 nm) 18 subjects 20% ALA-solution 1,2 or 3 hours of application
Approximately 90% resolution of AKs and improvement skin appearance by reducing wrinkling, shallowness, and dyspigmentation
Dover et al.* 2005 (Dover et al., 2005) IPL 20 subjects 20% ALA-solution 0.5 to 1 hour of application
Significantly greater improvement in global photodamage, mottled pigmentation, and fine lines in the ALA-PDT-IPL group than treatment with IPL alone group
Marmur et al.*2005 (Marmur et al., 2005) IPL 7 subjects 20% ALA-solution 1 hour of application
An increase in type I collagen fibers was seen in the ALA-PDT-IPL group than treatment with IPL group alone in the electron microscopic ultrastructural analysis
Lowe et al. 2005 (Lowe and Lowe, 2005) LED (633nm) 6 subjects 5% 5-ALA cream 0.5 hour of application
Significant treatment response in four out of six subjects (67%) with a reduction in fine lines in the periorbital area, and skin softness was shown in all subjects.
Gold et al.* 2006 (Gold et al., 2006) IPL 16 subjects 20% ALA-solution 0.5 to 1 hour of application
Improvement of crow’s feet appearance, tactile skin roughness, mottled hyperpigmentation, and telangectasias, and clearance rate of AK lesions were higher in the ALA-PDT-IPL group than the in IPL-alone group
Orringer et al. 2008 Orringer et al., 2008) PDL 25 subjects 20% ALA-solution 3 hour of application
Upregulation of collagen production was shown with increase in procollagen type I and III mRNA
In photoaged skin, a decrease in type I and III collagen is more prominent than in intrinsic aged
skin. Ultraviolet induces MMPs that can degrade collagen in human skin. MMP-1 initiates
cleavage of type I and III collagen, and the damaged collagen can be further degraded by
MMP-3 and MMP-9 (Sternlicht and Werb, 2001). Chronic UV exposure likely causes the accumulation
of MMP-mediated collagen damage; eventually the total collagen volume in the dermis will
decline as a result. In the present study, the expression of MMP-1 and MMP-3 was found to be
decreased one month after ALA-PDT.
In addition to destruction of mature collagen, UV irradiation impairs synthesis of new collagen,
which is reflected by the down-regulation of type I and type III procollagen gene expression
(Fisher et al., 2000). In addition, the TGF-β/Smad pathway is impaired by down-regulation of TβRII and contributes to the pathology (Quan et al., 2004). TGF-β is a major cytokine that stimulates fibroblast proliferation important to enhancing collagen synthesis in the dermis
(Inagaki et al., 1994). TGF-β initially binds to cell surface receptors, the TGF-β type I receptor and TβRII. Then the activated complex interacts with intracellular signal transducer Smad proteins that conveys TGF-β signaling (Quan et al., 2002). However, UV irradiation down-regulates TβRII, which eventually decreases type I procollagen expression in human skin (Quan et al., 2004). In the present study, the expression of type I and III procollagen, the precursors of
collagen, were constitutionally increased after treatment, which reflect the increased synthesis of
dermal collagen. In addition, the immunoreactivity of TGF-β as well as TβR II was significantly increased after treatment, which indicates that they contribute to the reconstitution of the ECM.
The increase of dermal collagen, TGF-β and TβR II expression was more significant in patients who received two more additional PDT treatments. This suggested a cumulative effect of the
ALA-PDT on photorejuvenation.
Another prominent feature of photoaged skin is the accumulation of dystrophic elastotic
material in the reticular dermis, known as solar elastosis (Montagna et al., 1989). Elastic fibers
consist of a central core of elastin and surrounding fibrillin-rich microfibrils (Mecham RP, 1991).
Increased tropoelastin gene expression, both in the epidermal keratinocytes and fibroblasts of
human skin, has been shown in vivo with UVB irradiation (Seo et al., 2001); this may be the
process by which elastotic materials accumulate in photodamaged skin. Moreover, in the
reticular dermis of photodamaged skin, an increased expression and deposition of fibrillin were
demonstrated (Bernstein et al., 1994). In addition to the increased production of tropoelastin and
fibrillin, degradation of elastic fibers contributes to the solor elastosis. MMP-12, the active
degradating enzyme against elastin, was reported to co-localize with the elastotic material in
photoaged skin, and the expression of MMP-12 mRNA and protein was induced by UV
irradiation in human skin in vivo (Chung et al., 2002). In addition, diverse proteinases from the
chronic UV induced inflammatory infiltrate can destroy the microfibrils (Kielty et al., 1994).
Therefore, we investigated the effect of ALA-PDT on solar elastosis, and the expression of
tropoelastin, fibrillin-1, MMP-12, and inflammatory cells. ALA-PDT reduced the accumulation
of dystrophic elastotic material in the dermis, and resulted in more normal elastin fiber. In
addition, tropoelastin and fibrillin-1, which were mainly co-localized with the elastotic materials,
showed reduced expression after the PDT, and therefore represented an improvement of the solar
elastosis. The expression of MMP-12 and inflammatory infiltrates significantly decreased after
treatment, which likely was associated with the changes in the solar elastosis as well.
following treatment with ALA-PDT. This conflicts with the findings of some other studies that
have reported on the effects of ALA-PDT on the expression of MMPs. For example, when
normal or scleroderma fibroblast cells were treated with ALA-PDT, the levels of MMP-1 and
MMP-3 proteins increased; this was interpreted as antisclerotic effects of ALA-PDT (Karrer et
al., 2003). The expressed level of MMPs increased in a time-dependent manner with the
maximal induction at 48 hours following the PDT, thereafter with a decreasing tendency.
Recently, in an in vivo study of PDT using a pulsed dye laser in human skin, MMP-1 gene
expression was acutely elevated and then returned to baseline levels within 24 hours (Orringer et
al., 2008). However, it was also shown that MMP-2 expression was down-regulated 24 hours
after Hexvix mediated PDT in a medulloblastoma cell line (TE-671) (Chu et al., 2008).
Our results regarding decreased levels of MMPs might be explained as follows. We evaluated
the histological changes one month after the PDT, suggesting that the point in time of the
assessment might be an important consideration. In addition, various cell lines used in the in
vitro studies might differ from results from our in vivo study. TGF-β might play a role as well.
TGF-β does not only up-regulate procollagen synthesis, but it also down-regulates the expression of MMPs (Massague, 1990). In the present study, a marked increase of TGF-β expression was noted after the PDT. In addition, a change in the inflammatory infiltrates could
affect the decrease in the MMPs. It is known that UV-induced MMPs are secreted by diverse
cells, including infiltrated inflammatory cells (Hase et al., 2000). We found that the
inflammatory infilates were significantly decreased after the PDT. Therefore, the net effects of
the induction of TFG-β and reduction of the degradating enzymes in the inflammatory cells likely contributed to the decreased expression of MMPs after the ALA-PDT (Rijken et al., 2005).
V. CONCLUSION
In conclusion, this study provided histological evidence of the beneficial effects of ALA-PDT
for photodamaged skin. In the photodamagend human skin, ALA-PDT induces deposition of
collagen, type I and type III procollagen in the dermis and TGF-β and TβR II expression in the epidermis. In addition, MMP-1 and -3 expression was decreased after ALA-PDT. Solor elastosis
was improved accompanying with decreased expression of tropoelastin, fibrillin-1 and MMP-12
as well. These results suggest that ALA-PDT could be of therapeutic benefit in the treatment of
photoaging. Further controlled split –face studies on the mechanism of the effects of ALA-PDT
on photoaging, including signaling pathways, are necessary in order to fully elucidate the
REFERENCES
1. Alster TS, Tanzi EL, Welsh EC: Photorejuvenation of facial skin with topical 20% 5-aminolevulinic acid and intense pulsed light treatment: a split-face comparison study. J Drugs
Dermatol 4:35-38, 2005
2. Bernstein EF, Chen YQ, Tamai K, Shepley KJ, Resnik KS, Zhang H, Tuan R, Mauviel A, Uitto J: Enhanced elastin and fibrillin gene expression in chronically photodamaged skin. J
Invest Dermatol 103:182-186, 1994
3. Chu ES, Wong TK, Yow CM: Photodynamic effect in medulloblastoma: downregulation of matrix metalloproteinases and human telomerase reverse transcriptase expressions. Photochem
Photobiol Sci 7:76-83, 2008
4. Chung JH, Seo JY, Lee MK, Eun HC, Lee JH, Kang S, Fisher GJ, Voorhees JJ: Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J Invest Dermatol 119:507-512, 2002
5. Dover JS, Bhatia AC, Stewart B, Arndt KA: Topical 5-aminolevulinic acid combined with intense pulsed light in the treatment of photoaging. Arch Dermatol 141:1247-1252, 2005
6. Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ: c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest 106:663-670, 2000
7. Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ: Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 379:335-339, 1996
8. Fritsch C, Goerz G, Ruzicka T: Photodynamic therapy in dermatology. Arch Dermatol 134:207-214, 1998
1989
10. Gilchrest BA (1995) Biochemical and molecular changes in photodamaged skin. In:
P h o t o d a m a g e ( G i l c h r e s t B A e d ) B l a c k w e l l S c i e n c e : C a m b r i d g e , 1 6 8 – 1 8 4 .
11. Gold MH, Bradshaw VL, Boring MM, Bridges TM, Biron JA: Split-face comparison of photodynamic therapy with 5-aminolevulinic acid and intense pulsed light versus intense pulsed light alone for photodamage. Dermatol Surg 32:795-801; discussion 801-793, 2006
12. Hase T, Shinta K, Murase T, Tokimitsu I, Hattori M, Takimoto R, Tsuboi R, Ogawa H: Histological increase in inflammatory infiltrate in sun-exposed skin of female subjects: the possible involvement of matrix metalloproteinase-1 produced by inflammatory infiltrate on collagen degradation. Br J Dermatol 142:267-273, 2000
13. Inagaki Y, Truter S, Ramirez F: Transforming growth factor-beta stimulates alpha 2(I) collagen gene expression through a cis-acting element that contains an Sp1-binding site. J Biol
Chem 269:14828-14834, 1994
14. Kahari VM, Saarialho-Kere U: Matrix metalloproteinases in skin. Exp Dermatol 6:199-213, 1997
15. Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM: Influence of 5-aminolevulinic acid and red light on collagen metabolism of human dermal fibroblasts. J Invest
Dermatol 120:325-331, 2003
16. Kielty CM, Woolley DE, Whittaker SP, Shuttleworth CA: Catabolism of intact fibrillin microfibrils by neutrophil elastase, chymotrypsin and trypsin. FEBS Lett 351:85-89, 1994
17. Lavker RM, Kligman AM: Chronic heliodermatitis: a morphologic evaluation of chronic actinic dermal damage with emphasis on the role of mast cells. J Invest Dermatol 90:325-330, 1988
the treatment of facial ageing. J Cosmet Laser Ther 7:159-162, 2005
19. Marmur ES, Phelps R, Goldberg DJ: Ultrastructural changes seen after ALA-IPL photorejuvenation: a pilot study. J Cosmet Laser Ther 7:21-24, 2005
20. Massague J: The transforming growth factor-beta family. Annu Rev Cell Biol 6:597-641, 1990
21. Mecham RP HJ (1991) The elastic fiber. In: Cell Biology of the Extracellular Matrix Plenum Press: New York.
22. Montagna W, Kirchner S, Carlisle K: Histology of sun-damaged human skin. J Am Acad
Dermatol 21:907-918, 1989
23. Orringer JS, Hammerberg C, Hamilton T, Johnson TM, Kang S, Sachs DL, Fisher G, Voorhees JJ: Molecular effects of photodynamic therapy for photoaging. Arch Dermatol 144:1296-1302, 2008
24. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ: Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol 119:499-506, 2002
25. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ: Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol 165:741-751, 2004
26. Rijken F, Kiekens RC, Bruijnzeel PL: Skin-infiltrating neutrophils following exposure to solar-simulated radiation could play an important role in photoageing of human skin. Br J
Dermatol 152:321-328, 2005
27. Seo JY, Lee SH, Youn CS, Choi HR, Rhie GE, Cho KH, Kim KH, Park KC, Eun HC, Chung JH: Ultraviolet radiation increases tropoelastin mRNA expression in the epidermis of human skin in vivo. J Invest Dermatol 116:915-919, 2001
28. Sternlicht MD, Werb Z: How matrix metalloproteinases regulate cell behavior. Annu Rev
Cell Dev Biol 17:463-516, 2001
29. Szeimies RM, Landthaler M, Karrer S: Non-oncologic indications for ALA-PDT. J
Dermatolog Treat 13 Suppl 1:S13-18, 2002
30. Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest BA: A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch
-국문 요약-
광선
광선
광선
광선 각화증
각화증
각화증
각화증 환자에게서
환자에게서
환자에게서 5-Aminolevulinic Acid 를
환자에게서
를
를 이용한
를
이용한
이용한
이용한
광역동
광역동
광역동
광역동 치료
치료
치료 후
치료
후
후
후 나타난
나타난
나타난
나타난 광회춘
광회춘 효과
광회춘
광회춘
효과
효과
효과: 조직학적
조직학적
조직학적 분석
조직학적
분석
분석
분석
아주대학교 대학원 의학과 박민영 (지도교수: 김유찬) 연구 배경: 만성적인 자외선 노출은 내인성 노화와는 다르고 더 심한 양상을 나타내는 광노화를 유발한다. 조직학적으로 광노화된 피부는 collagen 합성의 감소, 기존 collagen 파괴의 증가와 함께 비정상적인 elastotic material 의 진피 침착을 특징으로 한다. 광노화된 피부를 회복시키기 위한 여러 가지 치료법 중에서 광역동 치료가 이에 효과가 있다는 연구들이 있다. 그러나 이들 대부분의 보고는 임상적인 관찰에 근거한 것이었다.연구 목적: 본 연구에서는 5-aminolevulinic acid (ALA)를 이용한 광역동 치료가 광 회 춘 효 과 를 나 타 내 는 조 직 학 적 변 화 를 일 으 키 는 지 확 인 하 고 자 하 였 다 .
연구 방법: 얼굴 부위에 한 개에서 세 개의 광선 각화증을 가진 환자를 대상으로,
1200W metal halogen lamp 를 광원으로 하여 한 달 간격으로 2 회 ALA 광역동 치료를
시행하였다. 치료 전과 최종 치료 1 달 후 조직 검사를 시행하였고 총 25 쌍의 피부 조직을 얻었다. 각각에 대하여 기본 염색과 여러 면역 조직 화학 염색을 시행하였다.
연구 결과: ALA 를 이용한 광역동 치료 후, 평균 표피 두께가 감소하였고 진피의 염증 세포 침윤이 줄어들었다. 진피의 collagen 부피의 증가가 뚜렷하였고 이는 type
I, III procollagen 발현의 증가와 동반되었다. 또한 표피에는 TGF-β 와 TGF-β type II receptor 가 치료 후 더 높게 나타났다. elastotic material 에 함께 위치한 fibrilline-1 과
tropoelastin 의 발현이 광역동 치료 후 감소되는 소견을 보였고, MMP-1, 3, 12 또한 감소하는 경향을 보였다. 결론: 본 연구 결과는 ALA 를 이용한 광역동 치료가 광노화 된 피부의 회복에 도움을 준다는 것에 대한 조직학적 증거를 제시하였다. 5-ALA 를 이용한 광역동 치료는 앞으로 광회춘에 대한 한가지 치료 방법으로 사용될 수 있을 것이다. 아울러 추 후 이 에 대 한 자 세 한 기 전 을 밝 히 기 위 한 조 직 화 된 연 구 가 필 요 하 겠 다 . 핵심어: 광노화, 광역동 치료, 조직학적 변화