저작자표시-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. l 이 저작물을 영리 목적으로 이용할 수 있습니다. 다음과 같은 조건을 따라야 합니다: l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
Homing of
111In-Labeled
Bone Marrow Mesenchymal Stem Cells
in Acute Brain Trauma Model
by
Bok-Nam Park
Major in Molecular Medicine
Department of Biomedical Sciences
Homing of
111In-Labeled
Bone Marrow Mesenchymal Stem Cells
in Acute Brain Trauma Model
by
Bok-Nam Park
A Dissertation Submitted to The Graduate School of Ajou University
in Partial Fulfillment of the Requirements for the Degree of
Ph. D. in Biomedical Sciences
Supervised by
Joon-Kee Yoon, M.D., Ph.D.
Young Hwan Ahn, M.D., Ph.D.
Major in Molecular Medicine
Department of Biomedical Sciences
The Graduate School, Ajou University
This certifies that the dissertation
of Bok-Nam Park is approved.
SUPERVISORY COMMITTEE
Jae-Ho Lee
Joon-Kee Yoon
Young Hwan Ahn
Young-Sil An
Sang-Yoon Lee
The Graduate School, Ajou University
- ABSTRACT -
Homing of
111In-Labeled Bone Marrow Mesenchymal
Stem Cells in Acute Brain Trauma Model
Bok-Nam Park
Department of Biomedical Sciences The Graduate School, Ajou University
(Supervised by Assistant Professor Joon-Kee Yoon and Associate Professor Young Hwan Ahn)
This study was to evaluate the in vivo distribution of intravenously transplanted bone marrow-derived mesenchymal stem cells (BMSCs) in an acute brain trauma model by 111 In-tropolone labeling and to perform the effect of 111In-labeling on the viability and functions of BMSCs. Rat BMSCs were labeled with 37 MBq 111In-tropolone. Their labeling efficiency and in vitro retention rate were measured. To evaluate dose-dependent effect of 111In-labeling, BMSCs were labeled with various doses (0.4-11.1 Bq/cell) of 111In-tropolone, and growth curve analysis, fluorescent activated cell sorter (FACS) analysis after staining with
5-bromo-2-deoxy-uridine (BrdU), and microscopic evaluation after 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-gal) staining were performed until the 14th day. FACS analysis after staining with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) was performed at early (3 and 12 hr) and late (7 days) stages with higher doses of 111In (11.1 and 33.3 Bq/cell) to evaluate apoptotic or necrotic change of labeled BMSCs. The biodistribution of 111In-BMSCsin trauma models was compared with those in sham-operated rats and normal rats by gamma camera images. The migration of 111In-BMSCs to the traumatic brain was evaluated using confocal microscope. The labeling efficiency of 111In-BMSCs was 66 ± 5%, and their retention rate was 85.3% at 1 h after labeling. There was no difference in the number of viable cells between 111In-BMSCs and controls at 48 h after labeling. However, the proliferation of 111In-BMSCs was inhibited after the third day of labeling, and it did not reach confluency. For lower doses of 111In (0.4 and 1.1 Bq/cell), the growth of labeled stem cells was not significantly different from that of control, whereas, labeling with higher doses of 111In (4.4 and 11.1 Bq/cell) led to a significant proliferative inhibition from the 3rd day to the 14th day. FACS analysis also revealed less BrdU positive cells in BMSCs labeled with 1.1, 4.4 and 11.1 Bq/cell compared with controls on the 3rd day after labeling. Of these, the patterns of cell cycle in BMSCs labeled with 0.4 and 1.1 Bq/cell of 111In were restored similar to controls on the 14th day. On the contrary, BMSCs labeled with 4.4 and 11.1 Bq/cell of 111In could not recover from cell cycle arrest. Senescence-associated β-gal (SA- β-gal) staining was not prominent in all concentrations until the 14th day after labeling. FACS analysis with Annexin V-FITC and PI also revealed no significant apoptosis or necrosis in both early and late stages. On gamma camera images, most of the 111In-BMSCs uptake was
observed in the liver and spleen at the second day of injection. The brain uptake of 111 In-BMSCs was more prominent in trauma models (1.4%) than in sham-operated (0.5%) or normal rats (0.3%). Radiolabeled BMSCs were observed at the marginal region of traumatic brain on the confocal microscope. We observed the dose-dependent growth inhibition of BMSCs by 111In-labeling, which was developed by dose-dependent, transient cell cycle arrest, not by cellular senescence or apoptosis/necrosis. Although growth inhibition by 111 In-labeling need to be evaluated further prior to use in humans, 111In-BMSCsare useful for the tracking of intravenously transplanted mesenchymal stem cells in brain disease models.
Key words: 111In-tropolone, Bone marrow mesenchymal stem cells, Cell tracking,
TABLE OF CONTENTS
ABSTRACT ··· i
TABLE OF CONTENTS ··· iv
LIST OF FIGURES ··· vi
I. INTRODUCTION ··· 1
II. MATERALS AND METHODS ··· 3
A. Isolation and culture of rat BMSCs ··· 3
B. Generation of an animal model of acute brain trauma ··· 4
C. Synthesis and radioabeling of mesenchymal stem cells with 111In-tropolone ··· 4
D. In vitro stability and cell viability of mesenchymal stem cells with 111In-tropolone ··· 5
E. Dose-dependent effect of 111In on the growth of BMSCs ··· 6
F. In vivo tacking of 111In-BMSCs by gamma camerain animal models ··· 6
G. PKH 26 labeling of mesenchymal stem cells ··· 7
H. Tissue preparation with DAPI staining for confocal microscopy ··· 7
I. In vitro BrdU labeling for 111In-BMSCs ··· 8
J. Annexin V-FITC/PI double staining for 111In-BMSCs ··· 9
K. Cytochemical staining with SA-β-galactosidase for 111In-BMSCs ··· 9
L. Statistical analysis ··· 10
III. RESULTS ··· 11
B. Dose-dependent growth of 111In-BMSCs ··· 12
C. In vivo tracking of 111In-BMSCs by gamma camera in trauma models and controls ··· 13
D. Histological analysis of transplanted BMSCs in animal model of trauma ··· 14
E. Cell cycle analysis by flow cytometry ··· 16
F. Annexin V-FITC/PI double staining flow cytometry ··· 18
G. Senescence-associated-β-galactosidase histochemistry ··· 20
IV. DISCUSSION ··· 22
V. CONCLUSION ··· 29
REFERENCES ··· 30
LIST OF FIGURES
Fig. 1. Retention rate of 111In-BMSCs ··· 11
Fig. 2. Growth curve of 111In-BMSCs ··· 12
Fig. 3. In vivo distribution of 111In-BMSCs in acute brain trauma rats, sham-operated control rats and normal rats at 24 h after intravenous transplantation ··· 14
Fig. 4. Confocal laser scanning microscope image of transplanted BMSCs in the margin of traumatic regions··· 15
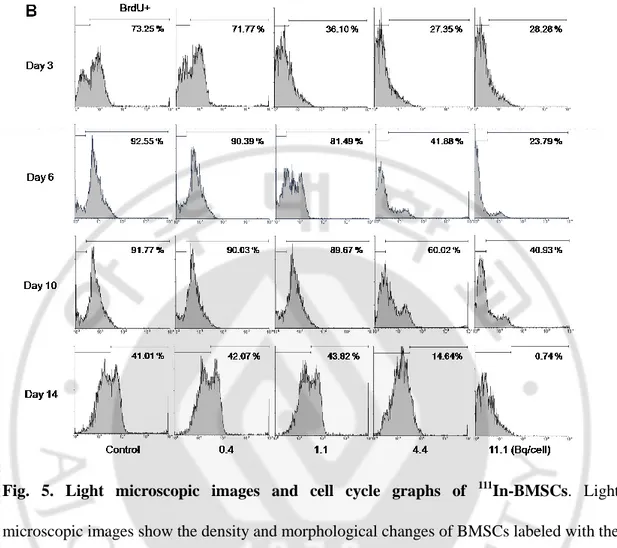
Fig. 5. Light microscopic images and cell cycle graphs of 111In-BMSCs ··· 17
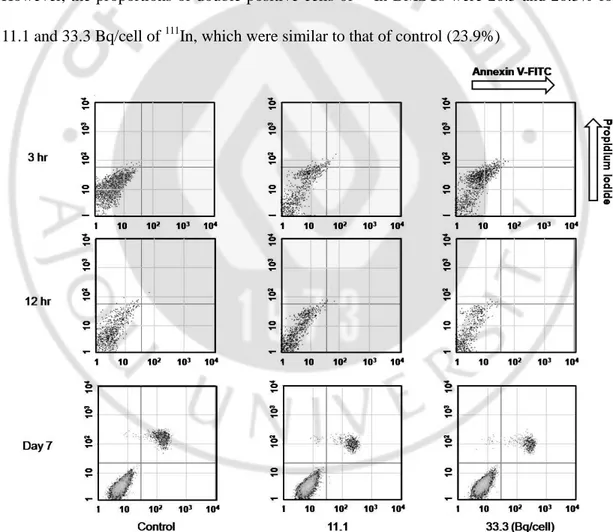
Fig. 6. Apoptotic and necrotic change of 111In-BMSCs ··· 19
I. INTRODUCTION
Recent studies have demonstrated that transplantation of bone marrow-derived mesenchymal stem cells (BMSCs) reduced the size of infarction and increased functional recovery in animal models of brain disease (Li et al., 2002; Honma et al., 2006). Moreover, it was reported that intravascularly injected, ex vivo cultured, autologous BMSCs induce functional recovery in patients with stroke (Bang et al., 2005) and multiple system atrophy (MSA) (Lee et al., 2008). Bone marrow-derived mesenchymal stem cells are multipotent and capable of differentiating into mesodermal lineages, such as bone, fat, cartilage and muscle and even into ectodermal lineages, including neurons and astrocytes, both in vitro and in vivo (Pittenger et al., 1999; Mackay et al., 1998; Woodbury et al., 2000; Sekiya et al., 2004; George et al., 2006). BMSCs s can also differentiate into tumor cells (Studeny et al., 2004; Riggi N et al., 2005; Zhu et al., 2006), and thus, their migration and proliferation should be monitored carefully after transplantation.
Of the various techniques associated with in vivo cell tracking, nuclear medicine imaging is the most clinically friendly method. Nuclear medicine imaging has been widely used to label various types of blood cells, such as leukocytes, platelets and neutrophils (Thakur et al., 1997; Scheffel et al., 1982; Gunter et al., 1983). Moreover, it can provide the quantitative measurement of transplanted cells in each organ as a percentage of the injected dose of a radioisotope (Kang et al., 2006; Hofmann et al., 2005; Brenner et al., 2004). 111 In-oxine/tropolone is a well-known cell-labeling agent that has been used to localize infections in the form of labeled leukocytes since the 1970s (Thakur et al., 1997). Moreover, 111In was
recently used to evaluate the migration and transplantation of therapeutic endothelial progenitor cells and mesenchymal stem cells in myocardial infarction (Brenner et al., 2004; Chin et al., 2003; Barbash et al., 2003; Jin et al., 2005). However, 111In has not been used to track grafted mesenchymal stem cells in brain disease. Therefore, in this study, the migration of intravenously injected rat BMSCs to the injured region in an animal model of acute cerebral trauma was investigated by labeling with 111In-tropolone.
On the other hand, in a few recent studies, it has been reported that 111In has an adverse effect on the viability, the growth or the differentiation of labeled cells (Brenner et al., 2004, Bindslev et al., 2006). The viability of CD34+ hematopoietic cells was impaired about 30% at 48 and 96 hr after 111In-labeling and the proliferation of BMSCs was significantly inhibited by a high dose of 111In (800 Bq/cell). Therefore, the effects of 111In-labeling on the proliferation, cell cycle, and cellular death were also evaluated. For this purpose, the growth curve was obtained and the cell cycle analysis by fluorescent activated cell sorter (FACS) after staining with 5-bromo-2-deoxy-uridine (BrdU) was performed. In addition, 3 kinds of ionizing radiation-induced cell deaths, that is, apoptosis, necrosis and premature senescence were evaluated by FACS after double staining with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) and cytochemical staining with 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-gal).
II. MATREALS AND METHODS
A. Isolation and culture of rat BMSCs
Adult Sprague-Dawley rats (300 - 320 g, Orient, Sungnam, Korea) were housed in groups of two or three under environmentally controlled conditions at 23 ± 2°C and 50 ± 10% humidity and given free access to food and water. All experimental procedures were approved by the Care of Experimental Animals Committee of Ajou University School of Medicine, Suwon, Republic of Korea. Rat BMSCs were isolated from the femurs of 6- to 7-week-old male Sprague-Dawley rats. Both ends of the femurs were removed, and the remaining bones were centrifuged (Hanil, co., Seoul, Korea) at 1500 rpm for 20 min. The supernatant was discarded, and the cell pellets were resuspended in phosphate-buffered saline (PBS, WelGene, Korea). After centrifugation, the cells were resuspended and incubated in low-glucose Dulbecco modified Eagle's media (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a 5% fully humidified CO2 incubator. Three days later, non-adherent cells were removed by replacing the medium (passage 0). On the 10th day of incubation, the cells were detached with 0.25% trypsin/0.1% EDTA (Sigma, St. Louis, MO, USA) and replated on 100-mm culture dishes (passage 1). When these primary cultures reached 80% confluence, the cells were harvested using 0.25% trypsin and subcultured. At passage 4, rat BMSCs were characterized by immunofluorescence and fluorescence-activated cell sorter analysis (Park et al., 2008).
B. Generation of an animal model of acute brain trauma
A controlled cortical impact injury device with an impactor tip (5-mm diameter), which is controlled by gas pressure (Amscience, USA), was used to generate an animal model of brain trauma, as previously described (Chen et al., 2003, Kim et al., 2007). Following the induction of anesthesia through intraperitoneal injection of 10% chloral hydrate (Fluka, USA), the left scalp was incised, and a small hole (6-mm diameter) was made between the bregma and lambda. The exposed dura was stuck by the pneumatic impactor at approximately 2 mm lateral to the central suture at a depth of 8 mm, a speed of 7 m/s and a contact time of 100 ms. The scalp was sutured, and the rats were allowed to rest for 24 h. Meanwhile, the sham-operated rats underwent craniostomy and suture.
C. Synthesis and radiolabeling of mesenchymal stem cells with
111In-tropolone
One to two milligrams of tropolone (Sigma, St. Louis, MO, USA) was dissolved in 1 mL of normal saline, and then 80 μL of tropolone solution was mixed with 37 to 111 MBq (1-3 mCi) of 111InCl3 (physical half-life=2.83 days, γ-energy=245 and 173 keV; PerkinElmer, Waltham, MA, USA) in 0.05 N HCl. The reaction mixture was incubated for 15 min at room temperature (pH 7.2) (Gunter et al., 1983). Before labeling, the BMSCs were washed with PBS, centrifuged at 1000 rpm for 3 min and resuspended in 1 mL PBS. 111In-tropolone was then added to the BMSC suspension and incubated at room temperature for 20 min. After incubation, BMSCs were centrifuged at 1000 rpm for 3 min, and supernatant and cell pellets were collected separately to calculate the labeling efficiency. For in vivo monitoring in acutebrain trauma model and controls, 111In-BMSCswere resuspended in 1.0 mL of normal saline and injected via the lateral tail vein using 25-gauge needles.
D. In vitro stability and cell viability of mesenchymal stem cells with
111In-tropolone
To examine the retention rates, 111In-BMSCs were divided into three 60-mm culture dishes, which included 4 mL of culture medium, and incubated at 37°C in the presence of 5% CO2 for 1, 3, 6, 24, and 48 h. The supernatant was collected and the culture medium was replaced at each time point. After 48 h of incubation, cells were detached and centrifuged at 1000 rpm for 3 min. The radioactivity of the cell pellets and supernatant at each time point was counted with a dose calibrator. The retention percentage of 111In-BMSCs was calculated by dividing the activity in BMSCs by the total activity. To investigate the viability of 111 In-BMSCs, the numbers of residual viable cells were measured by 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide inner salt (XTT) assay (Roche Applied Science, Mannheim, Germany). Approximately 1.0 × 104 rat BMSCs were plated in a 96-well plate and incubated at 37°C in 5% CO2 for 48 h. XTT and phenazine methosulfate were then added at a 50:1 ratio. The plates were incubated at 37°C for 4 h, and the absorbance was measured at 450 nm, subtracted from 590 nm, using a microplate reader (Bio-Rad Lab, Hercules, CA, USA).
E. Dose-dependent effect of
111In on the growth of BMSCs
To evaluate dose-dependent influence of 111In-tropolone labeling on the growth of BMSCs, cells labeled with 4 different doses of 111In-tropolone (0.4, 1.1, 4.4 and 11.1 Bq/cell) were divided into 5 dishes (3.0 × 104 cells/60 mm culture dish) respectively, and their growth was monitored for 14 days. As a control, unlabled BMSCs (3.0 × 104 cells/60-mm culture dish) were used. We counted the numbers of both 111In-BMSCs and unlabled BMSCs on the day of labeling and on the 3th, 6th, 10th, and 14th days. This experiment was performed in triplicate for accuracy.
F. In vivo tracking of
111In-BMSCs in by gamma camera animal models
Static planar images were acquired after intravenous injection of 111In-BMSCs (1.0 × 106 cells for each rat) with a dual head gamma camera (MultiSPECT2; Siemens, Erlangen, Germany) equipped with medium energy collimators. Rats were placed in a prone position and scanned for 10 min from the anterior and posterior projection. The matrix size was 256×256. A 7.4-MBq 111In-BMSCs were intravenously injected in normal rats (n=3), sham-operated rats (n=2) and brain trauma models (n=3), and then the brain uptake was compared on the 2nd day images. For the comparison of biodistribution, on both anterior and posterior images, polygonal and circular region of interests (ROI) were drawn for each organ and right thigh (background), respectively, and then geometric means were used for calculation of percentage uptake/whole body.
G. PKH 26 labeling of mesenchymal stem cells
For detection of mesenchymal stem cells in brain, the cells were labeled using a PKH 26 red fluorescence cell linker kit (Sigma, St. Louis, MO, USA) according to the manufacture's instruction (Lee-MacAry et al., 2001). Briefly, cells were detached and centrifuged at 1300 rpm for 3 min in a 15 mL conical tube, and then supernatant was discarded. One milliliter of diluent C and 4.0 × 106 M of PKH 26 dye were added to the cell pellet and incubated at room temperature for 5 min with gentle inversion. Subsequently, the mixture was incubated with 2 mL of serum for 1 min to stop the staining reaction. Stained cells were centrifuged at 1300 rpm for 10 min and washed with 10 mL of complete medium for injection or further labeling of 111In. Radiolabeling of BMSCs and intravenous injection in rat models were done at 30 min after PKH 26 dye labeling.
H. Tissue preparation with DAPI staining for confocal microscopy
Animals were sacrificed on the second day of injection, and the brain tissues were fixed overnight with 4% paraformaldehyde dissolved in 0.1 M PBS. They were embedded in paraffin block and then sectioned on a sliding microtome to obtain a 5-μm-thick coronal section. For deparaffinization, brain tissue slides were incubated for 20 min at 55°C and rinsed thrice with 1 × PBS (Berger et al., 1997). Deparaffinized brain tissue slides were incubated with 4, 6-diamidino-2-phenylindole (DAPI, Fluka, Switzerland) for 15 min at room temperature to counterstain nuclei. Brain tissue slides were then washed twice with 1 × PBS and mounted using Prolong Antifade Kit (Molecular Probes, Eugene, OR, USA). Fluorescent signals were captured from the sham-operated control brains and traumatic
brains injected with PKH 26-labeled BMSCs or PKH 26-111In colabeled BMSCs, and analyzed using an LSM 510 confocal laser scanning microscope (Carl Zeiss, Germany).
I. In vitro BrdU labeling for
111In-BMSCs
To detect synthetic proportions, BrdU (Sigma, St. Louis, MO, USA) was labeled with 111
In-BMSCs in vitro. The halogenated pyrimidine analogue BrdU can be incorporated into newly synthesized DNA in place of thymindine and can be quickly detected using a monoclonal antibody against BrdU (Philip et al., 1997). BMSCs labeled with each dose of 111
In-tropolone (0.4, 1.1, 4.4 and 11.1 Bq/cell) were divided into 5 dishes (3.0 × 104 cells/60-mm dish) respectively, and grown until the 14th day. 111In-lableled BMSCs were incubated with 10 µM BrdU at 37°C in 5% CO2 for 1 h, washed with PBS, and harvested. Thereafter, the cells were resuspended ice cold PBS, 10% fetal bovine serum, and 1% sodium azide and incubated for overnight with anti-BrdU antibody (Abcam, USA). And then, cells were washed with cold PBS and incubated for 30 min at 4oC in the dark with AlexaFluor488-conjugated anti–mouse IgG antibody (Invitrogen, Carlsbad, CA, USA) in 3% bovine serum albumin (BSA)/PBS. The stained cells were analyzed by FACS with CellQuest software (Beckton Dickinson). In addition, to evaluate the cell density and morphological changes of 111
In-labeled BMSCs, cells were observed using a light microscope (Olympus Optical Co, Ltd, Tokyo, Japan).
J. Annexin V-FITC/PI double staining for
111In-BMSCs
To investigate the apoptosis/necrosis of 111In-BMSCswhich tested for higher doses of In-111 (11.1 and 33.3 Bq/cell) at early (3 and 12 h) and late (7 days) stages, double staining with Annexin V-FITC and PI for flow cytometric analysis was performed using commercial kit (BD Pharmingen™, USA) according to the manufacture's instruction (Vermes et al., 1995). In brief, 111In-BMSCsand untreated BMSCs were detached with 0.25% trypsin/0.1% EDTA, and then the cells were washed twice with cold PBS, and resuspended in binding buffer (10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2). Annexin V-FITC was added resulting in a final concentration of 1 mg/ml Annexin V. Then 10 mg/ml PI was added. The mixture was incubated for 15 min at room temperature in the dark and then analyzed by flow cytometry within 1h.
K. Cytochemical staining with SA-β-galactosidase for
111In-BMSCs
To investigate the premature senescence of BSMCs, we stained cells with X-gal (InnoprotTM, Spain) (Kazuaki et al., 1999). 111In-BMSCs(1.1, 4.4 and 11.1 Bq/cell) were seeded on 12 well plates at the density of 5,000 cells/cm2 and incubated under 37°C in 5% CO2 until 14
th
day. For comparison, nonlabeled BMSCs were also grown. After removing culture medium and rinsing cells, cells were fixed by incubation with 1 mL of working fixing solution for 5 min at room temperature. And then, BMSCs were stained with X-gal solution (pH 6) at 37oC in 5% CO2 for 24 h, protected from light, and examined using a light microscope. The development of premature senescence was determined by the presence of
blue-stained cells. For positive staining, we prepared the cells which starved for 1 week without media change at 37oC in 5% CO2, and stained with the same protocol.
L. Statistical analysis
All data are presented as mean ± standard error. A paired t-test and Student-Newman-Keuls post hoc test was used to compare cell numbers between the control and 111In-labeled BMSCs. A p value of less than .05 was considered to be significant. Error bar means standard error.
II. RESULTS
A. Radiolabeling efficiency and viability of
111In-BMSCs
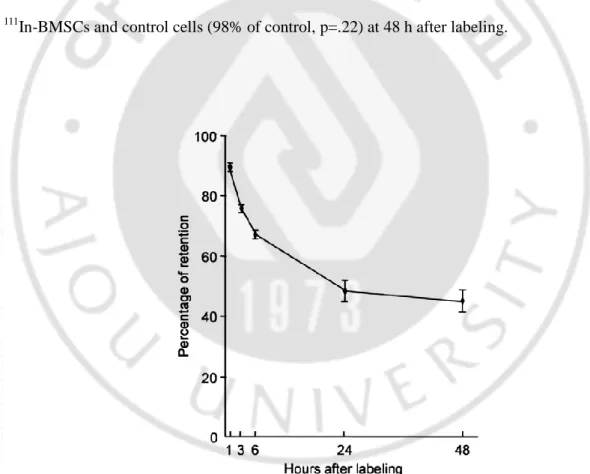
The labeling efficiency of 111In-BMSCs was 65.6 ± 5.3% (n=9), containing approximately 38 Bq/cell. The in vitro retention rates of 111In-BMSCs at 1, 3, 6, 24 and 48 h were 85.3%, 75.7%, 67.1%, 48.2% and 45.1%, respectively (Fig. 1). The XTT assay revealed that there was no significant difference in the number of viable cells between the 111
In-BMSCs and control cells (98% of control, p=.22) at 48 h after labeling.
Fig. 1. Retention rate of 111In-BMSCs. Retention rate of 111In-BMSCs slowly decreased over time. Error bar means standard error.
B. Dose-dependent growth of
111In-BMSCs
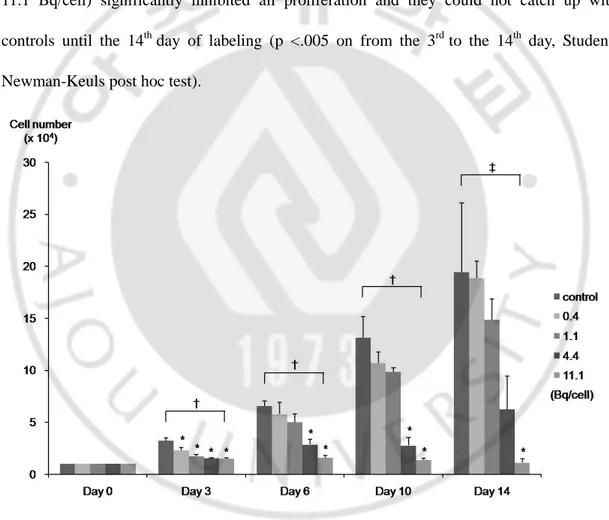
A growth curve of 111In-BMSCs showed the dose-dependent effect of 111In-tropolone. For cells labeled with the lowest and second lowest doses (0.4 and 1.1 Bq/cell, Fig. 2), their growth was not significantly different from that of controls until the 14th day (p <.001 on from the 3rd to 10th day, p=.004 on the 14th day, ANOVA). However, higher doses (4.4 and 11.1 Bq/cell) significantly inhibited all proliferation and they could not catch up with controls until the 14th day of labeling (p <.005 on from the 3rd to the 14th day, Student-Newman-Keuls post hoc test).
Fig. 2. Growth curve of 111In-BMSCs. A growth curve of 111In-BMSCs shows the dose-dependent effect of 111In-labeling. This experiment was performed in triplicate with error bars showing standard error. Concentrations of 111In were presented as Bq/cell. Bq =
becquerel, *p < 0.05, †p < 0.001, ‡p = 0.004.
C. In vivo tracking of
111In-BMSCs by gamma camera in trauma models and
controls
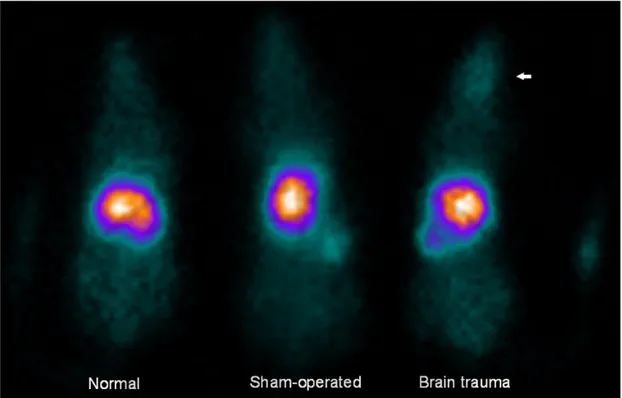
Planar gamma camera images revealed that most of 111In-BMSCs migrated to the liver at 24 h after injection in all three groups (Fig. 3), whereas the brain uptake was minimal. In the sham-operated rats and normal rats, brain uptake was not discernable visually. Unlike normal (0.3%) and sham-operated rats (0.5%), mild brain uptake (1.4%) was visible in trauma models injected with 111In-BMSCs. Other than brain uptake, the radio-uptake values of each organ among three groups were similar on gamma camera images.
Fig. 3. In vivo distribution of 111In-BMSCs in acute brain trauma rats, sham-operated control rats and normal rats at 24 h after intravenous transplantation. Most of the injected radioactivity was taken up by the liver (36%, 37%, and 35.1%, respectively normal, sham-operated, brain trauma), spleen (3%, 3.4%, and 4.2%) and lungs (2.1%, 2.4%, and 1.5%) in all three groups. The uptake of 111In-BMSCs in brain trauma rats was relatively prominent than those in sham-operated rats or normal rats.
D. Histological analysis of transplanted BMSCs in animal model of trauma
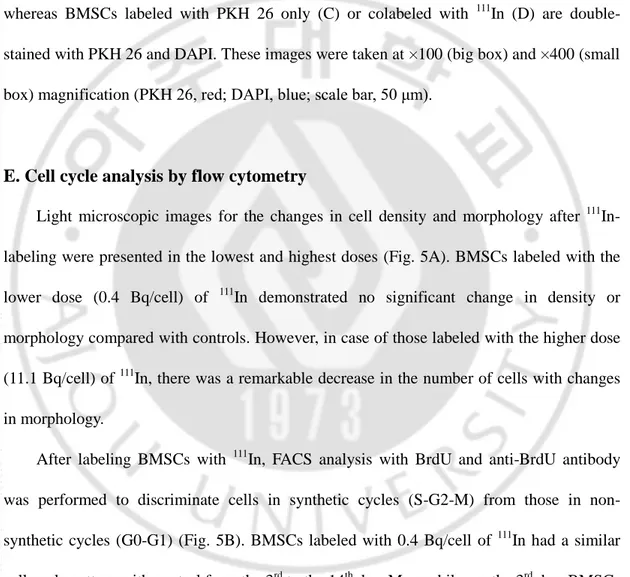
Migration of BMSCs was analyzed by fluorescent detection of cell on the margin of traumatic brain regions (Fig. 4A). In sham-operated controls, there were no PKH 26-positive (red) cells (Fig. 4B), whereas BMSCs labeled with PKH 26 only were found in the traumatic brains (Fig. 4C). As shown in Fig. 4D, PKH 26 and 111In colabeled BMSCs were alsodetected in the traumatic brains. The ratio of BMSCs stained with both PKH 26 and DAPI to total cells in the traumatic brain was 0.38 for PKH 26 labeling and 0.40 for PKH 26 and 111In colabeling. These confocal microscopy images revealed that BMSCs labeled with 111In migrated to the traumatic brains at 24 h after injection and their migratory ability seemed not to be affected by 111In-labeling. In trauma models, contralateral brain tissues did not contain BMSCs (data are not shown).
Fig. 4. Confocal laser scanning microscope image of transplanted BMSCs in the margin of traumatic regions. Rats were sacrificed at 24 h after injection and the existence of BMSCs in the margin of traumatic brain regions was identified by PKH 26-positive cells. Diagram of brain section (A). Sham-operated control shows no BMSCs in their brain (B), whereas BMSCs labeled with PKH 26 only (C) or colabeled with 111In (D) are double-stained with PKH 26 and DAPI. These images were taken at ×100 (big box) and ×400 (small box) magnification (PKH 26, red; DAPI, blue; scale bar, 50 μm).
E. Cell cycle analysis by flow cytometry
Light microscopic images for the changes in cell density and morphology after 111 In-labeling were presented in the lowest and highest doses (Fig. 5A). BMSCs labeled with the lower dose (0.4 Bq/cell) of 111In demonstrated no significant change in density or morphology compared with controls. However, in case of those labeled with the higher dose (11.1 Bq/cell) of 111In, there was a remarkable decrease in the number of cells with changes in morphology.
After labeling BMSCs with 111In, FACS analysis with BrdU and anti-BrdU antibody was performed to discriminate cells in synthetic cycles (S-G2-M) from those in non-synthetic cycles (G0-G1) (Fig. 5B). BMSCs labeled with 0.4 Bq/cell of 111In had a similar cell cycle pattern with control from the 3rd to the 14th day. Meanwhile, on the 3rd day, BMSCs labeled with more than 1.1 Bq/cell of 111In showed less BrdU positive cells than control by FACS (36.1%, 27.4%, and 28.3% for 1.1, 4.4 and 11.1 Bq/cell, respectively). After that, BMSCs labeled with 111In of 0.4 and 1.1 Bq/cell were recovered and had similar cell cycle
patterns to controls. On the contrary, BMSCs labeled with 111In of 4.4 and 11.1 Bq/cell could not recover and showed single peak (G0-G1) on the 14th day.
Fig. 5. Light microscopic images and cell cycle graphs of 111In-BMSCs. Light microscopic images show the density and morphological changes of BMSCs labeled with the lowest and the highest doses of 111In (A, original magnification- X200). Cell cycle graphs of 111
In-BMSCsare presented according to the dose and day of labeling (B). Proportions of BrdU positive cells are presented as percentage of total cell count. Concentrations of 111In were presented as Bq/cell. Bq = becquerel.
F.
Annexin V-FITC/PI double staining flow cytometryThe Annexin V-FITC and PI staining of 111In-BMSCswere performed at early (3 and 12 h) and late (7 days) stages for higher doses of 111In (11.1 and 33.3 Bq/cell, Fig.6).
Apototic (Annexin V+/PI-)/necrotic (Annexin V-/PI+) fractions were 0/0.9, 0.2/2.3 and 0.2/3.0% at 3 h, 0.1/1.2, 0.1/0.4 and 0/0.9% at 12 h and 0.4/2.8, 0.8/3.3 and 0.7/3.3% on the 7th day for 0 (control), 11.1 and 33.3 Bq/cell of 111In, respectively. FACS analysis with Annexin V-FITC and PI revealed no significant difference in apoptosis or necrosis from controls at all time points (3, 12 h and 7 days) and at all doses (11.1 and 33.3 Bq/cell). On day 7, double positive (late apoptotic) cells were observed in both 111In-BMSCs and control. However, the proportions of double positive cells of 111In-BMSCs were 20.5 and 20.3% for 11.1 and 33.3 Bq/cell of 111In, which were similar to that of control (23.9%)
Fig. 6. Apoptotic and necrotic change of 111In-BMSCs. Apoptosis and necrosis of 111 In-BMSCswas evaluated by staining with Annexin V-FITC and propidium iodide. Horizontal
and vertical axes show the number of Annexin V-FITC (+) cells and propidium iodide (+) cells, respectively. Concentrations of 111In were presented as Bq/cell. Bq = becquerel.
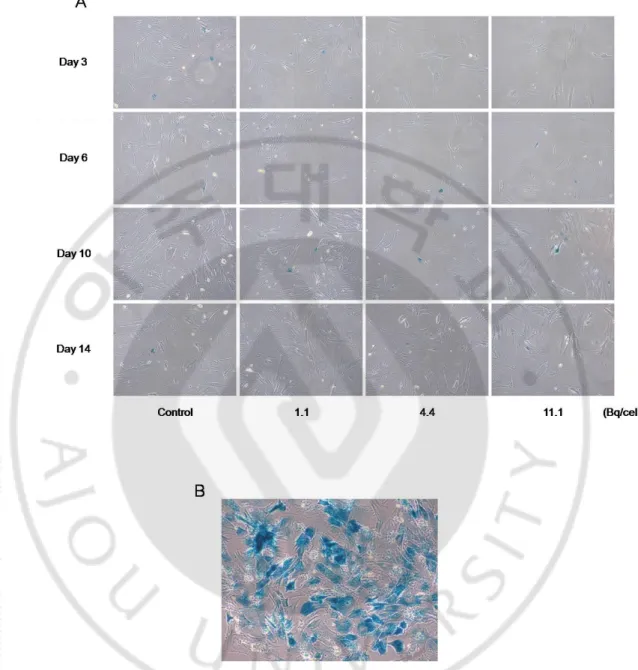
G. Senescence associated β-galactosidase histochemistry
SA-β-galactosidase was used as a marker for assessing senescence in cells. After X-gal staining with various doses of 111In from 1.1 to 11.1 Bq/cell, the cytochemical assay for 111 In-BMSCswas performed until the 14th day. Senescent cells were stained blue. As shown in Fig. 7A, most of the 111In-labeled BMSCs were not stained for SA-β-galactosidase at all concentrations of 111In from the 3rd day to the 14th day. There was no difference in the number of stained cells between 111In-BMSCs and controls, which indicates that 111 In-labeling does not induce a cellular senescence. For reference, BMSCs incubated in a starved condition were stained blue in senescent cells (Fig. 7B).
Fig. 7. Cellular senescence of 111In-BMSCs. Cellular senescence of 111In-BMSCs was evaluated by staining with X-gal (A). Positive staining cells which incubated in senescent environment were stained with X-gal (B). Senescent cells are stained blue. Concentrations of 111
IV. DISCUSSION
This study was performed to evaluate the migration of intravenously injected therapeutic mesenchymal stem cells in a brain disease model by a direct cell-labeling method with 111In-tropolone and to elucidate the effects of 111In-labeling on the growth, cell cycle and cellular death of labeled BMSCs. As a result, intravenously injected BMSCs migrated to the injured brain area, which were imaged by gamma camera with a direct cell labeling with 111
In, and 111In-labeling did not induce a cellular death within the experimental dose range despite it led to cell cycle arrest transiently at lower doses and permanently at higher doses. These results indicate that 111In-labeling could be used for monitoring of intravenously transplanted BMSCs in brain disease models without deteriorating viability significantly.
In this study, the viability of 111In-BMSCs measured by XTT assay at 48 h was not different from that of unlabeled cells. However, the proliferation of labeled BMSCs began to decrease on the 3rd day after radiolabeling and the difference in the number of viable cells between 111In-labeled and unlabeled BMSCs reached a statistical significance on the 6th, 10th and 14th day. This proliferative inhibition of 111In-BMSCs also showed a dose-dependency. For cells labeled with lower doses (0.4 and 1.1 Bq/cell), their growth had no significant difference from controls during the measurement period and, on the 14th day, they caught up with controls. Meanwhile, BMSCs labeled with higher doses (4.4 and 11.1 Bq/cell) had significantly lower cell numbers that controls until the 10th day (4.4 Bq/cell) and 14th day (11.1 Bq/cell). This inhibition of proliferative capacity was not due to tropolone or other chemicals that were added during the labeling process, because cold compound-treated
BMSCs proliferated similar to controls (data are not shown).
There have been conflicting reports concerning the cytotoxic effect of 111In on BMSCs. The Trypan blue excursion test revealed that the viability of 111In-labeled swine BMSCs (40 Bq/cell) was more than 95% at 48 h after radiolabeling (Chin et al., 2003). In another study with canine BMSCs, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay revealed a slight difference in metabolic activity/proliferation between labeled cells and unlabeled cells at 48 h and after the sixth day of labeling; however, there was no dose dependency at concentrations ranging from 5 to 30 mCi (185–1110 MBq) of 111In per million cells. Moreover, 111In-labeled canine BMSCs retained the ability to differentiate into adipose cells as efficiently as unlabeled BMSCs (Kraitchman et al., 2005). On the other hand, in another recent study, while all canine BMSCs exposed to 111In-tropolone of more than 8 Bq/cell did not survive at 14 days after labeling, all other cells incubated with 111In-tropolone of less than 0.9 Bq/cell were viable (Jin et al., 2005). As with the human BMSCs, at concentrations from 30 to 260 Bq of 111
In/cell, the doubling time of 111In-labeled human BMSCs (labeling efficiency=26 ± 5%) was not significantly different from that of the controls, but their proliferation was significantly inhibited at a higher radioactivity concentration (800 Bq 111In/cell) (Bindslev et al., 2006). Similarly, in this study, 4.4 and 11.1 Bq/cell had an inhibitory effect on the growth of BMSCs (p <.005). Although the threshold was different among those studies, possibly because of the different cell types and cell-handling methods, it seems obvious that, at high concentrations, 111In has an inhibitory effect on BMSCs. For comparison, the growth of 111 In-labeled H9c2 cell line derived from embryonic rat ventricle was evaluated. Just like the 111
In-BMSCs, H9c2 cells labeled with lower doses (0.4 and 1.1 Bq/cell) showed no significant difference from controls, whereas those labeled with higher doses (4.4 and 11.1 Bq/cell) were significantly inhibited until the 14th day of labeling (data are not shown). Another study with hematopoietic progenitor cells also supported this result (Brenner et al., 2004). 111 In-labeled hematopoietic progenitor cells showed impaired viability at 48 and 96 h after labeling, and their migration was decreased to 74% of the controls as early as 24 h after labeling. Moreover, they lost their colony-producing capacity (Brenner et al., 2004). Although the data were not shown in the results of this study, a similar pattern of proliferative inhibition was observed with 111In-labeled human BMSCs. Therefore, this radiation effect of 111In-labeling should be overcome by careful dosimetry prior to clinical application, and it should be investigated in other radioisotopes, such as 18F-FDG or 99mTc, which are used to monitor therapeutic cells.
For further evaluation of the effect of 111In-labeling on BMSCs, cell cycle analysis and cell death studies were performed in this study. Cell cycle was analyzed by FACS with BrdU and BrdU-specific antibodies to discriminate the cycling cells from the non-cycling cells. As expected, the cells labeled with a lower dose (0.4 Bq/cell) demonstrated no significant density and morphological change compared with controls, whereas, a higher dose (11.1 Bq/cell) caused a significant difference. The results obtained in cell cycle by BrdU incorporation to DNA were also of interest. Over time, the synthetic fraction became similar to controls at concentrations from 0.4 to 1.1 Bq/cell of 111In. However, at concentrations from 4.4 to 11.1 Bq/cell, BMSCs had different patterns of cell cycle from control during the observation period, which implies that cells received ionizing radiation over a certain range
could not recover from cell cycle arrest. On the other hand, apoptosis/necrosis assay and a test for premature senescence were performed to investigate whether a cell cycle arrest would induce a cell death in BMSCs. Annexin V-FITC/PI would discriminate between apoptotic fraction and necrotic fraction which could be used with a flow cytometric assay (Istvan et al., 1995). For higher doses of 111In (11.1 and 33.3 Bq/cell), FACS analysis were performed for BMSCs at early (3 and 12 h) and late (7 days) stages. 111In-BMSCs showed no significant apoptosis or necrosis regardless of stage and dose. In addition, SA β-galactosidase histochemistry was applied to 111In-BMSCs to evaluate cellular senescence, and, as a result, no significant difference in the degree of SA β-gal staining between labeled BMSCs (0.4-11.1 Bq/cell) and control was observed. These results indicates that although 111In-labeling affects on the proliferation of BMSCs and cell cycle in a dose-dependent manner, it does not lead to a cellular death such as necrosis, apoptosis or premature senescence and cell cycle arrest might be transient. In a recent study, researchers observed that 7 and 12 Gy of gamma radiation resulted in a significant decrease in cellular proliferation and this was caused by increased apoptosis, G2 cell cycle arrest and premature senescence (Schonmeyr et al., 2008). This is inconsistent with the result of the present study in which G1 cell cycle arrest was observed and a cellular death was not prominent. This difference seems to result from the dose and type of radiation. 111In irradiated a relatively low dose to BMSCs and produced Auger electrons as well as gamma radiation.
Despite the antiproliferative influence of radiolabeling, 111In-BMSCs survived and migrated to the diseased brain at 24 h after intravenous injection, which was confirmed by confocal microscope in the present study. We labeled both PKH 26 and 111In to the BMSCs
and infused those cells into the traumatic rats, and, as a result, colabeled BMSCs migrated to the traumatic brain regions, but not to the contralateral site or untraumatized brains of sham-operated controls. This result implies that 111In-BMSCs might retain their migrating capability in vivo, even though their proliferating capacity decreases significantly in vitro. This result is consistent with that of an earlier study of 111In-labeled canine BMSCs in which, although 111In induced a slight difference in metabolic activity/proliferation between labeled cells and unlabeled cells, 111In-BMSCs showed more migration to the infarcted myocardium (3.03 ± 2.9%) than the noninfarcted myocardium (1.38 ± 2.2%, p<.02) on day 1 (Lee-MacAry et al, 2001). Although the brain uptake of trauma models and sham-operated controls was easily discriminated by visual interpretation, the quantitative measurement using the ROI method revealed only mild uptake in the traumatic brains (1.4%). In addition, the difference from controls could not reach statistical significance. This was probably caused by the technical limitation of intravenous injection and the low resolution of clinical gamma camera.
It has already been known that an obstacle to the migration of intravenously injected stem cells was an initial trapping in the lungs (Kang et al., 2006; Jin et al., 2005). In rat models, the lung uptake of BMSCs was significantly higher with intravenous infusion than with intracavitary infusion (53.3 ± 9.8 vs. 1.9 ± 0.56, p<.01), whereas the uptake of BMSCs by the heart was significantly lower with intravenous infusion than with intracavitary infusion (0.2 ± 0.02 vs. 0.9 ± 0.32, p<.001) (Barbash et al., 2003). Similarly, 111In-oxine labeled neural and hippocampal progenitor cells distributed mostly to the internal organs after intravenous injection, in contrast, a weak gamma camera signal was detected in the
ischemic brains after intraarterial injection (Lappalainen et al., 2008). Therefore, some authors preferred to use direct injection methods, such as intracoronary or intracavitary injection, instead of intravenous injection to enhance the migration of stem cells to the target tissue. Lee et al. (Lee et al., 2008) recently demonstrated the effects of BMSCs therapy in patients with MSA, an intractable neurodegenerative disease, using a combined intraarterial and intravenous infusion transplantation method. Although direct implantation of BMSCs into the target area of the target organ is the most effective method for therapeutic cell delivery, the issue of patient safety remains unclear, even with autologous cells. In this study, the comparison between the different injection methods was not done, because this experiment was performed as a preclinical study for intravenous stem cells tracking in human.
Another technical limitation of this study was that a clinical gamma camera was used instead of a small animal imaging camera. In recent years, studies with small animal imagers, which could give more accurate localization, have been increased (Lappalainen et al., 2008; Min et al., 2006; Huang et al., 2008). The resolution of the clinical gamma camera was not high enough for the discrimination between the traumatic hemisphere and the contralateral one, even though we acquired three-dimensional tomographic images (data are not shown in this article). Therefore, to compensate this limitation, a confocal microscope was adopted to confirm the presence of 111In-BMSCs in the traumatic brains. Confocal microscope demonstrated the migration of colabeled BMSCs to the peritraumatic cavity regions. Maintenance of BMSCs characteristics as stem cells is important to keep homing ability (Karp et al., 2009). Intraarterial delivery close to the target site was shown to enhance
homing to the brain significantly vs. distant intravenous injection by “passive entrapment” of BMSCs (Walczak et al., 2008). Demonstration of intravenously infused 111In-BMSCs at the traumatic brain region in this study is of value as it indicates that stem cell characteristics of BMSCs is still maintained in spite of radioisotope labeling.
As BMSCs are multipotent undifferentiated cells, these transplanted cells are able to migrate to the nontargeted organ and even proliferate in the unwanted direction in vivo (George et al., 2006; Friedenstein et al., 1974). It was recently reported that murine mesenchymal stem cells generated osteosarcoma-like lesions in the lung (Aguilar et al., 2007). Though this is rare evidence of unwanted differentiation of grafted murine mesenchymal stem cells, it should not be ignored. In the present study, continuous irradiation from 111In that was used to label BMSCs caused a significant reduction in the proliferation of radiolabeled cells, but it did not lead to tumor formation.
For this study, 111In-tropolone from 111In-chloride was manually synthesized based on previous reports concerning leukocyte labeling (Thakur et al., 1977; Scheffel et al., 1982; Gunter et al., 1983). As well, many of the previous studies used 111In-oxine rather than 111 In-tropolone. However, we used 111In-tropolone, because a commercial kit for 111In-oxine was not available, and the procedure is easier with 111In-tropolone than 111In-oxine. The resultant labeling efficiency of 111In-tropolone (n=9, 66%) to BMSCs was more than 20% higher than that of 111In-oxine (n=8, 37%) in this study (P<.01).
V. CONCLUSION
Although its influence on the viability and growth of BMSCs needs further elucidation, radiolabeling with 111In could be useful for tracking intravenously injected BMSCs in an animal model of brain disease. We observed the dose-dependent growth inhibition of BMSCs by 111In-labeling, which was developed by dose-dependent, transient cell cycle arrest, not by cellular senescence or apoptosis/necrosis.
REFERENCES
1. Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N, et al: Murine but not human mesenchymal stem cells generate osteosarcoma like lesions in the lung. Stem
Cells 25:1586–1594, 2007
2. Bang OY, Lee JS, Lee PH, Lee G: Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57: 874-882, 2005
3. Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al: Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108: 863–868, 2003
4. Berger MM, See DM, Redl B, Aymard M, Bruno L: Direct in situ transcriptase polymerase chain reaction for the detection of Enterovirus genome in liver tissues. J Virol
Methods 65: 55–66, 1997
5. Bindslev L, Haack-Sorensen M, Bisgaard K, Kragh L, Mortensen S, Hesse B, et al: Labelling of human mesenchymal stem cells with indium-111 for SPECT imaging: effect on cell proliferation and differentiation. Eur J Nucl Med Mol Imaging 33: 1171–1177, 2006
6. Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U, et al: 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med 45: 512–518, 2004
7. Chen S, Pickard JD, Harris NG: Time course of cellular pathology after controlled cortical impact injury. Exp Neurol 182: 87-102, 2003
8. Chin BB, Nakamoto Y, Bulte JW, Pittenger MF, Wahl R, Kraitchman DL: 111In-oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun 24: 1149–1154, 2003
9. Choi Bu-Young, Kim Hak-Yup, Lee Ki-Ho, Cho Youl-Hee, Kong Gu: Clofilium, a potassium channel blocker, induces apoptosis of human promyelocytic leukemia (HL-60) cells via Bcl-2-insensitive activation of caspase-3. Cancer Letters 147: 85-93, 1999
10. Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, et al: Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 2: 83–92, 1974
11. George J, Kuboki Y, Miyata T: Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng 95: 404–411, 2006
12. Gunter KP, Lukens JN, Clanton JA, Morris PJ, Janco RL, English D: Neutrophil labeling with indium-111: tropolone vs. oxine. Radiology 149: 563–566, 1983
13. Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al: Monitoring of bone marrow cell homing into the infarcted human myocardium.
Circulation 111: 2198–2202, 2005
14. Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, et al: Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 199: 56–66, 2006
15. Huang J, Lee CC, Sutcliffe JL, Cherry SR, Tarantal AF: Radiolabeling rhesus monkey CD34+ hematopoietic and mesenchymal stem cells with 64 Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for micro PET imaging. Mol Imaging 7:1-11, 2008
16. Istvan Vermes, Clemens Haanen , Helga Steffens-Nakken , Chris Reutelingsperger: A novel assay for apoptosis Flow cytometric detection of phosphatidylserine early apoptotic cells using fluorescein labeled expression on Annexin V. J Immuno Methods 184: 39-51, 1995
17. Jin Y, Kong H, Stodilka RZ, Wells RG, Zabel P, Merrifield PA, et al: Determining the minimum number of detectable cardiac-transplanted 111In-tropolone-labelled
bone-marrow-derived mesenchymal stem cells by SPECT. Phys Med Biol 50: 4445–4455, 2005
18. Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS: Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med 47: 1295–1301, 2006
19. Karp JM, Leng Teo GS: Mesenchymal stem cell homing: the devil is in the details. Cell
Stem Cell 4: 206–216, 2009
20. Kazuaki Mishima, James T. Handa, Amy Aotaki-Keen, Gerard A. Lutty, Lawrence S. Morse, Leonard M. Hjelmeland: Senescence-Associated β-Galactosidase Histochemistry for the Primate Eye. IOVS, 40: 1590-1593, 1999
21. Kim H, Kim S, Ahn Y: Animal model of traumatic brain injury induced by controlled cortical impact device. J Kor Neurotraumatol Soc 3: 91–98, 2007
22. Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al: Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation 112: 1451–1461, 2005
23. Lappalainen RS, Narkilahti S, Huhtala T, Liimatainen T, Suuronen T, Narvanen A, et al: The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci
Lett 440: 246–250, 2008
24. Lee-MacAry AE, Ross EL, Davies D, Laylor R, Honeychurch J, Glennie MJ, et al: Development of a novel flow cytometric cellmediated cytotoxicity assay using the fluorophores PKH-26 and TOPRO-3 iodide. J Immunol Methods 252: 83–92, 2001
25. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K: Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther 83: 723–730, 2008
26. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al: Human marrow stromal cell therapy for stroke in rat; neurotrophins and functional recovery. Neurology 59: 514–23, 2002
27. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF: Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow.
28. Min JJ, Ahn Y, Moon S, Kim YS, Park JE, Kim SM, et al: In vivo bioluminescence imaging of cord blood derived mesenchymal stem cell transplantation into rat myocardium. Ann Nucl Med 20: 165–170, 2006
29. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH: Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem 107: 141–151, 2008
30. Philip M. Bak and Ralph J. Panos: Protease Antigen Recovery Decreases the Specificity of Bromodeoxyuridine Detection In Formalin-fixed Tissue. J Histochem Cytochem 45: 1165–1170, 1997
31. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al: Multilineage potential of adult human mesenchymal stem cells. Science : 284:143– 147,1999
32. Riggi N, Cironi L, Provero P, Suva ML, Kaloulis K, Garcia-Echeverria C, et al: Development of Ewing's sarcoma from primary bone marrowderived mesenchymal progenitor cells. Cancer Res 65:11459–11468, 2005
33. Scheffel U, Tsan MF, Mitchell TG, Camargo EE, Braine H, Ezekowitz MD, et al: Human platelets labeled with In-111 8-hydroxyquinoline: kinetics, distribution, and estimates of
radiation dose. J Nucl Med 23: 149–156, 1982
34. Sekiya I, Larson BL, Vuoristo JT, Cui JG, Prockop DJ: Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res 19: 256– 264, 2004
35. Schonmeyr BH, Wong AK, Soares M, Fernandez J, Clavin N, Mehrara BJ: Ionizing radiation of mesenchymal stem cells results in diminution of the precursor pool and limits potential for multilineage differentiation. Plast Reconstr Surg 222: 64-76, 2008.
.
36. Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al: Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96: 1593–1603, 2004
37. Thakur ML, Coleman RE, Welch MJ: Indium-111-labeled leukocytes for the localization of abscesses: preparation, analysis, tissue distribution, and comparison with gallium-67 citrate in dogs. J Lab Clin Med 89: 217–228, 1977
38. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C: A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184: 39-51, 1995
39. Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al: Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 39: 1569–1574, 2008
40. Woodbury D, Schwarz EJ, Prockop DJ, Black IB: Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61: 364–370, 2000
41. Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al: Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 80: 267–274, 2006
- 국문요약 -
급성 brain trauma 모델에서
111In 으로 표지된
중간엽 줄기세포의 귀소성
아주대학교 대학원 의학과 박 복 남 (지도교수: 윤 준 기, 안 영 환) 본 연구는 급성 외상성 뇌 질환 동물모델에 111 In-tropolone으로 표지한 중간엽 줄기 세포를 정맥 내 주사하여 생체 내 분포를 알아보고, 111 In-표지가 세포의 생육성과 기능에 미치는 영향을 보고자 하는 것이다. Rat에서 유래한 중간엽 줄기 세포를 37 MBq 111 In-tropolone으로 표지하고, 그것의 표지율을 측정하고 생체 외에서 잔류를 측정하였다. 111 In의 방사선량에 따른 표지 영향을 보기 위해서, 줄기 세포를 다양한 방사선량의 111 In-tropolone (0.4–11.1 Bq/cell)으로 표지하였고, 각각 다른 방사선량으로 표지 후에 성장곡선 실험, 5-bromo-2-deoxy-uridine (BrdU)로 염색하여 fluorescent activated cells sorter (FACS) 분석을 하였으며, 5-bromo-4-chloro-3-indolyl-D-galactopyranoside (X-gal)로 염색 후에 현미경으로 분석하였다. Annexin V- fluorescein isothiocyanate (FITC)/ propidium iodide (PI)으로 염색한 후에 FACS 분석을 실시했는데, 높은 방사선량 (11.1 and 33.3 Bq/cell)의 In-111로 표지하여 세포 자멸사나 괴사 변화를 확인하기 위해 초기 (표지 3, 12 시간후)와 후기 (표지 7 일 후) 단계의 변화를 비교하였다. 111 In-tropolone으로 표지한 중간엽 줄기 세포를 투여한 후에 감마 카메라 영상으로 뇌 질환 동물모델, 겉보기 수술한 동물, 정상 모델에서의 생체 내 분포를 비교하였다. 111 In으로 표지한 중간엽 줄기 세포가 뇌 질환이 있는 부위로 이동하는 것을 confocal 현미경으로 확인하였다. 111 In-중간엽 줄기 세포의 표지율은 평균 66 ± 5%이고, 표지 1시간 후의 잔류율은 85.3%였다. 표지 48시간 후에, 111 In으로 표지한 세포와 대조군 사이에 살아있는 세포 수 차이는 없었다. 그러나, 표지한 지3일 후에, 111 In으로 표지한 중간엽 줄기 세포의 증식은 억제되었으며, confluency에 도달하지 못하였다. 낮은 방사성량 (0.4와 1.1 Bq/cell)의 In-111으로 표지한 세포의 경우, 성장에 있어서는 대조군과 다르지 않았으나, 높은 방사선량 (4.4 와 11.1 Bq/cell)의 In-111로 표지한 줄기세포의 경우 3 일째부터 14 일째까지 증식이 현저하게 제한되었다. FACS 분석 결과에서도 표지 3일째에 1.1, 4.4, 11.1 Bq/cell에서 대조군에 비해 BrdU에 대해 낮은 양성 반응을 보였다. 이들 중에서, 0.4, 1.1 Bq/cell의 In-111로 표지한 BrdU 양성 반응이 있는 줄기 세포는 대조군과 비교해 볼 때, 시간이 지난 후에 회복되는 것을 확인하였다. 반면에, 4.4, 11.1 Bq/cell로 표지한 줄기 세포는 그 이후에도 세포 주기 정지가 회복되지 않았다. 노화와 연관된 β-gal 염색은 표지 후 14일까지 보았지만, 모든 세포에서 보여지지 않았다. 또한, Annexin V-FITC와 PI를 이용한 FACS 실험에서도 초기와 후기 단계에서 모두 세포 자멸사나 괴사를 보이지 않음을 확인하였다. 감마 카메라 영상에서는 투여한지 이틀째에 대부분의 111 In-중간엽 줄기 세포가 간과 비장에서 섭취되는 것을 볼 수 있었다. 111 In-중간엽 줄기 세포의 뇌 섭취는 겉보기 수술한 동물 (0.5%)이나, 정상 쥐 (0.3%)에서 보다는 뇌 질환 동물모델 (1.4%)에서
현저하게 높게 관찰되었다. Confocal 현미경 상에서 방사성 표지된 중간엽 줄기 세포가 뇌 질환이 있는 곳에서 확인되었다. In-111 표지 후에, 방사선량에 의존한 중간엽 줄기 세포의 성장이 억제되는 것을 확인했는데, 이는 방사선량 혹은 일시적인 세포 주기 정지에 의한 것이며, 세포성 노화나, 세포 자멸사/괴사에 의한 것은 아닌 것으로 관찰되었다. 비록 111 In 표지로 인해 성장 억제가 임상 사용에 앞서 좀 더 검토가 필요하지만, 111 In-중간엽 줄기 세포는 뇌 질환 동물모델에서 정맥 내 투여한 줄기세포를 추적하는 데는 유용할 것으로 기대한다. 핵심어: 111
In-tropolone, Bone marrow mesenchymal stem cells, Cell tracking, Growth arrest, Traumatic brain, Radiotoxicity