TIS21 shifts p53-induced response of
EJ bladder carcinoma cells
from senescence to apoptosis
by
Ok Ran Choi
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
TIS21 shifts p53-induced response of
EJ bladder carcinoma cells
from senescence to apoptosis
by
Ok Ran Choi
A Dissertation Submitted to The Graduate School of
Ajou University in Partial Fulfillment of the Requirements
for the Degree of
Ph.D. in Medical Sciences
Supervised by
In Kyoung Lim M.D., Ph.D.
Major in Medicine
Department of Medical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Ok Ran Choi is approved.
SUPERVISORY COMMITTEE
Yi-Sook Jung
In Kyoung Lim
Sang Chul Park
Kyeong Sook Choi
Gyesoon Yoon
The Graduate School, Ajou University
June 23rd, 2011
- ABSTRACT -
TIS21 shifts p53-induced response of EJ bladder carcinoma cells
from senescence to apoptosis
It has been known that wild type p53 mediates senescence phenotypes and cell death in response to DNA damage signals both in human and animals. TIS21BTG2/PC3 has been known as a p53 target gene and functions as a tumor suppressor in carcinogenesis of thymus, prostate, kidney, and liver. Bladder carcinoma EJ cells contain both oncogenic H-ras and non-functional p53 tumor suppressor due to mutation in its exon 5, possibly contributing to conferring EJ cells cancer characteristic. In normal primary fibroblast cells, over-expression of H-ras activates p53 pathway and induces cellular senescence.
Employing adenoviral vectors carrying p53 (Ad-p53) or TIS21 (Ad-TIS21) gene, I evaluated the effect of TIS21 on the p53-induced senescence phenotypes in EJ cells. Adenoviral transfer with wild-type p53 significantly induced senescent phenotypes of EJ cells characterized by increase in size, flattened morphology and an increase in SA-b-galactosidase activity. p53 increased expression of paxillin which regulated senescent morphology localizing at the edge of cell membrane. EJ cells co-expressing p53 plus TIS21 significantly reduced the expression paxillin, which was induced by p53 expression.
The effect in cells infected with p53 plus TIS21 was quite distinct from that of the p53 alone. Expression of the tumor suppressors clearly reduced cell size, SA-b-galactosidase activity and expression of paxillin. Expressions of p53 plus TIS21 significantly induced cell death of the cells, as demonstrated by staining with EthD-1 and Annexin V. Moreover, the
expression of TIS21 increased apoptosis-associated acetylation of p53 molecule at lysine residues and its accumulation in the nuclei.
Based on the evidences presented herein, I conclude that TIS21 directs p53-induced senescence towards apoptosis in EJ bladder carcinoma cells.
Keywords: p53, TIS21BTG2/PC3, H-ras, paxillin, senescence, apoptosis, caspase 3 activity, acetylation of p53, p53 localization
TABLE OF CONTENTS
ABSTRACT ··· ⅰ TABLE OF CONTENTS ··· ⅲ LIST OF FIGURES ··· vi . Ⅰ INTRODUCTION ··· 1A. Background of EJ bladder carcinoma cell line ··· 1
B. Functions of TIS21/BTG2/PC3 ··· 2
C. Functions of tumor suppressor protein p53 ··· 3
D. Purpose of this study ··· 4
. Ⅱ MATERIALS AND METHODS ··· 5
A. MATERIALS ··· 5
B. METHODS ··· 5
1. Cell culture ··· 5
2. Adenoviral infection ··· 5
3. Senescence associated b-galactosidase (SA-b-gal) assay ··· 6
4. Immunoblot analysis ··· 6 5. Immunocytochemistry ··· 7 6. RT-PCR analysis ··· 7 7. siRNA trasnfection ··· 8 8. ROS generation ··· 8 9. Measurement of apoptosis ··· 8 (1) Annexin V staining ··· 8
(2) Calcein-AM and EthD-1 staining ··· 9
(3) Caspase 3/7 activity assay ··· 9
10. Soft agar colony forming assay ··· 9
11. Assay for cell plating efficiency assay ··· 10
12. Statistical analyses ··· 10
. Ⅲ RESULTS ··· 11
A. Role of TIS21/BTG2/PC3 in EJ cells ectopically expressing p53 ··· 11
B. TIS21 enhanced apoptosis in EJ cells ectopically expressing p53 ··· 13
C. Co-expression of p53 plus TIS21 decreased colony forming ability of EJ cells ··· 15
D. p53 increased senescence markers in TIS21-independent manner ··· 17
E. Effect of p53 plus TIS21 on paxillin expression in EJ cells ··· 19
F. Knockdown of p53 restored senescent phenotype and reduced the expressions of paxillin and H-ras ··· 21
G. Co-infected cells with p53 plus TIS21 increased apoptosis markers related with intrinsic pathway ··· 24
H. TIS21 posttranslationally modificated p53 and changes the cellular localization of p53 ··· 27
I. TIS21 siRNA abrogated p53 plus TIS21-mediated apoptosis ··· 32
J. p53 localized at the cytoplasm in the Doxorubucin-induced-senescent cells ··· 34
K. TIS21 induced p53-mediated apoptosis ··· 36
. Ⅳ DISCUSION ··· 41 . Ⅴ CONCLUSION ··· 45 REFERENCES ··· 46 국문요약 ··· 56
LIST OF FIGURES
Fig. 1. Expression of wild-type p53 in EJ cells induced senescence phenotypes, but co-expression of p53 plus TIS21 did not ··· 12 Fig. 2. Co-expression with p53 plus TIS21 increased apoptosis in EJ cells ··· 14 Fig. 3. Co-expression of p53 plus TIS21 reduced colony forming ability in EJ
cells ··· 16 Fig. 4. Expression of p53 increased the molecular markers of cellular
senescence ··· 18 Fig . 5. Expression of p53 upregulated paxillin expression in EJ cells, but
Co-expression with TIS21 attenuated ··· 20 Fig. 6. Confirmation of p53 effect on paxillin expression by transfection of p53-siRNA ··· 23 Fig. 7. Co-expression p53 plus TIS21 increased the expression of pro-apoptotic
proteins, Apaf-1 and p53AIP1 in EJ cells ··· 25 Fig. 8. Co-expression of wild-type p53 with TIS21 enhanced apoptosis in EJ cells ··· 26 Fig. 9. Co-expression of p53 plus TIS21 in EJ cells significantly increased the acetylation of p53 on the several lysine residues ··· 28 Fig. 10. Differential localization of p53 in the cells expressing p53 alone and
cells expressing p53 plus TIS21 ··· 29 Fig. 11. No difference in a-tubulin acetylation the cells expressing p53 alone
Fig. 12. Expression of TIS21 increased the translocation of p53 into the nuclei and its acetylation ··· 31 Fig. 13. TIS21-siRNAs inhibited apoptosis in EJ cells infected with p53 plus TIS21 ··· 33 Fig. 14. p53 localization in the replicative or the doxorubicin-induced
senescence of HDF cells ··· 35 Fig. 15. Co-infection with p53 plus TIS21 induced apoptosis in EJ cells ··· 38 Fig. 16. Inhibition of FoxM1 and Skp2 expression by wild-type p53 in EJ cells, which can stabilize TIS21 expression in the cells ··· 40
I. INTRODUCTION
A. Background of EJ Bladder carcinoma a cell line
Bladder cancer is the fourth common cancer in males in the United States (Jemal et al., 2009). In Korea, bladder cancer is the sixth frequent neoplasia in men (Song et al., 2010). In 2002, the global incidence of bladder cancer was estimated at 357,000 cases, making this the 7th common cancer in men and the 17th common in women worldwide (Parkin et al., 2005). Human bladder carcinoma cell line (EJ cell) contains both an oncogenically activated H-ras gene (Parada et al., 1982; Reddy et al., 1982) mutation and nonfunctional p53 due to a mutation in exon 5 (Rieger et al., 1995). Point mutations in p53 gene of the EJ cell line resulted in the amino acid changes of glutamate for lysine at codon 164. The ras genes produce a small family of highly related proteins with a potent transforming potential. Ras family consists of three members, including H-, N-, and K-ras. The Harvey sarcoma virus-associated oncogene was named Ha-ras (H-ras in mammals) (Parada et al., 1982), whereas that of Kirsten sarcoma virus was termed Ki-ras (K-ras in mammals) (Der et al., 1982). Mutant alleles of these ras sequences were soon discovered in many human cancer cell lines, including those of the bladder, colon and lungs. By 1983, the third member of the mammalian family of ras-related genes, N-ras had been cloned from neuroblastoma and leukaemia cells (Hall et al., 1983; Shimizu et al., 1983; Taparowsky et al., 1983). This gene was also found to contain activating point mutations in certain human tumours. On some experimental basis, oncogenic ras is incapable of transforming primary fibroblasts by itself, instead inducing growth arrest and premature senescence (Franza et al., 1986; Serrano et al.,
1997). This phenomenon is due to activation of p53, which is downstream of ras. Expression of wild-type p53 in EJ cell triggers senescence program in human tumor cells lacking functional p53 (Sugrue et al., 1997).
B. Functions of TIS21/BTG2/PC3
TIS21 (12-O-tetradecanoylphorbol-13-acetate-inducible sequence 21) was first identified as one of the immediate early response genes (Lim et al., 1987) in mouse 3T3 fibroblasts treated with 12-O-tetradecanoylphorbol-13-acetate (Fletcher et al., 1991) and belongs to anti-proliferative gene family along with BTG1 (Rouault et al., 1996), BTG3 (Guehenneux et al., 1997), and Tob (Matsuda et al., 1996) genes. TIS21/BTG2/PC3 represents orthologs of mouse, human, and rat (Lim, 2006), respectively.
TIS21 has been reported to function diverse biological process; (a) a transcriptional co-regulator (Rouault et al., 1998; Morel et al., 2003; Duriez et al., 2004), (b) a differentiation and anti-apoptotic factor in neurogenesis (Iacopetti et al., 1999; Corrente et al., 2002; el-Ghissassi et al., 2002; Canzoniere et al., 2004), (c) a key mediator of the stage-specific expansion of thymocyte and the negative regulator of hematopoietic progenitor expansion (Konrad and Zuniga-Pflucker, 2005), (d) a tumor suppressor gene in both mouse and human (Lim et al., 1995; Ficazzola et al., 2001; Kawakubo et al., 2004; Struckmann et al., 2004; Lim, 2006) (e) a pancell cycle regulator; G1/S arrest in pRBdependent and -independent manners, and G2/M arrest and cell death in the p53 null U937 cells (Lim et al., 1998; Guardavaccaro et al., 2000; Ryu et al., 2004; Hong et al., 2005), (f) a developmental regulator of vertebrate patterning in mouse, paraxial mesoderm development in zebra fish
and notochord development in Xenopus (Sakaguchi et al., 2001; Park et al., 2004; Sugimoto et al., 2005). Boiko et al. (Boiko et al., 2006) recently ascertained BTG2 as a tumor suppressor by showing that BTG2 is a major downstream effector of p53-dependent growth arrest of mouse and human fibroblasts transduced with oncogenic Ras, and that repression of BTG2 regulates the activities of cyclin D1 and cyclin E and phosphorylation of pRB, thus inducing neoplasic transformation of primary human fibroblasts.
So far only a few reports have been published regarding the role of TIS21/BTG2/PC3 in apoptosis; In U937 cells, for example, phosphorylation of serine 147 residue of TIS21/BTG2/PC3 by p-Erk1/2 induces Pin-1 binding in the cytoplasm and subsequent cell death (Hong et al., 2005). Also BTG2 enhances the susceptibility of HeLa cells to doxorubicin-induced oxidative damage (Lim et al., 2008). Despite the expanding pool of knowledge regarding the involvement of TIS21/BTG2/PC3 in various biological processes, its exact role in apoptosis and the detailed regulatory mechanism is largely unknown.
C. Functions of tumor suppressor protein p53
In normal cells under physiological conditions, the tumor suppressor protein p53 is expressed at low levels and has a short half-life due to rapid turnover mediated by ubiquitination and proteolysis (Oren et al., 1981; Maki et al., 1996). The activation of p53 allows it to carry out its function as a tumor suppressor through a number of growth controlling endpoints. These include cell cycle arrest (Shen et al., 1983), apoptosis (Yonish-Rouach et al., 1991), senescence (Levine, 1997), differentiation (Chandrasekaran et al., 1982) and anti-angiogenesis (Dameron et al., 1994). In the role of p53 as a tumor suppressor, it
serves as a ‘guardian of the genome’ (Lane, 1992), by regulating critical checkpoints in response to the distinct stresses.
The ability of p53 to induce cell growth arrest and apoptosis is relatively well-understood, and its importance in tumor suppression is firmly established. However, the molecular details of how p53 distinguishes between the genes of the different transcriptional programs remain still unclear.
D. Purpose of this study
In the present study, I have focused on the effect of TIS21 on p53-expressing bladder carcinoma EJ cells using Adenovirus system. Our results revealed that p53 expression significantly induced senescence phenotypes of EJ cells. However, p53 plus TIS21 significantly increased apoptotic phenotypes. Here, I discuss function of TIS21 in the induction of apoptosis of bladder carcinoma EJ cells.
.
Ⅱ
MATERIALS AND METHODS
A. MATERIALS
Antibodies against pRB, p53, p21sdi1, GAPDH, a-tubulin, HA, cyclin E1, caveolin 1, Paxillin, Skp2, FoxM1, and actin were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-H-ras antibody was from cytoskeleton Inc. (Denver, CO), those of PARP, caspase 3, pERK1/2, pAKT, pro-apoptotic proteins, p53S46 was from Cell Signaling. Anti-DR5, FAS, Apaf1, IGFBP3, p53AIP1 and anti-Ac-p53 were purchased from Abcam. Dulbecco’s modified Eagle's medium (DMEM), fetal bovine serum (FBS), and trypsin were purchased from Gibco-BRL (Grand Island, NY). FITC-labeled goat-anti-rabbit IgG was from Jackson Immuno Research Laboratories, Inc. (West Grove, PA). X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside) was from Sigma Chemical Co. (St. Louis, MO). Calcein-AM and EthD-1 were obtained from sigma. All other reagents were used in molecular biology grade.
B. METHODS
1. Cell culture
The EJ human bladder carcinoma cells were grown in DMEM supplemented with 5% FBS (Gibco-BRL) and penicillin-streptomycin (50 U/ml) and maintained in 5% CO2 at 37°C.
2. Adenoviral infection
performed with 40 multiplicity of infection (moi) for 4 hours, and then cultured the cells in the fresh medium until 48 hours.
3. Senescence associated
b-galactosidase (SA-b-gal) assay
SA-b-gal assay was performed as described previously (Dimri et al., 1995). Briefly, cells were washed twice with PBS and fixed in 3% formaldehyde solution for 5 min, and then incubated overnight in freshly prepared staining solution [40 mM citric acid/sodium phosphate, pH 6.0, 1 mg/ml of X-gal (5-bromo-4-3-indolyly-β-galactopyranoside), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl2] after washing with PBS. Stains were observed 24 h after incubation at 37 °C. By counting numbers of the blue staining and the total cells in the field of 0.5 × 0.5 cm under an inverted microscope, degree of senescence was calculated as the percentage of cells stained in blue over the total number of cells. Counts from the five fields were calculated and the average was measured.
4. Immunoblot analysis
Cells were washed twice with PBS and lysed with RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% NP40, 0.5% deoxycholic acid, 1 mM PMSF] for 30 min on ice. After centrifugation at 12,000 rpm for 30 min at 4 °C, lysates were collected. Equivalent amounts of proteins were resolved on SDS-PAGE and then transferred to a PVDF (Bio-Rad). Membranes were blocked with PBS containing 5% non-fat milk, and 0.1 % Tween-20 for 1 hr at room temperature and immunoblotted. Enhanced chemiluminescence
reagent (Amersham) was applied to visualize protein bands.
5. Immunocytochemistry
EJ cells plated on coverslips were washed twice with PBS and fixed with 4% paraformaldehyde in PBS at room temperature (RT) for 10 min, and then washed 3 times with PBST (1 x PBS, pH7.4 containing 0.1 % Tween-20). After permeabilization with PBST (at RT for 15 min), the cells were incubated with 5% BSA in PBST for 1 hr at RT, and then with a primary antibody for 1 hr at RT, followed by incubation with secondary antibody (1:500 in PBST) for 1 hr. After incubating with secondary antibody, DAPI was applied for 10 min and the cells were observed under fluorescence microscope. Images were captured with an LSM510 Zeiss confocal microscope. Images were quantified using an Axioimager M1 with Axiovision 4.5 software (Carl Zeiss, Jena, Germany).
6. RT-PCR analysis
Total RNA was extracted using TRIzol reagent (Gibco BRL) according to the manufacturer’s instruction. To prepare cDNAs, 1 mg of total cellular RNAs was reverse-transcribed by SuperScriptTM II reverse transcriptase (Invitrogen, Carlsbad, CA) with oligo dT primer according to manufacturer’s instruction. The primers used were: GAPDH sense and antisense primers, 5’-CCATGGAGAAGGCTGGGG-3’ and CAAAGTTGTCATGGATGACC-3’; BTG2 sense and antisense primers, 5’-CCTGGGCAGAGAGTGAAAAG-3’ and 5’-CCTTCCATCCTAACCCCAAT-3’; TIS21 sense and antisense primers, 5’-ATGAGCCACGGGAAGAG-3’ and 5’-CGGGGCCTCCTCATAC-3’; Skp2 sense and antisense primers,
5’-ATGCACAGGAAGCACCTCCAGG-3’ and 5’-TCATAGACAACTGGGCTTTTGC-3’, respectively. PCR products were evaluated by 2.0% agarose gel electrophoresis.
7. siRNA transfection
Transfection of p53-siRNA to EJ cells was performed for 4 hrs using oligofectamin (Invitrogen) according to the protocol provided by manufacturer after adenovirus infection (40 moi) for 4 hr in DMEM with 5% FBS.
8. ROS generation
H2O2 generation was measured by FACS analysis using 20 mM 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA, D-399, Molecular Probes, Eugene, OR). The infected- and transfected-cells were maintained for 3 days. H2-DCFDA was added 20 min prior to harvesting the cells. Samples were analyzed by flow cytometry using LSR-II Flow Cytometer and FACS DiVa 4.0 software (BD Biosciences).
9. Measurement of apoptosis
(1) Annexin V staining
FITC-Annexin V Apoptosis Detection Kit I was used according to manufacturer’s instruction. Briefly, cells were washed twice with cold PBS, resuspended in 1x binding buffer at a concentration of 1 x 105 cells/100 ml, and then 5 ml FITC- annexin V and 5 ml
propidium iodide were added before incubation for 15 min at RT in dark. Finally, 1x binding buffer (300 ml) was added to each tube and analyzed by flow cytometry within 1 hr.
(2) Calcein-AM and EthD-1 staining
After infection with LacZ, p53 and/or TIS21 for 2 days, Calcein-AM (2 mM) and EthD-1 (4 mM) were added to the cells and incubated at 37°C for 15 min. The cells were washed twice with PBS and observed using a fluorescence microscope (Zeiss Axiovision 200M).
(3) Caspase 3/7 activity assay
To determine apoptosis, caspase-Glo 3/7 assay (Promega, CA) was performed on p53 and/or TIS21 infected EJ cells. Cells (5x103) infected with adenovirus carrying with either LacZ or p53 and/or TIS21 for 2 days were seeded in 96-well plates. Caspase-Glo 3/7 reagent (100 ml) was added directly to the 96-well plates and incubated for 1 hr before recording luminescence using TD-20/20 luminometer (Turner Designs, CA).
10. Soft agar colony forming assay
To prepare of base agar, agar (1.6%) was dissolved in distilled water and autoclaved at 120 °C for 15 min, then cooled to 42 °C in water bath in sterilized environment. DMEM (2x) containing 10% FBS and antibiotics was warmed to 40 °C in water bath and equilibrated at least 30 minutes. Equal volumes of the two solutions were mixed to give 0.8% agar + 1x DMEM + 5% FBS. Finally, 1.5 ml of the mixture was added to each 6 well plate and the plates were kept for 5 min to solidify base agar. To prepare of top agar, agar (0.8%) was
autoclaved and cooled to 42 °C in water bath. Cells (2 x 105) were seeded in a 60-mm plate and infected with p53 and/or TIS21. One day after infection, cells were reseeded. Cells were trypsinized and counted using hemocytometer. Cells (5x103) were mixed with 2x complete medium by gentle swirling and then added 1.5 ml to each 6 well plate. The plates were kept for 5 min to solidify top agar. Two ml of complete medium was added on top of the layer and the plates were incubated at 37 °C in a humidified incubator for 2 weeks. Numbers of colonies in soft agar were counted under a microscope.
11. Assay for cell plating efficiency
Cells (1 x 105) were seeded in a 60-mm plate and infected with p53 and/or TIS21. Infected-EJ cells were maintained for 3 days. Colonies containing more than 50 cells were evaluated as positive after staining with crystal violet.
12. Statistical analyses
Numerical data were presented as mean + standard deviation (SD) of the three independent determinations. The statistical analysis was performedwith Student's t test. For analyzing multiple treatment groups, a factorialANOVA followed by Bonferroni's t test was applied. Differenceswith p values of <0.05 were considered to be statistically significant.
III. RESULTS
A. Role of TIS21/BTG2/PC3 in EJ cells ectopically expressing p53
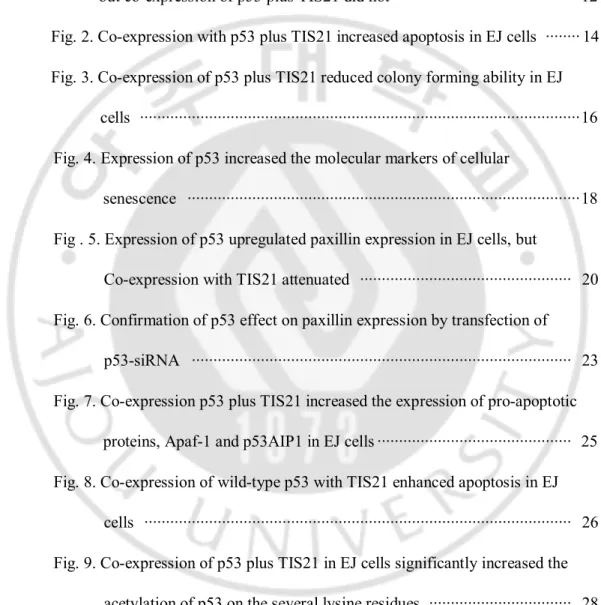
It has been already known that wild-type p53 mediates senescence phenotypes and cell death in response to DNA damage signals both in human and animals. Introduction of p53 triggers a rapid senescence program in EJ cells lacking functional p53 (Sharma et al., 1993; Sugrue et al., 1997). Here, I evaluated whether TIS21 affected the senescence phenotypes which was induced by exogenous wild-type p53 in EJ cells. Cells with p53 infection showed enlarged cell size, (Figs 1A, 1C), reduced cell proliferation (Fig 1B), and increased SA-b-galactosidase activity (Fig. 1D), suggesting the induction of cellular senescence. However, co-infection with TIS21 enhanced the effect of p53 on the inhibition of cell proliferation, but decreased cell size and SA-b-galactosidase activity (Fig. 1). TIS21 thus inhibited flattening of cells, enlarged cell size, cell proliferation and SA-b-galactosidase activities in EJ cells infected with p53.
Fig. 1. Expression of wild-type p53 in EJ cells induced senescence phenotypes, but co-expression of p53 plus TIS21 did not. (A) Cells were seeded at a density of 1 x 105 in a 60 mm dish, infected with p53 and/or TIS21, cell morphology was observed 3 days of infection and photographs were taken under an inverted microscope. (B) Cells were plated at a density of 6 ´ 103 cells/well in a 12-well plate. On the indicated times, cell numbers were counted using hemocytometer. (C) Cells were prepared as described in (A). Cells size was measured using Image J software. (D) Cells were seeded in a density of 1 x 105 cells in a 60 mm dish. After 3 days, cells were reseeded and then incubated for another 3 days. Cells were stained 6 days after adenovirus infection by using SA-b-galactosidase assay.
B. TIS21 enhanced apoptosis in EJ cells ectopically expressing p53
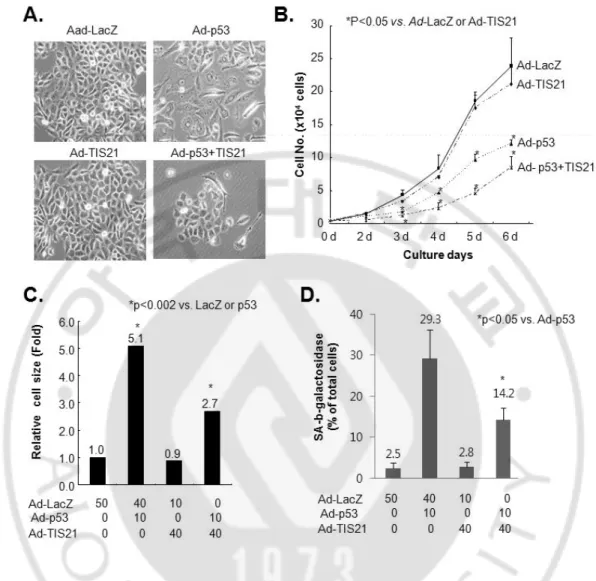
It has been reported that phosphorylation of TIS21 by pErk1/2 induces binding of TIS21 to Pin-1 or cyclin B1-Cdc2 complex and leads to mitochondrial depolarization (Hong et al., 2005). Furthermore, BTG2 enhances the susceptibility of HeLa cells to doxorubicin-induced oxidative damage (Lim et al., 2008). In the present study, I assessed the role of TIS21 on cell death in the presence p53. Infection with either LacZ or TIS21 did not show any sign of cell death. Cells infected with p53 alone induced senescence phenotype and slight cell death (Fig. 2B and C). Co-infection with p53 plus TIS21, however, increased dead cells (Fig. 2A) and enhanced EthD-1 (20.4%) and Annexin V (11.2%) positive EJ cells (Fig. 2B and 2C). These results suggest that TIS21 enhances apoptosis in p53-dependent manner.
Fig. 2. Co-expression with p53 plus TIS21 increased apoptosis in EJ cells. (A) Cells were plated at a density of 2 ´ 105 cells and cell numbers were counted using hemocytometer at the indicated time points. (B) Adenovirus infected EJ cells were prepared at 2 days and cell viability was assessed by double labeling with 2 mM Calcein-AM and 4 mM EthD-1. The calcein-positive live cells and ethidium-positive dead cells were visualized using a fluorescence microscope and counted. (C) Cells were washed in cold PBS and resuspended in binding buffer. The cells were incubated with 5 ml FITC Annexin-V and 5 ml propidium iodide for 15 min in darkness. Cells were immediately analyzed by flow cytometry.
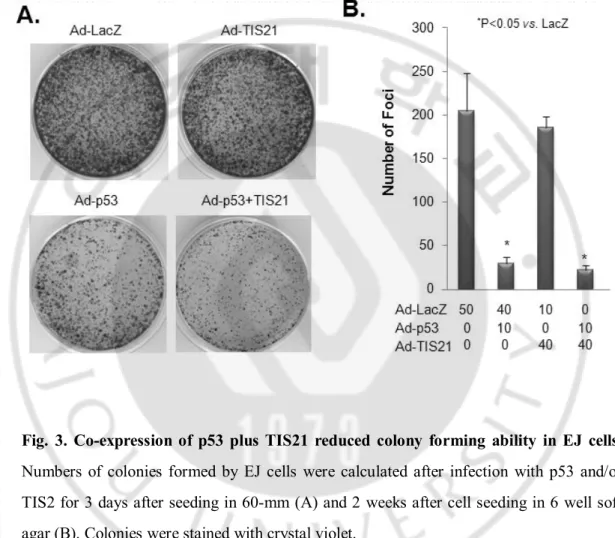
C. Co-expression of p53 plus TIS21 decreased colony forming ability of EJ cells
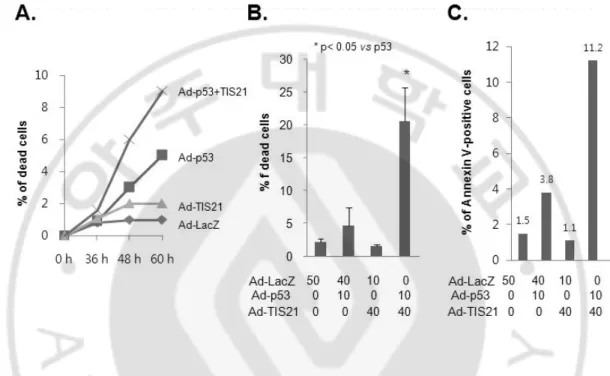
Wild-type p53-induced senescence is a tumor-suppressing mechanism that needs to be disrupted during cancer development. We thus analyzed the contribution of TIS21-mediated apoptosis on p53-infected EJ cells in semisolid soft agar medium. Combination of p53 plus TIS21 resulted in efficient inhibition of anchorage-dependent growth in soft agar (Fig. 3A) and anchorage-independent colony formation (Fig. 3B). Therefore, TIS21 enhances the apoptotic effect of p53 on EJ cells.
Fig. 3. Co-expression of p53 plus TIS21 reduced colony forming ability in EJ cells. Numbers of colonies formed by EJ cells were calculated after infection with p53 and/or TIS2 for 3 days after seeding in 60-mm (A) and 2 weeks after cell seeding in 6 well soft agar (B). Colonies were stained with crystal violet.
D. p53 increased senescence markers in TIS21-independent manner
I has previously reported that over-expression of TIS21 inhibits 293 cells growth by delayed synthesis of cyclin E and CDK4 activity independent of pRB expression (Lim et al., 1998). I, therefore, assessed the level of cyclin E and pRB in EJ cells infected with p53 and/or TIS21. The level of pRB and cyclin E1 decreased in TIS21-independent manner in EJ cell expressing p53 (Fig.4). I further analyzed the cell cycle profile of EJ cells by flow cytometry. After infection for 2 days, majority of the p53 and the p53 plus TIS21-expressing cells progressed into S phase (30.6% vs. 33.4%, respectively), whereas S phase population of TIS21-expressing cells remained similar to that of the control (Fig. 4B). Expressions of senescence marker molecules such as pErk1/2, pGSK3b and pAkt (Fig. 4C) and generation of ROS (Fig. 4D) were increased both by p53 alone and co-expression of p53 plus TIS21, whereas expression of PARP was significantly decreased. Expression of TIS21 alone, however, did not show any difference from expression of LacZ alone.
Fig.4. Expression of p53 increased the molecular markers of cellular senescence. Expression of cell cycle-related proteins (A) and senescent markers (C) was examined after infection with or without p53 or TIS21 at the indicated time points (A) and 2 days after infection (B-D). Cells were seeded at a density of 1 x 105 per 60 mm dish and infected with p53 and/or TIS21. Cells were harvested at 2 days after infection. At least 10,000 cells were counted in each experiment and analysis of cell cycle phase was repeated 4 times by flow cytometry.
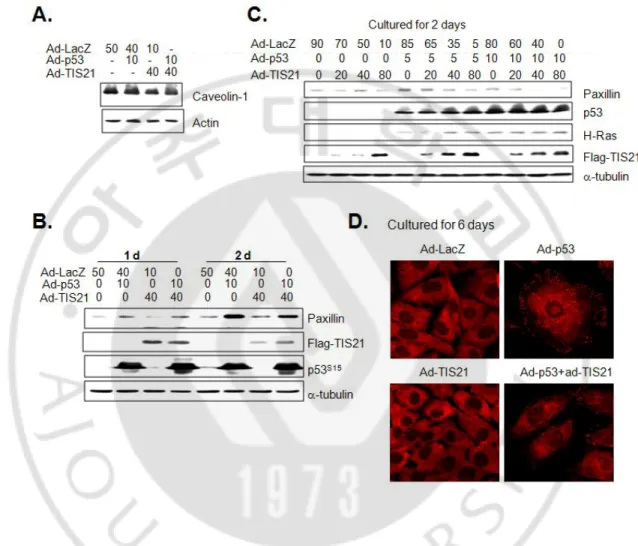
E. Effect of p53 plus TIS21 on paxillin expression in EJ cells
It has been reported that expressions of caveolin-1 and paxillin, focal adhesion protein, regulate the morphology of senescent cells (Chen et al., 2000; Cho et al., 2004; Nishio and Inoue, 2005). I therefore assessed their expression after p53 infection into EJ cells with or without TIS21 infection. Although p53 failed to regulate caveolin-1 expression (Fig. 5A), expression of paxillin was significantly increased by p53 expression (Fig. 5B), as opposed to the slight reduction by co-expression with TIS21 (Fig. 5C). Moreover, paxillin was distributed mostly at the edge of cell membrane in the cells expressing p53 alone, whereas it was cytoplasm in the other cells (Fig. 5D). These results indicate that expression of TIS21 in EJ cells attenuated p53-enhanced focal adhesion complex formation, regulated by paxillin.
Fig. 5. Expression of p53 upregulated paxillin expression in EJ cells, but co-expression with TIS21 attenuated. Cells infected with p53 and/or TIS21 were subjected to Western blot analysis (A-C) at the indicated time points. Actin and a-tubulin were used as their loading control. (D) Six days after adenovirus infection, paxillin expression was evaluated by immunocytochemistry and confocal microscopy.
F. Knockdown of p53 restored senescent phenotype and reduced the expressions of paxillin and H-ras
To determine whether p53 regulates paxillin expression, siRNA based experiment was performed. Cells were infected with p53 and then transfected with either p53-siRNA or control siRNA, and the protein expressions were assessed. Knockdown of p53 expression abrogated the expression of paxillin in concentration dependent manner, along with cellular senescence morphology (Fig. 6A and B). Additionally, expression of H-ras, a downstream of p53, was also decreased by the elimination of p53 expression. The data clearly indicate the role of p53 in morphological changes in senescence of EJ cells. In addition, transfection with p53-siRNA over 25 nM was sufficient to eliminate ROS generation by p53 expression in EJ cells (Fig. 6C).
Fig. 6. Confirmation of p53 effect on paxillin expression by transfection of p53-siRNA. EJ cells were infected with p53 for 4 hrs and then transfected with p53-siRNA. The cells were harvested at 2 days and subjected to immunoblot analysis (A and D). Morphological change was also assessed by microscopic examination (B and E). Intracellular ROS levels were determined by staining cells with 20 mM H2-DCFDA fluorescence dye for 15 min at 37°C and the fluorescent intensities were also quantified by flow cytometry (C).
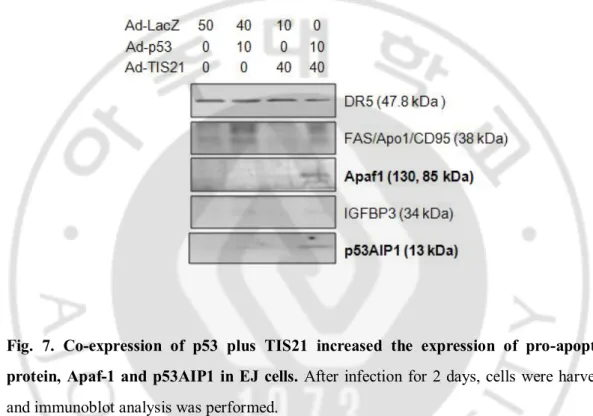
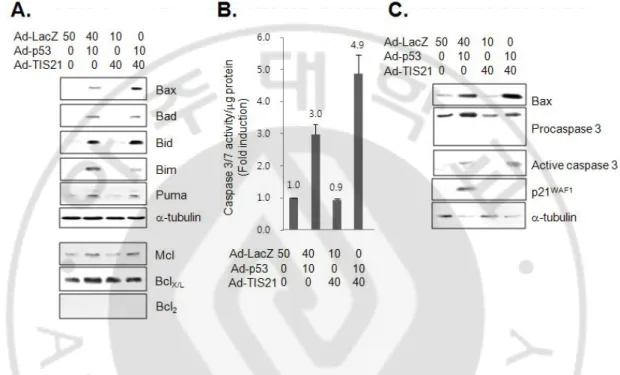
G. Co-infected cells with p53 and TSI21 increased the apoptosis markers related with intrinsic pathway
It has been reported that p53-dependent apoptosis is dependent on Apaf-1/Caspase-9 pathway (Soengas et al., 1999) and involves mitochondrial cytochrome c release (Schuler et al., 2000). A number of p53-regulated genes with p53 response elements have been identified, and some of these represent the potential downstream mediators of p53-dependent apoptosis. Therefore, I investigated the expressions of the p53 target genes in the apoptotic cells by p53 plus TIS21 co-expression, and found the cleaved Apaf1 and increased the expression of p53AIP1 in the cells (Fig. 7). Expressions of DR5, Fas and IGFBP3 were not changed, suggesting that p53 plus TIS21 may not induce apoptosis via intrinsic pathway. Among the known pro-apoptotic proteins, Bax expression was significantly increased in the co-infected cells (Fig. 8A). Activity of caspase 3/7 was induced by over-expression of p53 alone, however, it was higher in cells co-expressing p53 plus TIS21 (Fig. 8B). Indeed, expression of cleaved caspase 3 was more abundant in cells expressing p53 plus TIS21 along with loss of p21WAF1 expression (Fig. 8C). These findings collectively indicate that co-expression of p53 plus TIS21 induced apoptosis.
Fig. 7. Co-expression of p53 plus TIS21 increased the expression of pro-apoptotic protein, Apaf-1 and p53AIP1 in EJ cells. After infection for 2 days, cells were harvested and immunoblot analysis was performed.
Fig. 8. Co-expression of wild-type p53 with TIS21 enhanced apoptosis in EJ cells. (A) After infection for 2 days, cell lysates were prepared for detection of pro-apoptotic proteins. Specific antibodies were used to detect the proteins of interest. (B) To determine caspase 3/7 activity, caspase-Glo 3/7 assay was performed in p53, TIS21 and p53 plus TIS21 infected EJ cells. Five thousand cells which were infected with adenovirus for 2 days were seeded in 96 wells plates. 100 ml of caspase-Glo 3/7 reagent was added directly to the cells in 96-well plates and incubated for 1 hr before recording luminescence using TD-20/20 luminometer. For detection of cleaved caspase 3, immunoblot analysis was employed.
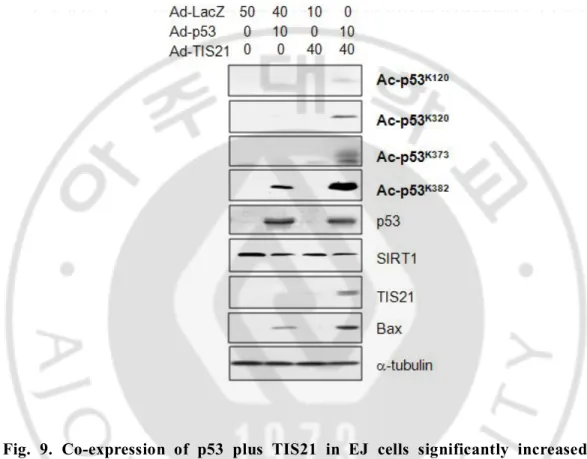
H. TIS21 posttranslationally modificated p53 and changes the cellular localization of p53
Protein levels and activity of p53 as a transcription factor are regulated by numerous stress-induced posttranslational modifications that converge on two distinct domains of the protein: p53 NH2-terminal region (phosphorylation site) and p53 COOH terminus (acetylation site, (Tang et al., 2006). Distinct p53 acetylation cassettes differentially influence gene-expression pattern and fate of the cells (Knights et al., 2006). So I assessed acetylation of p53 on lysine residues. Interestingly, acetylation status of p53 increased in the cells infected with p53 plus TIS21 (Fig. 9). I also observed that co-expression with p53 plus TIS21 significantly enhanced accumulation of p53 in the nuclei, as compared with that of the p53 alone (Fig. 10). Next I attempted to find out the mechanism of nuclear translocation of p53 in the co-infected cells. Two recent studies have identified that kinetic stabilization of microtubule enhances microtubule-mediated transport of p53 into the nuclei (Giannakakou et al., 2000; Rathinasamy and Panda, 2008). I also speculated a possible involvement of acetylated a-tubulin (Ac-tubulin) in the translocation of p53 in the TIS21-dependent manner. When I measured the expression of Ac-tubulin in EJ cells co-infected with p53 plus TIS21, I observed that expression of Ac-tubulin was not changed, thus ruled out the potential (Fig. 11). Next, I checked the localization of p53 and acetylated p53. Acetylated p53 in the cells expressing p53 did not localize in the nucleus. In cells infected with p53 plus TIS21, I observed that total p53 and acetylated p53 existed in the nucleus (Fig. 12.).
Fig. 9. Co-expression of p53 plus TIS21 in EJ cells significantly increased the acetylation of p53 on the several lysine residues. EJ cell were infected with adenovirus and harvested after 48 hrs. Acetylation level of p53 was determined by immunoblot analysis.
Fig. 10. Differential localization of p53 in the cells expressing p53 alone and cells expressing p53 plus TIS21. For visualization of localization of p53 plus TIS21, cells were fixed with 4% paraformaldehyde solution and stained with anti-p53 and anti-Flag antibodies. Images were captured with LSM510 confocal microscope (Magnification x1600).
Fig. 11. No difference in a-tubulin acetylation the cells expressing p53 alone and the cells co-expressing p53 plus TIS21. EJ cells were harvested after infection with p53 and/or TIS21 for 48 hrs, and the cell lysates were subjected to immunoblot analysis with anti Ac-tubulin and a-Ac-tubulin antibodies.
Fig. 12. Expression of TIS21 increased the translocation of p53 into the nuclei and its acetylation. (A) To show intracellular location of total p53 and acetylated p53, cells were fixed with 4% paraformaldehyde solution and stained with anti-p53 and anti-Ac-p53K382 antibodies. Images were captured with LSM510 confocal microscope (Magnification x400). (B) Quantification of nuclear p53 was determined by counting cells more than 1000.
I. TIS21 siRNA abrogated p53 plus TIS21-mediated apoptosis.
To confirm the contribution of TIS21 in the determination of cellular fate into apoptosis by TIS21 plus p53, exogenous TIS21 was down-regulated by transfection of TIS21-siRNAs. EJ cells were transfected with either TIS21-siRNA (#1 and/or #2) or control siRNA, and the efficiency of knockdown was measured by RT-PCR. As shown in Fig. 13B, expression of TIS21 mRNA was downregulated in the transfected cells, whereas the expression of glyceraldehydes-3-phosphate dehydrogenase was unaffected. Notably, transfection of TIS21-siRNA exhibited significant recovery of p53-induced senescent morphology in addition to reduction of cell death (Fig. 13A). Tranfection of EJ cells co-expressing p53 plus TIS21 with #1 and/or #2 significantly reduced the effects of p53 plus TIS21 on the cleavage of caspase 3, acetylations of p53, and loss of p21WAF1 expression (Fig. 13C). These results clearly confirmed the pro-apoptotic activity of TIS21 in the p53 plus TIS21 infected EJ cells. Knockdown of TIS21 switches the apoptosis induced by p53 plus TIS21 into senescence.
Fig. 13. TIS21-siRNAs inhibited apoptosis in EJ cells infected with p53 plus TIS21. EJ cells were co-infected with p53 plus TIS21 for 4 h, and then transfected with TIS21-siRNAs (#1 and/or #2) for 4 h. In 48 h of the infection, cells were harvested and subjected to RT-PCR and immunoblot analyses. (A) Microscopic images of cells were taken at 2 days after p53 plus TIS21 infection. (B) RT-PCR analysis. (C) Cleaved form of caspase 3, acetylation of p53, and expression of p21WAF1 were observed 48 h of infection by immunoblot analysis.
J. p53 localized at the cytoplasm in the doxorubicin-induced senescent cells
p53 is localized at the cytoplasm in senescent EJ cells (Figs. 10 and 12). To clarify whether the cytoplasmic localization of p53 in senescent cells is a general phenomenon of senescence, I employed replicative senescent (by cell division of 70-80 doublings in culture) or doxorubicin-induced senescent human diploid fibroblasts (HDF). First, I confirmed characteristics of cell senescence such as high expressions of b-gal (Fig.14A) and SA-pERK1/2 (Fig.14B). Expression of p53 protein was not observed in the replicatively senescent cells, however, it was easily observed in the cytoplasm of the senescent cells induced by treatment with 200 ng/ml of doxorubicin for 4 hrs and then cultured for 6 days in fresh medium (Fig.14C), supporting that cytoplasmic localization of p53 may be common phenomenon observed in p53- or doxorubicin-induced senescent cells. Accumulation of p53 protein in the nuclei was about 5.4% in the cells (Fig. 14D).
Fig. 14. p53 localization in the replicative or the doxorubicin-induced senescence of HDF cells. Cells were incubated at 90 % confluency in 60 mm culture dish and then treated with 200 ng/ml doxorubicin for 4 hr. Cells were reseeded and incubated for 6 another days. (A) SA-b-galactosidase activity (B) Senescent markers were examined. (C) Intracellular localizations of p53 protein were visualized by immunofluorescence staining using anti-p53 antibody and observed under a fluorescence microscope. (D) Quantification of nuclear p53 was determined by counting the cells. DIS: doxorubicin-induced senescence
K. TIS21 induced p53-mediated apoptosis
To confirm and elucidate induction of apoptosis by TIS21 plus p53, I infected EJ cells with p53 for 4 hr and subsequently imposed TIS21 infection at 0, 6, 12, 24 and 36 hr after p53 infection. The cells were harvested 48 hr after p53 infection. As shown in Fig. 15, expression of TIS21 induced cell death of the p53-expressing EJ cells, but not cellular senescence (Fig. 15). I also observed the increased level of cleaved caspase 3 and lysine acetylation in p53 molecule following the co-expression of TIS21 (Fig. 15C).
Fig. 15. Co-infection with p53 plus TIS21 induced apoptosis in EJ cells. EJ cells were infected with p53 for 4 hr, and the cells were subsequently infected with TIS21 for 4 hrs in 6, 12, 24, and 36 hrs of the p53 infection. All of the cells were harvested 48 hr after the p53 infection. (A) Diagram showing time for TIS21 infection. (B) Cell morphology was assessed under inverted microscope. (C) Cleaved caspase 3 and acetylated p53 were increased in the cells infected with TIS21 plus p53
L. Regulation of TIS21 stability by p53
Protein level of TIS21 in cells co-infected with p53 plus TIS21 was found to be more than that in cells transfected with TIS21 alone (Figs. 4A and 4C). I have previously reported that skp2, a downstream target of FoxM1, enhances polyubiquitnation and degradation of TIS21 (Park et al., 2009). Here, I observed that p53 decreased both protein and RNA expressions of skp2 (Figs. 16A-C). I also found that p53 downregulated the expression of FoxM1, a transcription factor for skp2 (Fig. 16D), thus p53 may contribute to the stabilization of TIS21 expression through repression of FoxM1.
Fig. 16. Inhibition of FoxM1 and Skp2 expression by p53 in EJ cells, which can stabilize TIS21 expression in the cells. Cells were harvested at the indicated times (A, B and D) and expressions of Skp2 and FoxM1 were analyzed by immunoblot analyses. (C) Total RNAs were isolated from the cells and mRNA expressions were determined by reverse-transcription PCR. GAPDH was used as an internal control.
IV. DISCUSSION
I evaluated the effect of TIS21 on senescence program induced by p53 expression in EJ cells. Considering the report that TIS21 enhances cell death in HeLa (Lim et al., 2008) and U937 (Hong et al., 2005) cells, I assumed that TIS21 expression might induce apoptosis in cancer cells which express p53-induced senescence phenotypes. The phenomenon was confirmed in the present study by subsequent infections of EJ cells, they were already expressing p53, with TIS21 virus and TIS21-siRNAs (Figs. 14 and 15). To the best of our knowledge, this is the first report indicating that the activity of TIS21 that can switch the cellular fates from p53-dependent senescence to p53-dependent apoptosis.
First, I observed that over-expression of p53 in EJ cells induced senescence; however, simultaneous infection with p53 plus TIS21 failed to manifest senescence phenotypes (Fig. 1). Senescent morphology by p53 was accompanied by the increased expression of paxillin in the cells. However, paxillin expression was significantly reduced in the co-infected cells. Our data showed that knockdown of p53 lost senescent morphology and reduced paxillin and H-ras expressions (Fig. 6A). These results suggest that paxillin might be an important in senescent morphology. However further work is needed to get a deep insight regarding the correlation between paxillin expression and senescence phenomenon. It has been reported that degradation of focal adhesion proteins, paxillin and p130CAS, were related with caspase 3 activation (Kook et al., 2000; Shim et al., 2001), suggesting that paxillin might act as a potential substrate of active caspase 3 in the process of apoptosis.
Here, I found that TIS21 enhances posttranslational modification and nuclear accumulation of p53 in p53-infected EJ cells (Figs. 10-12). Next, I tested the mechanism of
nuclear translocation of p53 in the co-infected cells. Nuclear localization of p53 also depends on the ability of p53 to interact with microtubules, and it appears that p53 uses the microtubule networks and the molecular motor dynein to move through the cytoplasm toward the nucleus and the nuclear import machinery (Giannakakou et al., 2000; Rathinasamy and Panda, 2008). However, in our present model, I could not observe any change in acetylation of a-tubulin (Fig.11). p53 selectively triggers either senescence or apoptosis depending on the posttranslational modification, such as acetylation or phosphorylation. Among them, lysine acetylation of p53 is very important in apoptosis, because acetylation of p53 C-terminal domain can activate the genes containing p53-specific DNA binding elements (Gu and Roeder, 1997), thus acetylation of the p53 DNA-binding domain regulates induction of apoptosis (Sykes et al., 2006). Therefore, I focused on posttranslational modification of p53, specifically, acetylation of p53. Our data showed that acetylation of p53 was increased in EJ cells infected with p53 plus TIS21 (Fig. 9). In our study, p53 is more acetylated in cells co-expressed with p53 plus TIS21 than in cells expressed with p53 alone (Fig. 12). Therefore, localization of p53 in the induced senescent cells existed mostly in the cytoplasm, whereas higher amount of p53 was observed in the nuclei of cells co-infected with p53 plus TIS21 (Fig. 10 and 11). I also examined whether our observations were general phenomenon in cellular senescence program by employing HDF cells; p53 protein was not detected by immunocytochemistry in the replicatively senescent HDF cells, whereas p53 protein was mostly present in the cytoplasm of the induced senescence of HDF cells with 5% in the nuclei (Fig.12C). These data showed possibility to have additional function of cytoplasmic p53. An emerging area of research unravels additional activities of p53 in the cytoplasm, where it triggers apoptosis (Mihara et al., 2003;
Leu et al., 2004) and inhibits autophagy (Tasdemir et al., 2008).
Histone deacetylases (HDACAs) down-regulate p53-dependent transactivation by deacetylation of p53 protein. It has been reported that HDAC regulates Apaf-1 and caspase 3 expression in the developing mouse retina (Wallace et al., 2006). Deacetylation of p53 by HDACs is likely to be a part of the mechanisms that control the physiological activity of p53 (Juan et al., 2000; Wallace and Cotter, 2009). Mammalian HDAC-1, HDAC-2, and HDAC-3 are all capable of down-regulating p53 function (Juan et al., 2000).
Co-expression of p53 plus TIS21 strongly inhibited the colony formation as compared with the expression of p53 alone (Fig. 3). Induction of either senescence or apoptosis is very important for growth inhibition of cancer cells and protects from tumor cell development. It has been reported that senescence environment contributes to alter epithelial cell, alveolar epithelial morphogenesis, functional differentiation and branching morphogenesis, via MMP3 (Parrinello et al., 2005). In other words, secretion of senescent cells is able to induce growth of cancer cells. These results suggest that induction of senescence in cancer cells may not contribute effectively to the suppression of cancer cell growth, in this respect, apoptosis may be more efficient inhibition mechanism with regard to cancer associated therapeutic actions.
In conclusion, our data showed that p53 alone induced senescence in EJ bladder carcinoma cells, however, TIS21 induced up-regulation of pro-apoptotic gene expression such as Apaf-1, p53AIP1, cleaved caspase 3, and Bax in p53-dependent manner. Taken together, acetylation of p53 was increased in EJ cells co-expressing p53 plus TIS21. Based on the evidences presented herein, I conclude that TIS21 shifts the p53-induced cellular
response from senescence to apoptosis in EJ cells, and thus acts and one of the downstream targets.
V. CONCLUSION
TIS21/BTG2/PC3 has many functions: control of cell differentiation, cell cycle regulator, transcriptional co-regulator, and apoptosis or cell death. In the present study, I showed that expression of TIS21 induced the apoptosis through p53-dependent manner. In EJ human bladder carcinoma cells, TIS21 upregulated the expression of the pro-apoptotic genes such as Aparf-1, p53AIP1, cleavage caspase 3, and Bax in a p53-dependent manner. Moreover, TIS21 induces posttranslational modifications of p53, which may be involved in the induction of apoptosis and nuclear translocation of p53 protein.
This is the first report to indicate that co-expression of TIS21 and p53 induces apoptosis of EJ human bladder carcinoma cells, whereas expression of p53 induces senescence.
REFERENCES
1. Boiko AD, Porteous S, Razorenova OV, Krivokrysenko VI, Williams BR, Gudkov AV: A systematic search for downstream mediators of tumor suppressor function of p53 reveals a major role of BTG2 in suppression of Ras-induced transformation. Genes Dev 20: 236-252, 2006
2. Canzoniere D, Farioli-Vecchioli S, Conti F, Ciotti MT, Tata AM, Augusti-Tocco G, Mattei E, Lakshmana MK, Krizhanovsky V, Reeves SA, Giovannoni R, Castano F, Servadio A, Ben-Arie N, Tirone F: Dual control of neurogenesis by PC3 through cell cycle inhibition and induction of Math1. J Neurosci 24: 3355-3369, 2004
3. Chandrasekaran K, Mora PT, Nagarajan L, Anderson WB: The amount of a specific cellular protein (p53) is a correlate of differentiation in embryonal carcinoma cells. J
Cell Physiol 113: 134-140, 1982
4. Chen QM, Tu VC, Catania J, Burton M, Toussaint O, Dilley T: Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. J Cell Sci 113 ( Pt 22): 4087-4097, 2000 5. Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, Kim KT, Jang IS, Park SC:
Morphological adjustment of senescent cells by modulating caveolin-1 status. J Biol
Chem 279: 42270-42278, 2004
6. Corrente G, Guardavaccaro D, Tirone F: PC3 potentiates NGF-induced differentiation and protects neurons from apoptosis. Neuroreport 13: 417-422, 2002
7. Dameron KM, Volpert OV, Tainsky MA, Bouck N: Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 265: 1582-1584, 1994
8. Der CJ, Krontiris TG, Cooper GM: Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A 79: 3637-3640, 1982
9. Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al.: A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92: 9363-9367, 1995
10. Duriez C, Moyret-Lalle C, Falette N, El-Ghissassi F, Puisieux A: BTG2, its family and its tutor. Bull Cancer 91: E242-253, 2004
11. el-Ghissassi F, Valsesia-Wittmann S, Falette N, Duriez C, Walden PD, Puisieux A: BTG2(TIS21/PC3) induces neuronal differentiation and prevents apoptosis of terminally differentiated PC12 cells. Oncogene 21: 6772-6778, 2002
12. Ficazzola MA, Fraiman M, Gitlin J, Woo K, Melamed J, Rubin MA, Walden PD: Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis 22: 1271-1279, 2001
13. Fletcher BS, Lim RW, Varnum BC, Kujubu DA, Koski RA, Herschman HR: Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. J Biol Chem 266: 14511-14518, 1991
14. Franza BR, Jr., Maruyama K, Garrels JI, Ruley HE: In vitro establishment is not a sufficient prerequisite for transformation by activated ras oncogenes. Cell 44: 409-418, 1986
15. Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T: p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat
Cell Biol 2: 709-717, 2000
16. Gu W, Roeder RG: Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90: 595-606, 1997
17. Guardavaccaro D, Corrente G, Covone F, Micheli L, D'Agnano I, Starace G, Caruso M, Tirone F: Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol 20: 1797-1815, 2000
18. Guehenneux F, Duret L, Callanan MB, Bouhas R, Hayette S, Berthet C, Samarut C, Rimokh R, Birot AM, Wang Q, Magaud JP, Rouault JP: Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia 11: 370-375, 1997
19. Hall A, Marshall CJ, Spurr NK, Weiss RA: Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature 303: 396-400, 1983
20. Hong JW, Ryu MS, Lim IK: Phosphorylation of serine 147 of tis21/BTG2/pc3 by p-Erk1/2 induces Pin-1 binding in cytoplasm and cell death. J Biol Chem 280: 21256-21263, 2005
21. Iacopetti P, Michelini M, Stuckmann I, Oback B, Aaku-Saraste E, Huttner WB: Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division.
22. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ: Cancer statistics, 2009. CA Cancer
J Clin 59: 225-249, 2009
23. Juan LJ, Shia WJ, Chen MH, Yang WM, Seto E, Lin YS, Wu CW: Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem 275: 20436-20443, 2000
24. Kawakubo H, Carey JL, Brachtel E, Gupta V, Green JE, Walden PD, Maheswaran S: Expression of the NF-kappaB-responsive gene BTG2 is aberrantly regulated in breast cancer. Oncogene 23: 8310-8319, 2004
25. Knights CD, Catania J, Di Giovanni S, Muratoglu S, Perez R, Swartzbeck A, Quong AA, Zhang X, Beerman T, Pestell RG, Avantaggiati ML: Distinct p53 acetylation cassettes differentially influence gene-expression patterns and cell fate. J Cell Biol 173: 533-544, 2006
26. Konrad MA, Zuniga-Pflucker JC: The BTG/TOB family protein TIS21 regulates stage-specific proliferation of developing thymocytes. Eur J Immunol 35: 3030-3042, 2005 27. Kook S, Shim SR, Choi SJ, Ahnn J, Kim JI, Eom SH, Jung YK, Paik SG, Song WK:
Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol
Biol Cell 11: 929-939, 2000
28. Lane DP: Cancer. p53, guardian of the genome. Nature 358: 15-16, 1992
29. Leu JI, Dumont P, Hafey M, Murphy ME, George DL: Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 6: 443-450, 2004
30. Levine AJ: p53, the cellular gatekeeper for growth and division. Cell 88: 323-331, 1997 31. Lim IK: TIS21 (/BTG2/PC3) as a link between ageing and cancer: cell cycle regulator
32. Lim IK, Lee MS, Lee SH, Kim NK, Jou I, Seo JS, Park SC: Differential expression of TIS21 and TIS1 genes in the various organs of Balb/c mice, thymic carcinoma tissues and human cancer cell lines. J Cancer Res Clin Oncol 121: 279-284, 1995
33. Lim IK, Lee MS, Ryu MS, Park TJ, Fujiki H, Eguchi H, Paik WK: Induction of growth inhibition of 293 cells by downregulation of the cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21. Mol Carcinog 23: 25-35, 1998
34. Lim RW, Varnum BC, Herschman HR: Cloning of tetradecanoyl phorbol ester-induced 'primary response' sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene 1: 263-270, 1987
35. Lim YB, Park TJ, Lim IK: B cell translocation gene 2 enhances susceptibility of HeLa cells to doxorubicin-induced oxidative damage. J Biol Chem 283: 33110-33118, 2008 36. Maki CG, Huibregtse JM, Howley PM: In vivo ubiquitination and proteasome-mediated
degradation of p53(1). Cancer Res 56: 2649-2654, 1996
37. Matsuda S, Kawamura-Tsuzuku J, Ohsugi M, Yoshida M, Emi M, Nakamura Y, Onda M, Yoshida Y, Nishiyama A, Yamamoto T: Tob, a novel protein that interacts with p185erbB2, is associated with anti-proliferative activity. Oncogene 12: 705-713, 1996 38. Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM: p53 has
a direct apoptogenic role at the mitochondria. Mol Cell 11: 577-590, 2003
39. Morel AP, Sentis S, Bianchin C, Le Romancer M, Jonard L, Rostan MC, Rimokh R, Corbo L: BTG2 antiproliferative protein interacts with the human CCR4 complex existing in vivo in three cell-cycle-regulated forms. J Cell Sci 116: 2929-2936, 2003
40. Nishio K, Inoue A: Senescence-associated alterations of cytoskeleton: extraordinary production of vimentin that anchors cytoplasmic p53 in senescent human fibroblasts.
Histochem Cell Biol 123: 263-273, 2005
41. Oren M, Maltzman W, Levine AJ: Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol 1: 101-110, 1981
42. Parada LF, Tabin CJ, Shih C, Weinberg RA: Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature 297: 474-478, 1982
43. Park S, Lee YJ, Lee HJ, Seki T, Hong KH, Park J, Beppu H, Lim IK, Yoon JW, Li E, Kim SJ, Oh SP: B-cell translocation gene 2 (Btg2) regulates vertebral patterning by modulating bone morphogenetic protein/smad signaling. Mol Cell Biol 24: 10256-10262, 2004
44. Park TJ, Kim JY, Park SH, Kim HS, Lim IK: Skp2 enhances polyubiquitination and degradation of TIS21/BTG2/PC3, tumor suppressor protein, at the downstream of FoxM1. Exp Cell Res 315: 3152-3162, 2009
45. Parkin DM, Bray F, Ferlay J, Pisani P: Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108, 2005
46. Parrinello S, Coppe JP, Krtolica A, Campisi J: Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 118: 485-496, 2005
47. Rathinasamy K, Panda D: Kinetic stabilization of microtubule dynamic instability by benomyl increases the nuclear transport of p53. Biochem Pharmacol 76: 1669-1680, 2008
48. Reddy EP, Reynolds RK, Santos E, Barbacid M: A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene.
Nature 300: 149-152, 1982
49. Rieger KM, Little AF, Swart JM, Kastrinakis WV, Fitzgerald JM, Hess DT, Libertino JA, Summerhayes IC: Human bladder carcinoma cell lines as indicators of oncogenic change relevant to urothelial neoplastic progression. Br J Cancer 72: 683-690, 1995 50. Rouault JP, Falette N, Guehenneux F, Guillot C, Rimokh R, Wang Q, Berthet C,
Moyret-Lalle C, Savatier P, Pain B, Shaw P, Berger R, Samarut J, Magaud JP, Ozturk M, Samarut C, Puisieux A: Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet 14: 482-486, 1996
51. Rouault JP, Prevot D, Berthet C, Birot AM, Billaud M, Magaud JP, Corbo L: Interaction of BTG1 and p53-regulated BTG2 gene products with mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional regulatory complex. J Biol
Chem 273: 22563-22569, 1998
52. Ryu MS, Lee MS, Hong JW, Hahn TR, Moon E, Lim IK: TIS21/BTG2/PC3 is expressed through PKC-delta pathway and inhibits binding of cyclin B1-Cdc2 and its activity, independent of p53 expression. Exp Cell Res 299: 159-170, 2004
53. Sakaguchi T, Kuroiwa A, Takeda H: Expression of zebrafish btg-b, an anti-proliferative cofactor, during early embryogenesis. Mech Dev 104: 113-115, 2001
54. Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR: p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol
55. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW: Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593-602, 1997
56. Sharma S, Schwarte-Waldhoff I, Oberhuber H, Schafer R: Functional interaction of wild-type and mutant p53 transfected into human tumor cell lines carrying activated ras genes. Cell Growth Differ 4: 861-869, 1993
57. Shen DW, Real FX, DeLeo AB, Old LJ, Marks PA, Rifkind RA: Protein p53 and inducer-mediated erythroleukemia cell commitment to terminal cell division. Proc Natl
Acad Sci U S A 80: 5919-5922, 1983
58. Shim SR, Kook S, Kim JI, Song WK: Degradation of focal adhesion proteins paxillin and p130cas by caspases or calpains in apoptotic rat-1 and L929 cells. Biochem Biophys
Res Commun 286: 601-608, 2001
59. Shimizu K, Goldfarb M, Suard Y, Perucho M, Li Y, Kamata T, Feramisco J, Stavnezer E, Fogh J, Wigler MH: Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci U S A 80: 2112-2116, 1983
60. Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW: Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284: 156-159, 1999
61. Song YS, Cho KH, Kim KW, Yoon JH, Doo SH, Yang WJ, Cho JY, Lee DW: A Case of Bladder Cancer Found during a Workup for Urge Incontinence. Int Neurourol J 14: 130-132, 2010
62. Struckmann K, Schraml P, Simon R, Elmenhorst K, Mirlacher M, Kononen J, Moch H: Impaired expression of the cell cycle regulator BTG2 is common in clear cell renal cell carcinoma. Cancer Res 64: 1632-1638, 2004
63. Sugimoto K, Hayata T, Asashima M: XBtg2 is required for notochord differentiation during early Xenopus development. Dev Growth Differ 47: 435-443, 2005
64. Sugrue MM, Shin DY, Lee SW, Aaronson SA: Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci U
S A 94: 9648-9653, 1997
65. Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, McMahon SB: Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell 24: 841-851, 2006
66. Tang Y, Luo J, Zhang W, Gu W: Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell 24: 827-839, 2006
67. Taparowsky E, Shimizu K, Goldfarb M, Wigler M: Structure and activation of the human N-ras gene. Cell 34: 581-586, 1983
68. Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G: Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10: 676-687, 2008
69. Wallace DM, Cotter TG: Histone deacetylase activity in conjunction with E2F-1 and p53 regulates Apaf-1 expression in 661W cells and the retina. J Neurosci Res 87: 887-905, 2009
70. Wallace DM, Donovan M, Cotter TG: Histone deacetylase activity regulates apaf-1 and caspase 3 expression in the developing mouse retina. Invest Ophthalmol Vis Sci 47: 2765-2772, 2006
71. Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M: Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352: 345-347, 1991
-국문요약 -
정상 p53 유전자를 발현하는 EJ 방광암 세포주에서
TIS21 의 세포사멸 유도기전 연구
아주대학교 대학원의학과 최 옥 란 (지도교수 : 임 인 경) 암 억제유전자 p53 는 인간과 동물에서 DNA 손상신호를 받으면 세포노화 또는 세포사멸을 유도하는 중요한 단백질로 알려져 있다. 사람에서 발생하는 모든 암의 약 50% 에서는 p53 유전자의 돌연변이가 발생하여서 p53 의 기능이 억제되어 있음이 보고되었다. TIS21BTG2/PC3 는 세포성장억제유전자 중 하나로 알려져 있으며 p53 에 의존적으로 또는 비의존적으로 그 발현이 조절되며 흉선, 전립선, 신장, 그리고 간장의 발암과정에서 암 발생을 억제함이 보고되었다. 방광암세포주인 EJ 세포는 항상 활성화 되어있는 돌연변이형 H-ras 와 DNA 에 결합하는 부위에 돌연변이가 있어서 암억제유전자의 기능을 수행하지 못하는 p53 을 가지고 있다. H-ras 는 정상세포에서 과발현 되면 p53 을 활성화시켜서 세포노화를 유도하는 것으로 알려져 있으나 EJ 세포의 경우 H-ras 와 함께 p53 돌연변이형이 발현되므로 세포노화 대신에 암세포로서 무한증식하는 특징을 가지고 있다.본 연구는 adenoviral vector 를 사용하여서 p53 과 TIS21 유전자를 발현 (Ad-p53 and Ad-TIS21) 시킬 경우 (Ad-p53 에 의해 유도되는 세포노화 현상이 억제되고 오히려 세포 사멸을 유도하는 TIS21 의 역할을 규명하였다. EJ 세포에서 p53 과 TIS21 을 동시발현 시키면 p53 만을 발현시키는 것과는 다른 세포 모양을 나타내는데 p53 만을 발현하는 세포는 세포노화의 특징을 보이는 반면 TIS21 을 동시발현 하는 세포는 세포 크기가 작아지고 SA-b-galactosidase 의 활성도가 떨어지며 세포 수가 감소되었다. 이때, 세포의 모양 변화에 영향을 미치는 focal adhesion protein 인 paxillin 의 발현양이 p53 과 TIS21 을 발현하는 세포에서 의미 있게 줄어드는 것을 관찰하였다. Paxillin 의 발현양 감소와 함께 paxillin 의 세포 내 위치변화 역시 p53 단독발현 시에는 세포막이 기저부에 접촉하는 끝부분에서 발현되는데 반하여 p53 과 TIS21 동시발현 시에는 paxillin 이 세포질 전체에 퍼져서 존재하면서 paxillin 전체 양이 감소함을 관찰하였다. 위와 같은 현상이 apoptosis 유도에 의한 것인지 알아보기 위하여 calcein-AM 과 EthD-1 staining, Annexin V staining 그리고 caspase 3 활성도 측정 실험 등을 실시한 결과 p53 과 TIS21 동시발현 되는 세포에서는 세포사멸 현상이 의미 있게 증가함을 확인하였다. 이에 대한 분자기작으로서 p53 라이신 잔기의 acetylation 이 크게 증가함도 확인하였다. p53 acetylation 이 증가되면 p53 이 핵 안에 축적되면서 세포사멸관련 단백질의 발현을 증가시키고 세포를 죽음에 이르게 하는 사실을 규명함으로써 TIS21 에 의한 세포사멸 유도기전을 알게 되었다. 또한 세포노화 과정 중에 급증하는 p53 단백질은 세포사멸 시와는 다르게 거의 대부분 세포질에 존재하는 것을 정상 섬유아세포에 doxorubicin 을 처리하여 유도노화를 만든 후